Evaluating the Electrochemical Properties of Supercapacitors using the Three-Electrode System
Summary
The protocol describes the evaluation of various electrochemical properties of supercapacitors using a three-electrode system with a potentiostat device.
Abstract
The three-electrode system is a basic and general analytical platform for investigating the electrochemical performance and characteristics of energy storage systems at the material level. Supercapacitors are one of the most important emergent energy storage systems developed in the past decade. Here, the electrochemical performance of a supercapacitor was evaluated using a three-electrode system with a potentiostat device. The three-electrode system consisted of a working electrode (WE), reference electrode (RE), and counter electrode (CE). The WE is the electrode where the potential is controlled and the current is measured, and it is the target of research. The RE acts as a reference for measuring and controlling the potential of the system, and the CE is used to complete the closed circuit to enable electrochemical measurements. This system provides accurate analytical results for evaluating electrochemical parameters such as the specific capacitance, stability, and impedance through cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS). Several experimental design protocols are proposed by controlling the parameter values of the sequence when using a three-electrode system with a potentiostat device to evaluate the electrochemical performance of supercapacitors. Through these protocols, the researcher can set up a three-electrode system to obtain reasonable electrochemical results for assessing the performance of supercapacitors.
Introduction
Supercapacitors have attracted enormous attention as suitable power sources for a variety of applications such as microelectronic devices, electric vehicles (EVs), and stationary energy storage systems. In EV applications, supercapacitors can be used for rapid acceleration and can enable the storage of regenerative energy during the deceleration and braking processes. In renewable energy fields, such as solar power generation1 and wind power generation2, supercapacitors can be used as stationary energy storage systems3,4. Renewable energy generation is limited by the fluctuating and intermittent nature of these energy supplies; therefore, an energy storage system that can respond immediately during irregular power generation is required5. Supercapacitors, which store energy via mechanisms that differ from those of lithium-ion batteries, exhibit a high power density, stable cycle performance, and fast charging-discharging6. Depending on the storage mechanism, supercapacitors can be distinguished into double-layer capacitors (EDLCs) and pseudocapacitors7. EDLCs accumulate electrostatic charge at the electrode surface. Therefore, the capacitance is determined by the amount of charge, which is affected by the surface area and porous structure of the electrode materials. By contrast, pseudocapacitors, which consist of conducting polymers and metal oxide materials, store charge through a Faradaic reaction process. The various electrochemical properties of supercapacitors are related to the electrode materials, and developing new electrode materials is the main issue in improving the performance of supercapacitors8. Hence, evaluating the electrochemical properties of these new materials or systems is important in the progress of research and further applications in real life. In this regard, electrochemical evaluation using a three-electrode system is the most basic and widely utilized method in lab-scale research of energy storage systems9,10,11,12,13.
The three-electrode system is a simple and reliable approach for evaluating the electrochemical properties, such as the specific capacitance, resistance, conductivity, and cycle life of supercapacitors14. The system offers the benefit of enabling analysis of the electrochemical characteristics of single materials15, which is in contrast to the two-electrode system, where the characteristics can be studied through the analysis of the given material. The two-electrode system just gives information about the reaction between two electrodes. It is suitable for analyzing the electrochemical properties of the entire energy storage system. The potential of the electrode is not fixed. Therefore, it is not known at what voltage the reaction takes place. However, three-electrode system analyzes only one electrode with fixing potential which can perform a detailed analysis of the single electrode. Therefore, the system is targeted toward analyzing the specific performance at the material level. The three-electrode system consists of a working electrode (WE), reference electrode (RE), and counter electrode (CE)16,17. The WE is the target of research, assessment as it performs the electrochemical reaction of interest18 and is composed of a redox material that is of potential interest. In the case of EDLCs, utilizing high surface area materials is the main issue. Therefore, porous materials with a high surface area and micropores, such as porous carbon, graphene, and nanotubes, are preferred19,20. Activated carbon is the most common material for EDLCs because of its high specific area (>1000 m2/g) and many micropores. Pseudocapacitors are fabricated with materials that can undergo a Faradaic reaction21. Metal oxides (RuOx, MnOx, etc.) and conducting polymers (PANI, PPy, etc.) are commonly used22. The RE and CE are used to analyze the electrochemical properties of the WE. The RE serves as a reference for measuring and controlling the potential of the system; the normal hydrogen electrode (NHE) and Ag/AgCl (saturated KCl) are generally chosen as the RE23. The CE is paired with the WE and completes the electrical circuit to allow charge transfer. For the CE, electrochemically inert materials are used, such as platinum (Pt) and gold (Au)24. All components of the three-electrode system are connected to a potentiostat device, which controls the potential of the entire circuit.
Cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS) are typical analytical methods that use a three-electrode system. Various electrochemical characteristics of supercapacitors can be assessed using these methods. CV is the basic electrochemical method used to investigate the electrochemical behavior (electron transfer coefficient, reversible or irreversible, etc.) and capacitive properties of material during repeated redox processes14,24. The CV plot shows redox peaks related to the reduction and oxidation of the material. Through this information, researchers can evaluate the electrode performance and determine the potential where the material is reduced and oxidized. Furthermore, through CV analysis, it is possible to determine the amount of charge that material or electrode can store. The total charge is a function of the potential, and the capacitance can be easily calculated6,18. Capacitance is the main issue in supercapacitors. A higher capacitance represents the ability to store more charge. EDLCs give rise to rectangular CV patterns with linear lines so that the capacitance of the electrode can be calculated easily. Pseudocapacitors present redox peaks in rectangular plots. Based on this information, researchers can assess the electrochemical properties of materials using CV measurements18.
GCD is a commonly employed method for identifying the cycle stability of an electrode. For long-term use, the cycle stability should be verified at a constant current density. Each cycle consists of charge-discharge steps14. Researchers can determine the cycle stability through variations in the charge-discharge graph, specific capacitance retention, and Coulombic efficiency. EDLCs give rise to a linear pattern; thus, the specific capacitance of the electrode can be calculated easily using the slope of the discharge curve6. However, pseudocapacitors exhibit a nonlinear pattern. The discharge slope varies during the discharging process7. Furthermore, the internal resistance can be analyzed through the current-resistance (IR) drop, which is the potential drop owing to the resistance6,25.
EIS is a useful method for identifying the impedance of energy storage systems without destruction of the sample26. The impedance can be calculated by applying a sinusoidal voltage and determining the phase angle14. The impedance is also a function of the frequency. Therefore, the EIS spectrum is acquired over a range of frequencies. At high frequencies, kinetic factors such as the internal resistance and charge transfer are operative24,27. At low frequencies, the diffusion factor and Warburg impedance can be detected, which are related to mass transfer and thermodynamics24,27. EIS is a powerful tool for analyzing the kinetic and thermodynamic properties of a material at the same time28. This study describes the analysis protocols for evaluating the electrochemical performance of supercapacitors using a three-electrode system.
Protocol
1. Fabrication of electrode and supercapacitor (Figure 1)
- Prepare the electrodes prior to the electrochemical analysis by combining 80 weight (wt)% of the electrode active material (0.8 g activated carbon), 10 wt% of the conductive material (0.1 g carbon black), and 10 wt% of the binder (0.1 g polytetrafluoroethylene (PTFE)).
- Drop isopropanol (IPA; 0.1-0.2 mL) into the above-mentioned mixture, then spread the mixture thinly into a dough with a roller.
- Before attaching the electrode to stainless steel (SUS) mesh, cut the SUS mesh to dimensions of 1.5 cm (width) × 5 cm (length). After weighing the SUS mesh, coat the electrode (1 cm2) with a thickness of 0.1-0.2 mm on a SUS mesh and compress it with an electrode pressing machine. Here, the mass range of the electrode was 0.001-0.003 g.
- Dry the assembled supercapacitor electrode in an oven at 80 °C for about 1 day to evaporate the IPA.
- Weigh the SUS mesh to obtain the weight of the electrode and then immerse the mesh in the electrolyte (2 M H2SO4 aqueous solution).
- Place the SUS mesh in a desiccator to remove air bubbles at the surface of the supercapacitor electrode.
2. Preparation of sequence file for electrochemical analysis
- CV sequence settings to obtain the analysis results.
- Run the potentiostat measurement program to set the measurement experiment sequence file (Figure 2A).
- Click the Experiment button in the toolbar and go to Sequence File Editor > New or click the New Sequence button (Figure 2B). Click the Add button to add a sequence step (Figure 3A).
- In every step, set Control as Sweep, Configuration as PSTAT, Mode as CYCLIC, and Range as Auto. Set the Reference for Initial(V) and Middle(V) as Eref and put -200e-3 in the Value. Set the Reference for Final(V) as Eref and put 800e-3 in the Value.
- The voltage scan rate is set as the desired value by the user. Here, the scan rate was set to 10 mV/s. Put the value in Scanrate(V/s) as 10.0000e-3. Copy step-1 and click Paste[Dn] to paste it to step-2~5. Change the value of Scanrate(V/s) to either 20.000e-3, 30.000e-3, 50.000e-3, and 100.00e-3 respectively.
- Set Quiet time(s) as 0 and Segments as the number 2n+1 where n is the number of cycles. Here, 21 was applied for 10 cycles.
- Set Cut Off Condition as follows: for Condition-1 set Item as Step End and Go Next as Next.
- In the Controlling Miscellaneous Setting section, in the Sampling tab, set Item as Times(s), OP as >=, and DeltaValue as 0.333333 (step-1), 0.166666 (step-2), 0.111111 (step-3), 0.06667 (step-4), and 0.03333 (step-5) for each scan rate. This is the time interval for recording the data.
- Click Save As to save the CV analysis sequence file in any folder of the computer.
- GCD sequence settings to obtain the analysis results
- Run the potentiostat measurement program to set the measurement experiment sequence file (Figure 2A).
- Click the Experiment button in the toolbar and go to Sequence File Editor > New or click the New Sequence button (Figure 2B). Click the Add button to add a sequence step (Figure 4A,B).
- In Step-1, set Control as CONSTANT, Configuration as GSTAT, Mode as NORMAL, and Range as Auto. Set the Reference for Current(A) as ZERO. When the mass of the electrode is 0.00235 g, set Value as 1.8618e-3 which means the current density is 1 A/g.
- Set Cut Off Condition as follow: for Condition-1 set Item as Voltage, OP as >=, DeltaValue as 800e-3, and Go Next as Next.
- Set the following in the Controlling Miscellaneous setting section: in the Sampling tab, set Item as Times(s), OP as >=, and DeltaValue as 0.1.
- In Step-2, each set is the same as in Step-1, except set value of Current(A) as the negative value of Step-1 (-1.8618e-3). Set Condition-1 as follows: Item as Voltage, OP as <=, DeltaValue as -200e-3, and Go Next as Next.
- In Step-3, set Control as LOOP, Configuration as CYCLE, and set List-1 in Condition-1 of Cut Off Condition as Loop Next, Go Next as Step-1, and set List-2 as Step End, and Go Next as Next. Set the Iteration value as 10 which is the number of repeating cycles.
- Step-1, step-2, and step-3 form a single loop. Copy and paste them after step-4 and change the value of Current (A) to either 3.7236e-3, 5.5855e-3, 9.3091e-3, or 18.618e-3, calculated for various current densities of 2,3,5, and 10 A/g.
- Click Save As to save the GCD analysis sequence file in any folder of the computer.
- EIS sequence settings to obtain the analysis results
- Run the potentiostat measurement program to set the measurement experiment sequence file (Figure 2A).
- Click the Experiment button in the toolbar and go to Sequence File Editor > New or click the New Sequence button (Figure 2B). Click the Add button to add a sequence step (Figure 5A,B).
- In Step-1, set Control as CONSTANT, Configuration as PSTAT, Mode as TIMER STOP, and Range as Auto. Set the Reference for Voltage(V) as Eref and Value as 500e-3 which is half of the size of the voltage range.
- Set cut-off condition as follows: for Condition-1 set Item as Step Time, OP as >=, DeltaValue as 3:00, and Go Next as Next. This is the process for stabilizing the potentiostat device.
- In Step-2, set Control as EIS, Configuration as PSTAT, Mode as LOG, and Range as Auto. Set Speed of Initial (Hz) as Normal and value of Initial (Hz) and Middle (Hz) as 1.0000e+6 which is the high-frequency value and Final (Hz) as 10.000e-6, which is the low-frequency value.
- Set the Reference for Bias(V) as Eref and Value as 500e-3. To get a linear response result, set the amplitude (Vrms) as 10.000e-3. Set Density as 10 and Iteration as 1.
- Click Save as to save the EIS analysis sequence file in any folder of the computer.
3. Electrochemical analysis
- Operate the potentiostat device and run the measurement program to perform the CV, GCD, and EIS analyses. Fill 100 mL of 2 M H2SO4 aqueous electrolyte in a glass container (a beaker-shaped glass container was used).
- Before starting the measurement, in the potentiostat, connect the three types of lines: the working electrode (L-WE), the reference electrode (L-RE), and the counter electrode (L-CE), to the SUS mesh, reference electrode (Ag/AgCl), and counter electrode (Pt wire), respectively (Figure 6). Connect the fourth line, the working sensor (L-WS) to the L-WE.
- Cover the glass container with a cap, and immerse the three electrodes in the electrolyte through a perforation in the cap. Position the electrodes so that the WE is maintained at a constant distance between the CE and RE.
- Run the measurement program and open the prepared sequence. Click Apply to CH to insert the sequence to the potentiostat's channel. Start the measurement by clicking the Start button.
4. Data analysis
- CV data analysis for fitting the graph
- Open raw measurement data in the convert program to obtain the results in spreadsheet format. Click the File button and open the raw data. Select all cycles and click Export ASCII on the toolbar. Check the Cycle, Voltage, and Current in Columns to Export on the right side of the program.
- Click Create Directory and then click Export to convert raw data to spreadsheet format.
- Open the spreadsheet file and extract the voltage and current values of cycles 10, 20, 30, 40, and 50, which are the last cycles at each scan rate.
- Plot the CV graph with the voltage as the X-axis and specific current density as the Y-axis.
- GCD data analysis for fitting the graph
- Open raw measurement data in the convert program to obtain the results in spreadsheet format. Click the File button and open the raw data. Select all cycles and click Export ASCII on the toolbar. Check the Cycle, Voltage, and CycleTime in Columns to Export on the right side of the program.
- Click Create Directory and then click Export to convert raw data to spreadsheet format.
- Open the spreadsheet file and extract the voltage and CycleTime values for cycles 10, 20, 30, 40, and 50, which are the last cycles at each current density.
- Plot the GCD graph with the cycle time as the X-axis and voltage as the Y-axis.
- EIS data analysis for fitting the graph
- Open raw measurement data in EIS program. Click the Open file icon and open raw data and click the file name that was applied to see the detailed data.
- Extract Z' [Ohm] as the X value and Z'' [Ohm] as the Y value and plot the EIS graph.
Representative Results
The electrodes were manufactured according to protocol step 1 (Figure 1). Thin and homogeneous electrodes were attached to SUS mesh with a size of 1 cm2 and 0.1-0.2 mm thickness. After drying, the weight of the pure electrode was obtained. The electrode was immersed in a 2 M H2SO4 aqueous electrolyte, and the electrolyte was allowed to sufficiently permeate the electrode before the electrochemical analyses. The production sequence and system setting for the electrochemical measurements were performed according to protocol steps 2 and 3 (Figure 2 – Figure 5). The glass container used in the system can have various shapes29 where the distance between each electrode is minimized. The measurement results were organized and interpreted according to protocol step 4. To confirm whether the analysis was successful, the real-time graph obtained during the analysis and the shape of the graph of the raw data obtained after the analysis should be checked (Figures 3B,4C,5C). In the case of CV, a box-shaped graph was obtained at 300 mV/s, whereas GCD showed a symmetrical triangle. In the case of EIS, it is possible to check whether the analysis is properly performed through the size of the equivalent series resistance and semicircle, and the pattern at a low frequency depending on the material characteristics.
Figure 7 presents the CV, GCD, and EIS data. CV is the most common technique for determining the capacitance of electrodes and the characteristics of materials as a function of the potential. The well-developed rectangle-shaped CV graph in the scan rate range from 10 to 200 mV/s indicates EDLC characteristics and confirms that the supercapacitor operated well as an EDLC with good rate capability30 (Figure 7A). However, when the scan rate was above 300 mV/s, the graph lost its rectangular shape and collapsed, which means that the electrode lost the EDLC characteristics (Figure 7B). The specific capacitance of supercapacitors can be calculated from the CV data at each scan rate using the following equation6:
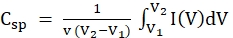 (1)
(1)
where Csp, v, V1, V2, and I(V) are the specific capacitance, scan rate, discharge voltage limit, charge voltage limit, and voltammogram current density (A/g), respectively. The specific capacitance was 126, 109, 104, 97, and 87 F/g at respective scan rates of 10, 20, 30, 50, and 100 mV/s.
GCD can be used to determine the cycle stability and resistance parameters of the electrode. As shown in Figure 7C, the GCD graph of the electrode presented a symmetric linear profile31 in all current densities within the potential range from −0.2 to 0.8 V. This is also a characteristic property of EDLCs. Subsequently, as the current density increased, the time on the x-axis decreased, and the area of the triangle decreased. The specific capacitance was calculated by dividing the discharge time by the voltage and multiplying by the current density, giving values of 153, 140, 135, 120, and 110 F/g at the respective current densities of 1, 2, 3, 5, and 10 A/g. The internal resistance (RESR) was calculated using the following equation32:
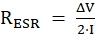 (2)
(2)
where ΔV is the IR drop, which is the potential drop due to the resistance (this is an additive effect of the cell components and electrolytes6,25), and I is the current density. The value of RESR was 0.00565 Ω at a current density of 1 A/g. The long-cycle test can be used to determine the cycle stability of the WE. The cycle stability is one of the main issues in energy storage systems when applied to an electrical device and can be confirmed by repeating many cycles at a constant current density. As shown in Figure 7D, the AC WE showed 99.2% capacitance retention over 10000 cycles at a current density of 10 A/g.
The EIS graphs are plotted in Figure 7E,F. EIS is a useful method for identifying the resistance of cell systems without destruction. The impedance of the cell is a function of the frequency (the typical frequency range is from 100 kHz to 10 MHz) with a small voltage (5 mV or 10 mV)14,33. In addition, the Nyquist plot is a common way to represent the impedance data, where the imaginary/real part of the impedance is plotted in the frequency range. The resulting data are recorded from the high-frequency domain to the low-frequency domain, and each part represents various types of resistance6. As shown in Figure 7E, the Nyquist plot can be divided into four parts. Part A corresponds to the equivalent series resistance, which is known as the sum of the resistance of the bulk electrolyte34,35 and the contact resistance between the electrode and the current collector36,37. Part B presents a semicircle, the diameter of which reflects the electrolyte resistance in the pores of the electrodes38 or charge transfer resistance34. Furthermore, the sum of parts A and B can be interpreted as the internal resistance, which is the sum of the bulk electrolyte resistance and the charge transfer resistance36. In part C, the 45° line region indicates the ion transport limitation of the electrode structures in the electrolyte34,39 or ion transport limitation in the bulk electrolyte35. Lastly, the vertical line in part D (Figure 7F) is attributed to the dominant capacitive behavior of the electric double layer formed at the electrode/electrolyte interface40. The EIS graph for the example system showed very small equivalent series resistance and semicircle (Rct) values, and the shape at low frequencies appeared close to vertical, which indicates the EDLC characteristics of the device6,41.
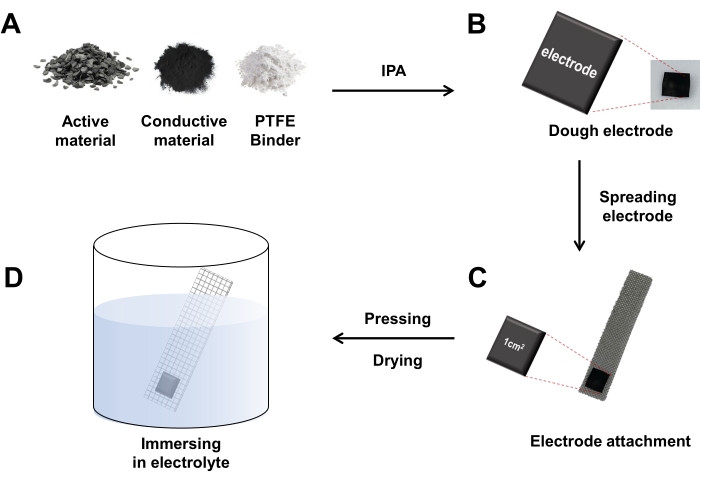
Figure 1. Fabricating process of supercapacitor. (A) Prepare the materials for electrode and mix with IPA. (B) Make an electrode in the form of a dough. (C) Spread the electrode thinly, cut it into 1 cm2 size with a thickness of 0.1-0.2 mm, and attach it to the stainless steel (SUS) mesh. (D) Immerse the supercapacitor in electrolyte after pressing and drying. Abbreviations: PTFE= polytetrafluoroethylene; IPA= isopropanol. Please click here to view a larger version of this figure.
Figure 2. Run the program for sequence settings. (A) Run the analysis program and (B) create the new sequence file with the editor. Please click here to view a larger version of this figure.
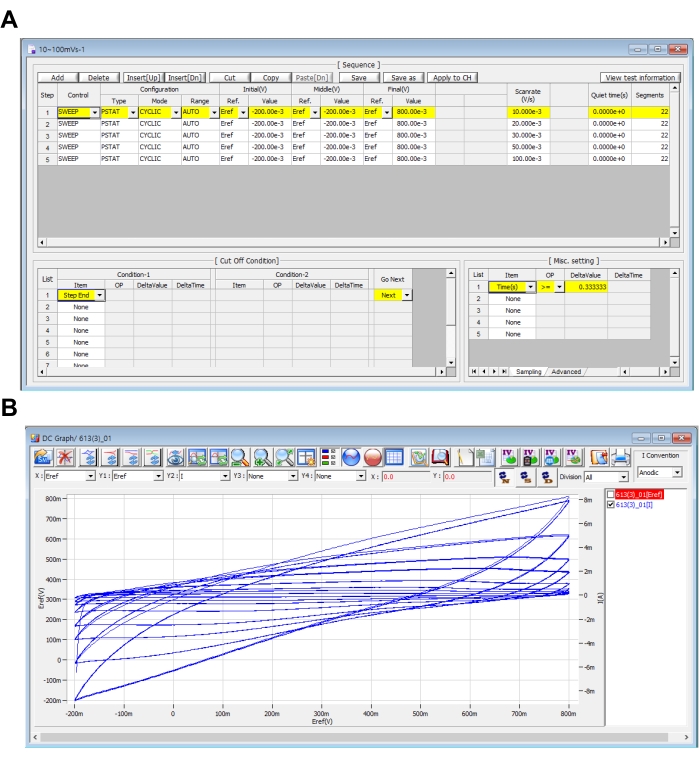
Figure 3. CV sequence settings. (A) CV sequence setting for each scan rate and (B) real-time measurement CV graphs. Please click here to view a larger version of this figure.
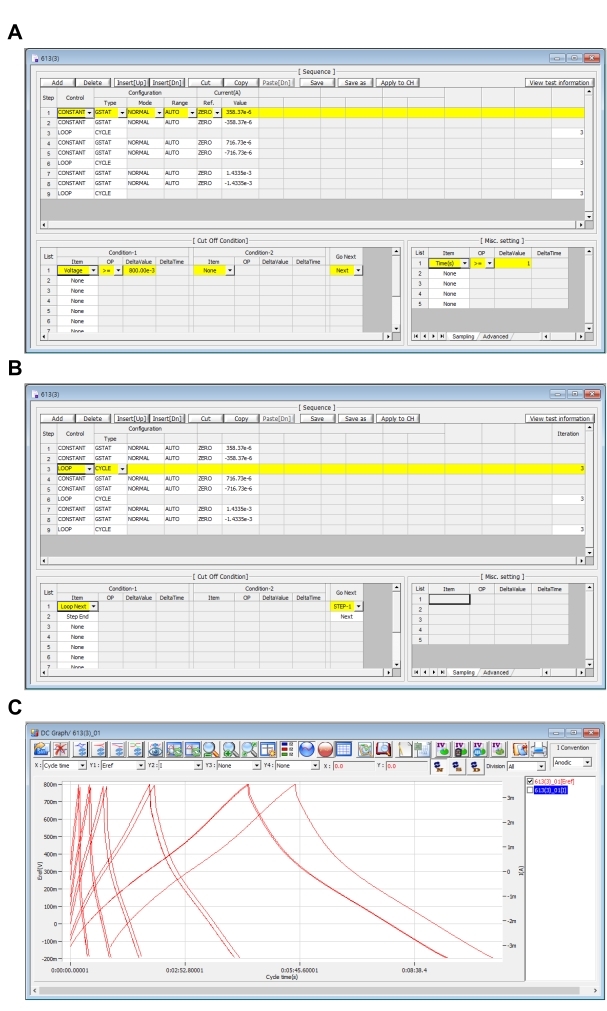
Figure 4. GCD sequence settings. (A, B) GCD sequence setting for each current density and (C) real-time measurement GCD graphs. Please click here to view a larger version of this figure.
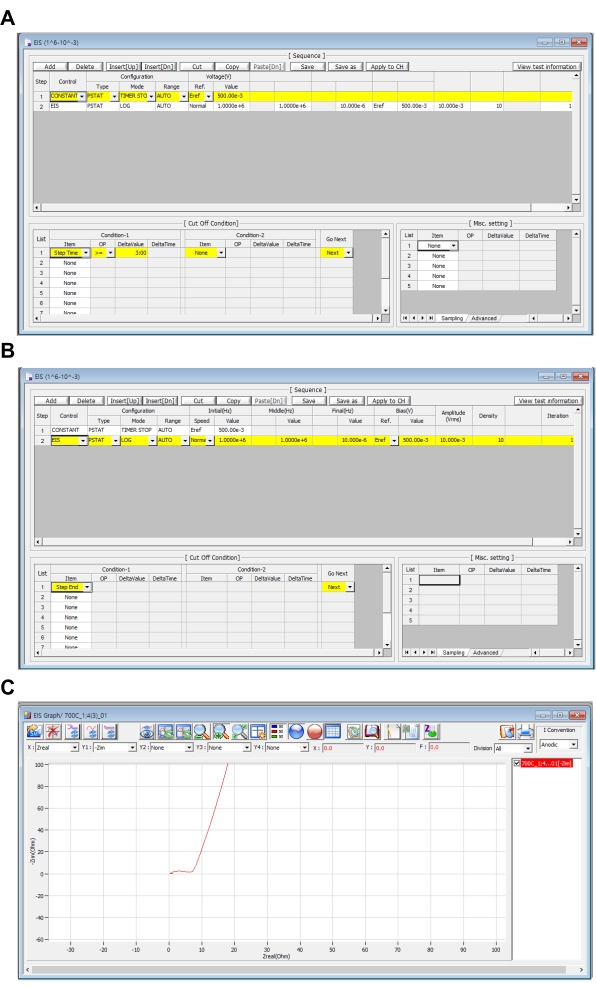
Figure 5. EIS sequence settings. (A, B) EIS sequence setting and (C) real-time measurement EIS graph. Please click here to view a larger version of this figure.
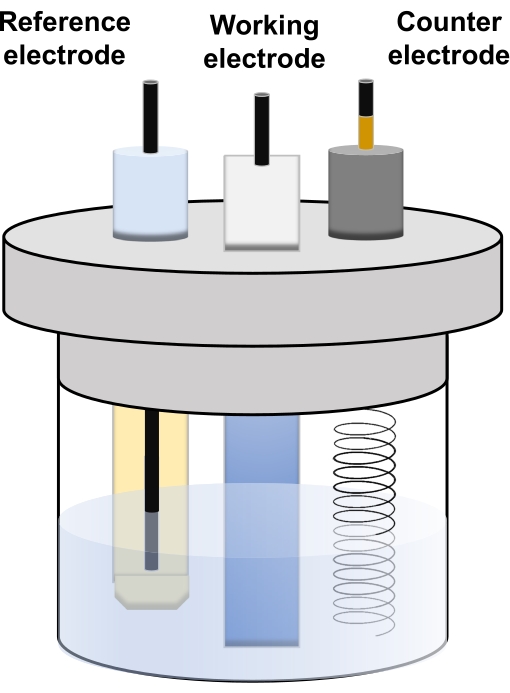
Figure 6. The basic composition of the three-electrode system for electrochemical measurement. Please click here to view a larger version of this figure.
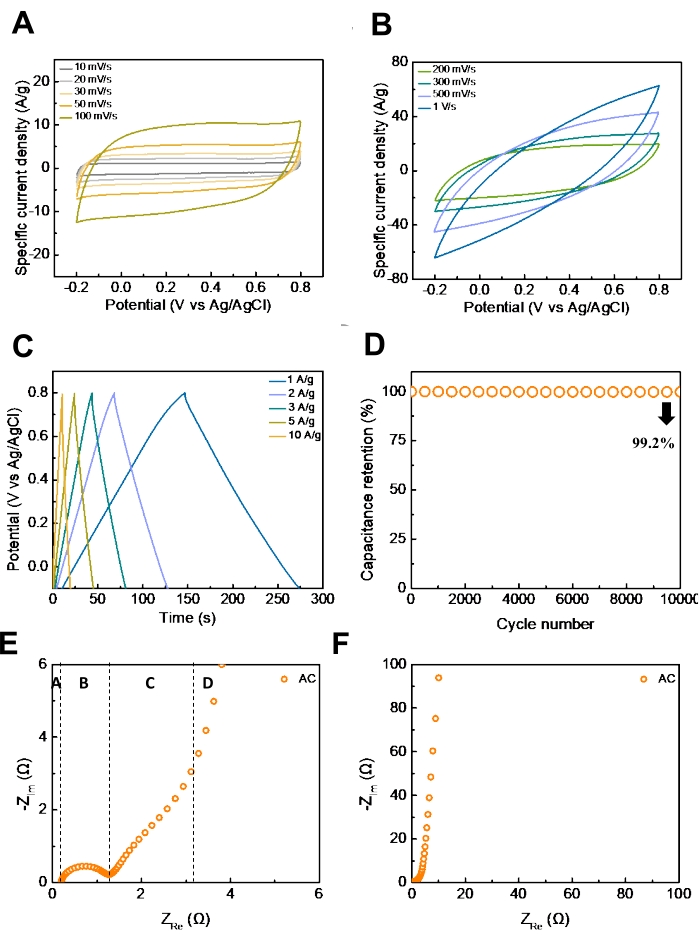
Figure 7. Electrochemical analyses graphs. (A) CV at low scan rates (10 mV/s – 100 mV/s); (B) CV at high scan rates (200 mV/s – 1000 mV/s); (C) GCD at a current density from 1 to 10 A/g; (D) Long cycle test at the current density of 10 A/g; (E, F) EIS Nyquist plots. Please click here to view a larger version of this figure.
Discussion
This study provides a protocol for various analyses using a three-electrode system with a potentiostat device. This system is widely used to evaluate the electrochemical performance of supercapacitors. A suitable sequence for each analysis (CV, GCD, and EIS) is important for obtaining optimized electrochemical data. Compared with the two-electrode system having a simple setup, the three-electrode system is specialized for analyzing supercapacitors at the material level15. However, the selection of appropriate experimental parameters such as the electrolyte42, potential range43, scan rate14, and current density14 is important for obtaining high-quality data. The parameters that must be judiciously set are summarized below.
The weight ratio may vary depending on the type of material used. The ratio can be adjusted according to the properties of the conductive material and binder used. The best ratio must maximize the amount of active material while maintaining the electrical conductivity and mechanical strength of the electrode. An 80 wt% ratio of the active material is widely used44,45,46,47.
The potential range is dependent on the electrochemical stability window (ESW) of the electrolyte. The ESW of an electrolyte can be determined by its reduction and oxidation potentials, which define the stable range within which the electrolyte can be used without decomposition48,49. The potential window for aqueous electrolytes is usually below 1.23 V, which is restricted by the thermodynamic potential of water electrolysis50. In the case of organic electrolytes, the potential window depends on the organic solvent used; organic electrolytes have a high voltage window (2.6 to 4.0 V)51. Researchers should set the optimal potential range in sequence according to the chosen electrolyte. In the case of an electrolyte that reacts upon contact with air, the container must be sealed.
The scan rate is the potential that varies linearly with the scan speed18 and has a crucial effect on the voltammetric behavior of materials. The optimal scan rate range cannot be specified because it depends on the material. At a higher scan rate, more redox reactions occur, and if the redox reaction is too fast, it is difficult to measure the electrochemical properties of the materials. At a lower scan rate, some peaks may be missing because there is sufficient time for activation during the redox reaction14. Researchers can select and adjust the optimal range using reference and empirical data. A scan rate from 50 mV/s to 1 V/s is commonly used. The current density is another parameter that affects the electrochemical parameters, including the capacitance14. If the current density is too high, the operating voltage is hardly measured. It is one of the reasons that the capacitance and energy density is decreased. An appropriate current density can be determined from the CV graph. The range of the y-axis shown for each scan rate may be used as the current density. A repeated cycle is applied in CV and GCD analyses to obtain the steady-state data. The cycle required to reach the steady state differs depending on the properties of the material. During cycling, the system attempts to achieve the equilibrium state and struggles to reach the same pattern14. Selecting a sufficient number of cycles for the materials is important. Ten cycles were applied in the present experiment.
Each parameter must be carefully determined because each parameter influences the next parameter value. Selecting the parameter values for obtaining optimal electrochemical data may involve modifying variables based on the initial experimental results. Evaluation of the electrochemical performance of a supercapacitor using the three-electrode system provides reliable data based on the values that the researcher has entered, but it is solely up to the user to set suitable parameters for analysis. The protocols specified in this report and the explanations supporting them will aid researchers in making a more informed decision.
To evaluate the electrochemical performance of supercapacitors, the mixing ratio of the electrode material and the electrode weight are vital parameters in the final step. The specific capacitance and current density can be obtained from the exact loading amount of the active material using the weight information. Inaccurate weight information may cause errors in the results. Finally, the installation of the appropriate equipment is important. The respective electrodes should not come into contact, but the distance between each electrode is indicated by the resistance of the system. Therefore, the electrodes should be placed as close as possible29. It is necessary to minimize external factors that may affect the evaluation of the supercapacitor by determining whether the electrode connection parts are corroded, or if the RE and the CE are in good condition.
The three-electrode system can perform detailed analysis, but through this, all performance of the supercapacitor cannot be evaluated. As mentioned earlier, the three-electrode system analyzes only one electrode at the material level. The final supercapacitor system consists of symmetrical or asymmetric electrodes and requires further evaluation of this system for application to real-life and industry. Many studies have conducted an evaluation using a three-electrode and two-electrode system together52,53,54,55. The system is also changing depending on the application. Not just evaluating supercapacitor, it is widely used in fuel cells56,57 and surface treatment58,59 fields. Various changes are taking place, such as giving flexibility60 or deviating from the existing form to another form61. The materials' characteristics can be easily evaluated with this system. Therefore, it will be applied in various forms to fields that require material analysis and evaluation.
In this paper, a supercapacitor was fabricated according to the proposed protocol. In addition, we evaluated the performance of a supercapacitor at the material level using various electrochemical analyses by utilizing the three-electrode system. The electrochemical properties of the electrodes were determined by adjusting the sequence parameters. This basic electrochemical protocol using the three-electrode system can be used to guide manufacturing and evaluation techniques for supercapacitor testing for beginners in this field of research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20214000000280), and the Chung-Ang University Graduate Research Scholarship 2021.
Materials
| Activated carbon | GS | Active material | |
| Ag/AgCl electrode | BASi | RE-5B | Reference electrode |
| Carbon black | Hyundai | Conductive material | |
| Desicator | Navimro | ||
| Electrode pressing machine | Rotech | ||
| Extractor | WonA Tech | Convert program (raw data to excel form) | |
| Isopropanol(IPA) | Samchun | I0346 | Solvent to melt the binder |
| Polytetrafluoroethylene(PTFE) | Hyundai | Binder | |
| Potentiostat | WonA Tech | Zive SP1 | |
| Pt electrode | BASi | MW-018122017 | Counter electrode |
| Reaction flask | Duran | Container for electrolyte | |
| SM6 | WonA Tech | Program of setting sequence and measuring electrochemical result | |
| Sulfuric acid | Samshun | S1423 | Electrolyte |
| SUS mesh | Navimro | Current collector | |
| Teflon cap | WonA Tech | Cap of the electrolyte continer | |
| Zman | WonA Tech | EIS program |
References
- El-Kady, M. F., et al. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proceedings of the National Academy of Sciences. 112 (14), 4233 (2015).
- Gee, A. M., Robinson, F. V. P., Dunn, R. W. Analysis of Battery Lifetime Extension in a Small-Scale Wind-Energy System Using Supercapacitors. IEEE Transactions on Energy Conversion. 28 (1), 24-33 (2013).
- Zhang, Z., et al. A high-efficiency energy regenerative shock absorber using supercapacitors for renewable energy applications in range extended electric vehicle. Applied Energy. 178, 177-188 (2016).
- Libich, J., Máca, J., Vondrák, J., Čech, O., Sedlaříková, M. Supercapacitors: Properties and Applications. Journal of Energy Storage. 17, 224-227 (2018).
- Cheng, Y. Super capacitor applications for renewable energy generation and control in smart grids. 2011 IEEE International Symposium on Industrial Electronics. , 1131-1136 (2011).
- Mathis, T. S., et al. Energy Storage Data Reporting in Perspective-Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Advanced Energy Materials. 9 (39), 1902007 (2019).
- González, A., Goikolea, E., Barrena, J. A., Mysyk, R. Review on supercapacitors: Technologies and materials. Renewable and Sustainable Energy Reviews. 58, 1189-1206 (2016).
- Yang, L., et al. Emergence of melanin-inspired supercapacitors. Nano Today. 37, 101075 (2021).
- Hendel, S. J., Young, E. R. Introduction to Electrochemistry and the Use of Electrochemistry to Synthesize and Evaluate Catalysts for Water Oxidation and Reduction. Journal of Chemical Education. 93 (11), 1951-1956 (2016).
- Licht, F., Aleman Milán, G., Andreas, H. A. Bringing Real-World Energy-Storage Research into a Second-Year Physical-Chemistry Lab Using a MnO2-Based Supercapacitor. Journal of Chemical Education. 95 (11), 2028-2033 (2018).
- Jakubowska, A. A Student-Constructed Galvanic Cell for the Measurement of Cell Potentials at Different Temperatures. Journal of Chemical Education. 93 (5), 915-919 (2016).
- González-Flores, D., Montero, M. L. An Advanced Experiment for Studying Electron Transfer and Charge Storage on Surfaces Modified with Metallic Complexes. Journal of Chemical Education. 90 (8), 1077-1081 (2013).
- Da Silva, L. M., et al. Reviewing the fundamentals of supercapacitors and the difficulties involving the analysis of the electrochemical findings obtained for porous electrode materials. Energy Storage Materials. 27, 555-590 (2020).
- Choudhary, Y. S., Jothi, L., Nageswaran, G. . Electrochemical Characterization. Spectroscopic Methods for Nanomaterials Characterization. , 19-54 (2017).
- Girard, H. -. L., Dunn, B., Pilon, L. Simulations and Interpretation of Three-Electrode Cyclic Voltammograms of Pseudocapacitive Electrodes. Electrochimica Acta. 211, 420-429 (2016).
- Bard, A. J., Inzelt, G., Scholz, F. . Electrochemical Dictionary. , (2012).
- Bard, A. J., Faulkner, L. R. . Electrochemical methods: fundamentals and applications. , (2000).
- Elgrishi, N., et al. A Practical Beginner’s Guide to Cyclic Voltammetry. Journal of Chemical Education. 95 (2), 197-206 (2018).
- Shiraishi, S., Tanaike, O. Application of Carbon Materials Derived from Fluorocarbons in an Electrochemical Capacitor. Advanced Fluoride-Based Materials for Energy Conversion. , 415-430 (2015).
- Inagaki, M., Kang, F. . Materials Science and Engineering of Carbon: Fundamentals. , (2014).
- Fleischmann, S., et al. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chemical Reviews. 120 (14), 6738-6782 (2020).
- Miao, Y. -. E., Liu, T. . Electrospinning: Nanofabrication and Applications. , 641-669 (2019).
- Yin, J., Qi, L., Wang, H. Antifreezing Ag/AgCl reference electrodes: Fabrication and applications. Journal of Electroanalytical Chemistry. 666, 25-31 (2012).
- Bard, A. J., Faulkner, L. R. . Electrochemical Methods: Fundamentals and Applications. , (2001).
- Wang, W., et al. Electrochemical cells for medium- and large-scale energy storage: fundamentals. Advances in Batteries for Medium and Large-Scale Energy Storage. , 3-28 (2015).
- Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. Journal of Applied Electrochemistry. 25 (3), 187-202 (1995).
- Murbach, M. D., Hu, V. W., Schwartz, D. T. Nonlinear Electrochemical Impedance Spectroscopy of Lithium-Ion Batteries: Experimental Approach, Analysis, and Initial Findings. Journal of The Electrochemical Society. 165 (11), 2758-2765 (2018).
- Macdonald, J. R., Johnson, W. B. . Impedance Spectroscopy. , 1-26 (2005).
- Chen, S. . Handbook of Electrochemistry. , 3-56 (2007).
- Xi, S., Zhu, Y., Yang, Y., Jiang, S., Tang, Z. Facile Synthesis of Free-Standing NiO/MnO2 Core-Shell Nanoflakes on Carbon Cloth for Flexible Supercapacitors. Nanoscale Research Letters. 12 (1), 171 (2017).
- Kim, M., Oh, I., Kim, J. Superior electric double layer capacitors using micro- and mesoporous silicon carbide sphere. Journal of Materials Chemistry A. 3 (7), 3944-3951 (2015).
- Stoller, M. D., Ruoff, R. S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy & Environmental Science. 3 (9), 1294-1301 (2010).
- Taberna, P. L., Simon, P., Fauvarque, J. F. Electrochemical Characteristics and Impedance Spectroscopy Studies of Carbon-Carbon Supercapacitors. Journal of The Electrochemical Society. 150 (3), 292 (2003).
- Yang, I., Kim, S. -. G., Kwon, S. H., Kim, M. -. S., Jung, J. C. Relationships between pore size and charge transfer resistance of carbon aerogels for organic electric double-layer capacitor electrodes. Electrochimica Acta. 223, 21-30 (2017).
- Arulepp, M., et al. Influence of the solvent properties on the characteristics of a double layer capacitor. Journal of Power Sources. 133 (2), 320-328 (2004).
- Mei, B. -. A., Munteshari, O., Lau, J., Dunn, B., Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. The Journal of Physical Chemistry C. 122 (1), 194-206 (2018).
- Nian, Y. -. R., Teng, H. Influence of surface oxides on the impedance behavior of carbon-based electrochemical capacitors. Journal of Electroanalytical Chemistry. 540, 119-127 (2003).
- Gamby, J., Taberna, P. L., Simon, P., Fauvarque, J. F., Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. Journal of Power Sources. 101 (1), 109-116 (2001).
- Coromina, H. M., Adeniran, B., Mokaya, R., Walsh, D. A. Bridging the performance gap between electric double-layer capacitors and batteries with high-energy/high-power carbon nanotube-based electrodes. Journal of Materials Chemistry A. 4 (38), 14586-14594 (2016).
- Fang, B., Binder, L. A modified activated carbon aerogel for high-energy storage in electric double layer capacitors. Journal of Power Sources. 163 (1), 616-622 (2006).
- Lei, C., et al. Activated carbon from phenolic resin with controlled mesoporosity for an electric double-layer capacitor (EDLC). Journal of Materials Chemistry A. 1 (19), 6037-6042 (2013).
- Lewandowski, A., Olejniczak, A., Galinski, M., Stepniak, I. Performance of carbon-carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes. Journal of Power Sources. 195 (17), 5814-5819 (2010).
- Dai, Z., Peng, C., Chae, J. H., Ng, K. C., Chen, G. Z. Cell voltage versus electrode potential range in aqueous supercapacitors. Scientific Reports. 5 (1), 9854 (2015).
- Kang, B., Ceder, G. Battery materials for ultrafast charging and discharging. Nature. 458 (7235), 190-193 (2009).
- Ban, C., et al. Nanostructured Fe3O4/SWNT Electrode: Binder-Free and High-Rate Li-Ion Anode. Advanced Materials. 22 (20), 145-149 (2010).
- Sun, Y., Hu, X., Luo, W., Xia, F., Huang, Y. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries. Advanced Functional Materials. 23 (19), 2436-2444 (2013).
- Lou, X. W., Deng, D., Lee, J. Y., Feng, J., Archer, L. A. Self-Supported Formation of Needlelike Co3O4 Nanotubes and Their Application as Lithium-Ion Battery Electrodes. Advanced Materials. 20 (2), 258-262 (2008).
- Chen, L., et al. Electrochemical Stability Window of Polymeric Electrolytes. Chemistry of Materials. 31 (12), 4598-4604 (2019).
- Ruschhaupt, P., Pohlmann, S., Varzi, A., Passerini, S. Determining Realistic Electrochemical Stability Windows of Electrolytes for Electrical Double-Layer Capacitors. Batteries & Supercaps. 3 (8), 698-707 (2020).
- Kang, J., et al. Extraordinary Supercapacitor Performance of a Multicomponent and Mixed-Valence Oxyhydroxide. Angewandte Chemie International Edition. 54 (28), 8100-8104 (2015).
- Pal, B., Yang, S., Ramesh, S., Thangadurai, V., Jose, R. Electrolyte selection for supercapacitive devices: a critical review. Nanoscale Advances. 1 (10), 3807-3835 (2019).
- Xie, K., et al. Carbon Nanocages as Supercapacitor Electrode Materials. Advanced Materials. 24 (3), 347-352 (2012).
- Demarconnay, L., Raymundo-Piñero, E., Béguin, F. A symmetric carbon/carbon supercapacitor operating at 1.6V by using a neutral aqueous solution. Electrochemistry Communications. 12 (10), 1275-1278 (2010).
- Frackowiak, E. Carbon materials for supercapacitor application. Physical Chemistry Chemical Physics. 9 (15), 1774-1785 (2007).
- Zhu, X., et al. Sustainable activated carbons from dead ginkgo leaves for supercapacitor electrode active materials. Chemical Engineering Science. 181, 36-45 (2018).
- Wang, Y., et al. Study on stability of self-breathing DFMC with EIS method and three-electrode system. International Journal of Hydrogen Energy. 38 (21), 9000-9007 (2013).
- Xin, L., Zhang, Z., Qi, J., Chadderdon, D., Li, W. Electrocatalytic oxidation of ethylene glycol (EG) on supported Pt and Au catalysts in alkaline media: Reaction pathway investigation in three-electrode cell and fuel cell reactors. Applied Catalysis B: Environmental. 125, 85-94 (2012).
- Fang, X., Kalathil, S., Divitini, G., Wang, Q., Reisner, E. A three-dimensional hybrid electrode with electroactive microbes for efficient electrogenesis and chemical synthesis. Proceedings of the National Academy of Sciences. 117 (9), 5074 (2020).
- Armstrong, E., sullivan, M., O’Connell, J., Holmes, J., O’Dwyer, C. 3D Vanadium Oxide Inverse Opal Growth by Electrodeposition. Journal of The Electrochemical Society. 162, 605-612 (2015).
- Wu, W. -. Y., Zhong, X., Wang, W., Miao, Q., Zhu, J. -. J. Flexible PDMS-based three-electrode sensor. Electrochemistry Communications. 12 (11), 1600-1604 (2010).
- Shitanda, I., et al. A screen-printed three-electrode-type sticker device with an accurate liquid junction-type reference electrode. Chemical Communications. 57 (23), 2875-2878 (2021).

