Subretinal Implantation of RPE on a Carrier in Minipigs: Guidelines for Preoperative Preparations, Surgical Techniques, and Postoperative Care
Summary
The subretinal implantation of retinal pigmented epithelium (RPE) is one of the most promising approaches for the treatment of degenerative retinal diseases. However, the performance of preclinical studies on large-eye animal models remains challenging. This report presents guidelines for the subretinal transplantation of RPE on a cell carrier into minipigs.
Abstract
Degenerative disorders of the retina (including age-related macular degeneration), which originate primarily at or within the retinal pigmented epithelial (RPE) layer, lead to a progressive disorganization of the retinal anatomy and the deterioration of visual function. The substitution of damaged RPE cells (RPEs) with in vitro cultured RPE cells using a subretinal cell carrier has shown potential for re-establishing the anatomical structure of the outer retinal layers and is, therefore, being further studied. Here, we present the principles of a surgical technique that allows for the effective subretinal transplantation of a cell carrier with cultivated RPEs into minipigs. The surgeries were performed under general anesthesia and included a standard lens-sparing three-port pars plana vitrectomy (PPV), subretinal application of a balanced salt solution (BSS), a 2.7 mm retinotomy, implantation of a nanofibrous cell carrier into the subretinal space through an additional 3.0 mm sclerotomy, fluid-air exchange (FAX), silicone oil tamponade, and closure of all the sclerotomies. This surgical approach was used in 29 surgeries (18 animals) over the past 8 years with a success rate of 93.1%. Anatomic verification of the surgical placement was carried out using in vivo fundus imaging (fundus photography and optical coherence tomography). The recommended surgical steps for the subretinal implantation of RPEs on a carrier in minipig eyes can be used in future preclinical studies using large-eye animal models.
Introduction
Age-related macular degeneration (AMD) is considered the main cause of central vision loss in developed countries and is one of many conditions related to retinal pigmented epithelium (RPE) dysfunction1,2. The RPE is found on the basally located Bruch's membrane (BM) and provides the necessary maintenance for the photoreceptors. The progressive degeneration of the RPE layer is a hallmark of the early atrophic form of AMD, and it also accompanies the development of the late exudative form of AMD as well. Despite many advances in retinal disease therapy, the development of an effective treatment modality remains challenging3. One of the promising methods is RPE replacement using an in vitro cultured RPE layer. This treatment is associated with progress in stem cell research using human embryonic stem cell-derived RPE (hESC-RPE) and induced pluripotent stem cell-derived RPE (iPSC-RPE)3,4,5,6,7. In recent years, many research groups have focused on developing different approaches for RPE replacement with the initially accepted proof-of-concept8,9,10,11,12,13,14,15. The RPE cells (RPEs) are usually delivered into the subretinal space in the form of a cell suspension, a self-supporting cell sheet, or a cell monolayer supported by an artificial carrier3,16,17,18,19,20,21. The injection of a cell suspension is the easiest method, but the compromised condition of the BM can often prevent the attachment of the transplanted cells. This can result in incorrect apicobasal orientation of the RPEs and failure to form a monolayer22,23. The main advantage of the other two methods (i.e., a self-supporting cell sheet and a cell monolayer supported by an artificial substrate) is that the cells are already in a differentiated monolayer state when implanted directly into the subretinal space24.
Many surgical techniques describing the delivery of cell carriers into the subretinal space have been published in recent years8,9,10,11,12,13,14,15. These studies described the use of large-eye animal models, the types of cellular carriers, the use of transplanted cellular cultures, the implantation instruments, as well as the surgical techniques, and the authors focused mainly on the results of subretinal implantation. In 2015, Popelka et al. reported the use of a frame-supported ultrathin electrospun polymer membrane for the transplantation of RPEs into porcine cadaver eyes8. The surgical technique described here with subretinal implantation of the cell carrier allowed for relatively precise handling of the carrier and easy positioning of the scaffold in the subretinal space. Kozak et al. assessed the feasibility of the delivery technique of a carrier with an approximate size of 2 mm x 5 mm in porcine eyes9. The unique design of the cell carrier permitted its correct placement, preventing the cellular monolayer from folding and wrinkling6. Al-Nawaiseh et al. first presented detailed step-by-step guidelines for subretinal scaffold implantation in rabbits25. Stanzel et al. then published a similar protocol in 2019 for transplantation in small rodents, rabbits, pigs, and nonhuman primates26. As published previously, the transplantation of a differentiated and polarized RPE monolayer on a solid carrier resulted in improved survival and better integration of the graft in comparison to other delivery techniques (Supplementary File 1)27.
The purpose of any preclinical animal studies performed in vivo is to reveal the various aspects of surgical transvitreal subretinal implantation of a cell carrier with a focus on the procedure safety, the survival of the transplanted cells, the tissue response to the subretinal maneuvers, and the short- and long-term postoperative outcomes. The use of porcine eyes as a large-eye animal model has been reported to be relevant in terms of the scope of the data obtained, which could be useful and potentially applicable to humans10,11,14. Our study reports the surgical technique used for the in vivo subretinal implantation of a cell carrier in a large-eye animal model. We present a detailed description of the preoperative preparations, the surgical technique of subretinal cell carrier implantation, and the postoperative care of the minipig eyes based on our experience over the last 8 years. We describe the basic surgical principles that can be used for in vivo experimental studies involving the implantation of different types of cells and cell carriers.
Large animal model
The experimental herd of Liběchov minipigs was founded by importing five animals from the Hormel strain from the USA in 1967. These animals were crossbred for porcine blood group studies with several other breeds or strains: Landrace, Large White, Cornwall, Vietnamese pigs, and miniature pigs of Göttingen origin28,29. At 5 months of age and approximately 20 kg body weight (BW), the minipigs reach sexual maturity. The survival of the parental minipig breeds (Hormel and Göttingen) is reported to be 12-20 years. The subretinal implantation of the cell carrier targets the central portion of the retina. The retina of minipigs lacks a macula and fovea. However, it has regions of highly concentrated cone photoreceptors called the area centralis and visual streaks30,31. These regions are responsible for the highest visual acuity.
The surgeries were performed by four experienced vitreoretinal surgeons with the assistance of an experienced surgical facility assistant (TA). Before the in vivo experiments, the surgeons were educated and obtained special knowledge of minipig eye anatomy, such as regarding the lower ratio of lens to vitreous volume, the shorter axial length (15-19 mm), the absence of the Bowman's membrane in the cornea, the smaller vitreous volume (2.8-3.2 mL), the absence of the macula and fovea, the absence of the annulus of Zinn, and the optic disc diameter (vertical/horizontal: 1.5 mm/2.1 mm). In all cases, the surgery was performed under general anesthesia in a specially organized operating room with the implementation of standard aseptic and antiseptic measures.
Protocol
This study adheres to the tenets of the Guidelines of the Declaration of Helsinki and ethical principles for medical research involving human subjects. All experiments were carried out according to the Guidelines for the Care and Use of Laboratory Animals and according to the Association for Research in Vision and Ophthalmology (ARVO) for the use of animals in ophthalmic and visual research. The study protocol was approved by the Resort Professional Commission of the CAS for Approval of Projects of Experiments on Animals at the Institute of Animal Physiology and Genetics of the Czech Academy of Sciences (Liběchov, Czech Republic) (Approved protocol No. 60/2016 and No. 64/2019).
1. Considerations during the subretinal transplantation of cells on a carrier into minipigs
- Animal selection
- Obtain and use Liběchov minipigs that are 12-36 months old, either sex, and around 40-80 kg of body weight (BW).
- Keep the minipigs indoors in an air-conditioned animal house with temperatures between 18-22 °C, exposure to an artificial 13 h/11 h light/dark cycle, standardized individual pens, free access to water, and twice daily feeding.
- Preparation prior to the surgery
- Check for the surgical orientation of the eye and draw the fundus schemes. To do so, schematically divide the retina of the minipigs on the fundus drawing using a pen with a vertical line into the temporal (from the optic disc toward the ear), nasal (from the optic disc toward the pig's snout), and central (between the major retinal vessels on the nasal side) regions (Figure 1A, B).
- Select only healthy animals without any behavioral and neurological pathology and with normal quality of the skin, body orifices, feces, and food consumption. Have a skilled veterinarian perform the clinical observation and select the animals.
- Inject intramuscularly 3 mg/kg BW of ceftiofur hydrochloride (1 mL/kg) on the day of surgery.
- Preoperative immunosuppression
- Prepare tacrolimus-eluting polymer microspheres as described in Wang et al. and Sevc et al. 32,33 with modification.
- Ensure that the concentration of tacrolimus in the polymer microspheres is 51.3 mg/g as determined by HPLC (Table of Materials).
- Perform a subcutaneous injection of tacrolimus-loaded polymer microspheres at a dose of 0.25 mg/kg BW 6 days before the eye surgery to impede cell graft rejection. This is done to ensure the survival of the human RPE donor cells during xenogeneic transplantation into the minipig eyes.
- Inject the animals intramuscularly on the day of the surgery with 80 mg of depo-medrol and benzylpenicillin at 1 mL/10 kg according to the body weight.
- Anesthesia
- Induce general anesthesia with an intramuscular injection of a mixture of tiletamine (2 mg/kg), zolazepam (2 mg/kg), ketamine (2 mg/kg), and xylazine (0.4 mg/kg)-TKX34,35 prior to the surgery. Check the depth of anesthesia by a state of unconsciousness and by checking the pedal reflex (a pinch of the interdigital skin of the hind leg), the corneal reflex (a slight touch of the cornea), the pupillary reflex (reaction to the light), and the palpebral reflex (a touch to the eyelid). Ensure that the animal does not blink. Confirm the heart and respiration rate regularity.
- After the induction of anesthesia, transport the sedated animal to the operating room on a lift trolley (Figure 2A).
- Place the animal on the operating table on its left side to enable surgery on the right eye (Figure 2B).
- Perform the adjustment of the animal's head using styrofoam pads to achieve the most suitable position of the central retina for implantation (i.e., horizontal and parallel to the floor) (Figure 2C).
- Place eyedrops of 0.5% proparacaine hydrochloride ophthalmic solution into the conjunctival sac three times 1 min apart to induce local anesthesia.
- Insert a vein cannula and intubate the animal with an endotracheal tube for the inhalation maintenance of anesthesia (1.5% isoflurane) using an anesthesia machine equipped with a patient monitor (Figure 2A, B).
- Administer an intramuscular injection of 1 mL of Eficur per 16 kg BW and 20 mg of Depo-Medrol 1 approximately 15 min before the beginning of the eye surgery (Table of Materials).
- Maintain the physiological body temperature by covering the animal with isothermal foil and perform the surgery as described in section 3.
- During the surgery, monitor the animal's temperature along with the heart rate and blood oxygen saturation using an ear clip and patient monitor. Avoid the lowering of the body temperature below 38 °C during the procedures, which is considered a safe limit36. Maintain the oxygen saturation (>96%) and pulse rate (70-90 beats per minute) during the whole experiment.
- When the surgery is complete, turn off the flow of isoflurane and extubate the animal.
- After spontaneous breathing and awakening, transfer the animals to their pens.
- Operating room setup
- Arrange the operating room according to the needs of the surgery on the eyes of the large-eye animal model (Figure 3A, B). Adjust the height of the surgeon's chairs, as well as the height of the microscope, to achieve a comfortable position for the surgeons with regard to the position of the pig's snout.
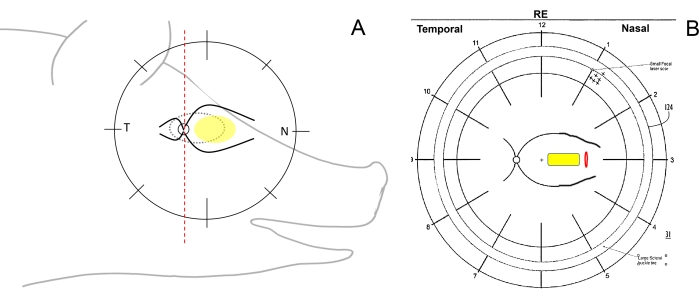
Figure 1: Schematic drawing of the retinal zones in minipigs. (A) Schematic drawing of the retinal zones in relation to the minipig's head; the yellow ellipse depicts the desired area of subretinal implantation, T refers to the temporal retinal area, and N refers to the nasal retinal area. (B) Example of the fundus scheme after subretinal implantation of the cell carrier (yellow) through retinotomy (red). Please click here to view a larger version of this figure.
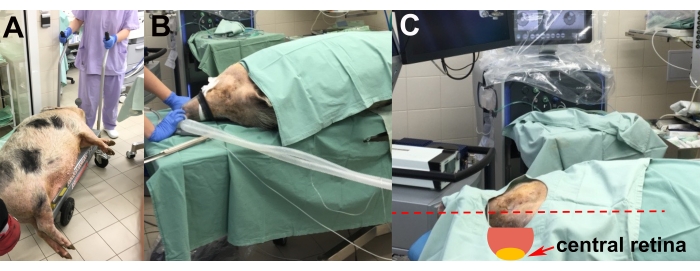
Figure 2: Transportation and placement of the animal. (A) Transportation of the sedated animal to the operating room. (B) Placement of the animal during intubation. (C) Adjustment of the head of the animal for optimal access to the central retina during the surgery (red arrow). Please click here to view a larger version of this figure.
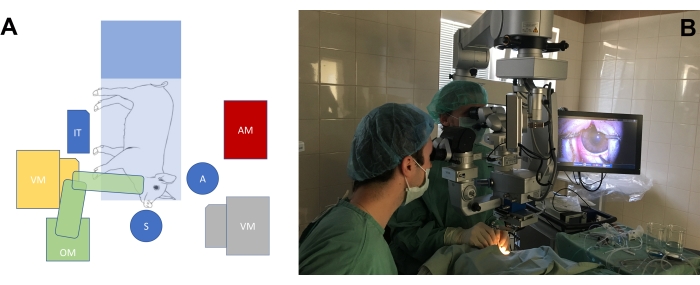
Figure 3: The standard operating room setup. (A) Schematic depiction of the surgeons' position (S = surgeon, A = assistant) in relation to the position of the operating table with the minipig, the operating microscope (OM), the vitrectomy machine (VM), the instrumental table (IT), and the anesthesiology machine (AM). There are two possible positions of the vitrectomy machine (yellow and gray). (B) Real-life setting in the operating room. Please click here to view a larger version of this figure.
2. Cell carrier, cultivated cells cultures, and implantation injector
- Cell carrier
- Cut the 30 µm wide oval frame with outer dimensions of 1.7 mm x 4.8 mm, equipped with triangular protrusions asymmetrically located on the frame (Figure 4A), from a bi-axially oriented 36 µm thick poly-ethylene-terephthalate (PET) foil using a femtosecond laser.
- Prepare poly(L-lactide-co-DL-lactide) (LLA/DLLA 90/10, MW 868, 270 g/mol) polymer solution in pyridine at a concentration of 11 wt% with the addition of 2.2 µL of formic acid per 1 g of solution.
- Prepare the nanofibrous membrane with an embedded supporting frame (Figure 4B) by the electrospinning of the polymer solution in three steps8: (1) deposit the first layer of nanofibers on a silicon substrate, (2) place the frame on the layer, and (3) deposit the second layer of nanofibers.
NOTE: Deposit every layer for 7 min to reach a total membrane thickness of 3.7 µm. Use the following parameters to obtain a membrane composed of 380 nm thick fibers and with an average pore size of 0.4 µm and a porosity of about 70%37: a 20 G all steel needle, a voltage of 7.1 kV, a gap of 10 cm, a flow rate of 250 µL/min, and a temperature of 25.0 °C ± 0.5 °C. - Carefully remove the membrane with the embedded frame from the silicon substrate and fix it to the body of a commercial 12 well cell culture insert devoid of the original membrane to facilitate the seeding and growth of cells.
- Treat the nanofibrous membrane before cell seeding in air-plasma for 30 s at a power of 70 W in a plasma cleaner.
- Cell cultures used for cultivation on the cell carrier
NOTE: The following cell carriers could be used: 1) nanofibrous cell carriers without any cells; 2) nanofibrous cell carriers with primary human RPEs (hRPEs); 3) nanofibrous cell carriers with human iPSC-derived RPE cells.- Culturing primary hRPEs
- Isolate primary hRPE cells from human donor eyes according to a previously reported technique38.
- Obtain the cells by enzymatic treatment of the retina for 30 min. Then, cultivate the primary hRPE cells (passage 0) for up to 2 weeks in DMEM/F12 supplemented with 10% fetal bovine serum (FBS).
- Once the cell cultures reach confluency, change the medium to 1% FBS and culture for an additional 30 days.
- Seed the primary hRPEs onto trans-well plates and onto a laminin coated nanofibrous cell carrier at a density of 2,000 cells/mm2. Following a further 30 days of incubation in 1% FBS, use the cell carriers with primary hRPEs for subretinal implantation into minipigs.
- Human iPSC-derived RPEs
- Use hiPSCs derived from MERTK associated retinitis pigmentosa patient-derived fibroblasts39 that are gene-corrected in two alleles using the CRISPR/Cas9 system (RP1-FiPS4F1-GC2)40, as well as hiPSCs derived from the fibroblasts of a healthy subject (Ctrl2-FiPS5F2)41 that are used as a control.
- Generate and subsequently differentiate both hiPSCs cell lines toward RPE cells (hiPSC-RPE) as reported previously42.
- Plate the hiPSC-RPEs at 200,000 cells/cm2 on laminin-coated cell culture inserts with nanofibrous membranes of poly(L-lactide-co-DL-lactide) with oval implantation frames in RPE medium containing knockout DMEM, 20% knockout serum, 0.1 mM non-essential amino acids, 0.23 mM β-mercaptoethanol, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 10 mM nicotinamide.
- Change the medium every other day, and culture the hiPSC-RPE for 2 months prior to implantation to encourage polarized growth.
- Implantation injector
- Prepare a plastic capillary with a rectangular cross-section of 2.8 mm x 0.8 mm by blow molding from a plastic tube of OD 1.75 mm/ID 1.10 mm.
- Cut a loading window of 4 mm x 2.2 mm in the plastic capillary 6 mm from the end.
- Assemble the injector from the plastic capillary, a silicone handle, a steel blade plunger, and a piston (Figure 5A).
- Load the carrier into the injector through a loading window and later eject it into the subretinal space by a push of the plunger, as described in step 3.5.2 (Figure 5B).
- Preparation of the nanofibrous carrier and loading of the injector
- Fill a small plastic Petri dish with 2 mL of phosphate-buffered saline (PBS). Take out an insert with the prepared cell layer, place it on a semi-soft polystyrene dish, and center it under a light microscope. Use a custom-modified punch to cut out the carrier along the oval frame using a microscope. The carrier dimensions should be 2 mm x 5 mm.
- Use a custom-made injector with flat transparent tubing for loading the carrier; 6 mm from the distal end of the capillary, there is a window for loading the carrier. Fill the injector's window with PBS.
- Using the forceps, release the sample from the bottom of the dish, lift it from the liquid, and transport it to the window of the injector while checking the side orientation marks on the frame first in order to detect the top side of the carrier with adherent cells. An oval frame facilitates manipulation with the carrier.
- Using a dental probe (a stainless steel dental instrument with a sharp ending), position the carrier in the window of the injector. Use the plunger to push the carrier into the closed and safe upper part of the injector. Then, prepare the carrier for surgery.
- Check the side orientation of the carrier at each step. Unload the nanofibrous carrier from the injector by pushing the metal plunger.
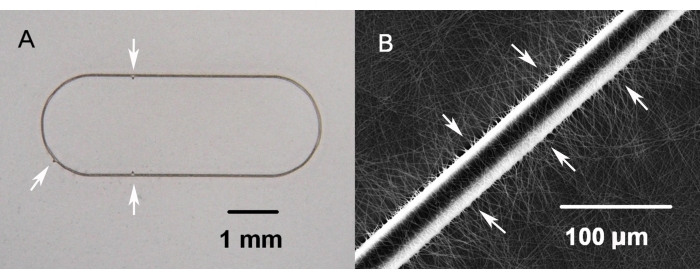
Figure 4: Nanofibrous carrier with an embedded supporting PET frame. (A) Three visible marks on the frame allow control of the side orientation of the carrier (white arrows). (B) Enlargement view of the PET frame fragment embedded in the nanofibrous membrane (white arrows) of the cell carrier. Please click here to view a larger version of this figure.
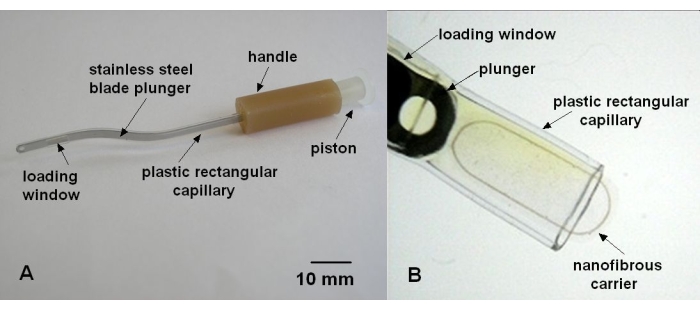
Figure 5: Implantation injector. (A) Parts of the injector. (B) Nanofibrous cell carrier with embedded supporting PET frame loaded to the plastic rectangular capillary of the implantation injector. Please click here to view a larger version of this figure.
3. Surgical procedure
- Surgical equipment
- Use the following surgical equipment: an ophthalmic surgical microscope, an operating system for surgeries on the anterior and posterior eye segments, a non-contact vitreoretinal surgical system, a laser photocoagulation device, and a digital camera.
NOTE: The standard parameters used in vitrectomy, exo- and endo-cautery, and laser photocoagulation equipment are depicted in Table 1.
- Use the following surgical equipment: an ophthalmic surgical microscope, an operating system for surgeries on the anterior and posterior eye segments, a non-contact vitreoretinal surgical system, a laser photocoagulation device, and a digital camera.
- Surgical instruments
- Sterilize the reusable surgical instruments with a mobile autoclave steam sterilizer or similar according to a standard protocol. The single-use surgical instruments and materials required during the surgery are listed in the Table of Materials.
- Preparation for the surgical steps
- After anesthetizing the animal as described in step 1.4.1, apply 1% tropicamide solution eye drops and 10% phenylephrine hydrochloride solution in the conjunctival sac 15 min before the procedure to provoke drug-induced mydriasis.
- Approach the operating table with the surgeon in the upper position and the assistant in the side position (Figure 3).
- Shave the area around the eye using a single-use shaving razor and remove the rough dirt.
- Disinfect the conjunctival sac with 5% povidone-iodine solution for 5 min.
- Disinfect the periorbital area with cotton swabs by scrubbing from the eyelids to the periphery. Repeat the process three times using a 10% povidone-iodine solution and leave on for 5 min.
- Cover the operating field with the eye in the middle using a standard sterile ophthalmic drape with sticky transparent foil. Move the eyelashes away from the eye globe. Avoid cutting the eyelashes to reduce the risk of postoperative endophthalmitis.
- Insert the lid speculum (Liberman-type or Cook eye speculum). Optionally, fix the nictitating membrane to the skin with 8-0 polyglactin suture.
- Open the conjunctiva on the nasal side 2 mm to 3 mm from the limbus in order to expose the sclera for the sclerotomies using surgical forceps and Westcott conjunctival scissors.
- Insert the three-valved 25 G trocars 2.5-3 mm from the limbus in the area of the pars plana (place them at 7 o'clock, 10 o'clock, and 11 o'clock). Use rotating movements during the insertion in a slightly oblique manner (100°-110°) toward the posterior retina and hold the trocar with the forceps (Figure 6).
- Keep the cornea wet or coat it with methylcellulose during the whole surgery to prevent osmotic corneal edema.
- Pars plana vitrectomy (PPV)
- Remove the middle portion of the vitreous body with the standard three-port pars plana vitrectomy approach. Carefully remove the vitreous behind the lens in the area of the future large sclerotomy.
- Use an intravitreal 2-4 mg of triamcinolone acetonide (TA) injection (50-100 µL) to stain the posterior vitreous, which usually remains adherent to the retina, in order to perform a controlled posterior vitreous detachment.
- After that, slowly perform a subretinal injection of 0.05-0.1 mL of BSS with a 41 G cannula more centrally, avoiding the formation of a bleb toward the periphery.
- Reduce the intraocular pressure settings with the irrigation system down to 15 mmHg during the subretinal injection in order to prevent a transient retinal vascular occlusion.
- Perform a linear large endodiathermy of the retina with a 27 G endodiathermy probe 3 mm near the nasal bleb base.
- Afterward, make a 3 mm large retinotomy with a 25 G MVR blade or vertical scissors with an elevated intraocular pressure (IOP) setting of the irrigation system up to 60 mmHg for 3 min to 5 min. Ensure that there is no bleeding from the retinotomy, and then reduce the IOP to 25 mmHg.
- Carry out an exodiathermy of the episcleral vessels between the two nasal trocars 2.5-3 mm from the limbus with a 27 G endodiathermy probe by applying a gentle touch onto the surface of the sclera.
- Check the fluid level of the infusion bottle before enlarging the sclerotomy, as after sclerotomy enlargement, fluid consumption is temporarily high.
- Make a 3.0 mm large sclerotomy, 3 mm from the limbus, using a 2.75 mm phaco knife.
- Pay attention to possible bleeding from the scleral vessels and ciliary body inside the large sclerotomy. In the case of bleeding, use a 27 G endodiathermy probe to coagulate the damaged vessels. Enlarge the sclerotomy to 3.0 mm with a satin knife to accommodate the tip of the injector (0.8 mm x 2.8 mm).
- Remove the prolapsed vitreous body on the site of the large sclerotomy with a vitrector. Maintain the infusion of BSS at the level of 25-30 mmHg with the vitrectomy system to avoid globe collapse.
- Implantation of the cell carrier
- Gently insert the injector with the dominant hand into the vitreous cavity through the large sclerotomy. In case of resistance, enlarge the size of the sclerotomy.
- Implant the cell carrier through the retinotomy into the subretinal space. If necessary, use a bimanual technique with additional sclerotomy and chandelier light in order to improve the control of the implantation.
- Withdraw the injector from the eye and close the large sclerotomy with an 8-0 polyglactin suture to avoid complications associated with intraocular hypotony.
- Perform a complete fluid-air exchange (FAX) and drainage of the subretinal fluid with a silicone-tipped cannula.
- After that, inject silicone oil (1,000 cSt) into the vitreous cavity using the vitrectomy system and tubing system for silicone oil injection until the IOP is normal.
- Intraoperative imaging and documentation
- Perform a video recording during the entire surgery with photo documentation of the key steps of implantation using a video recording system.
- Complete the fundus drawing by documenting the location of the sclerotomies, retinotomy, subretinal implant, and any complications that occurred using fundus drawing schemas.
- Post-surgery steps
- At the end of the surgery, remove the trocars and close the three sclerotomies and the conjunctiva with 8-0 polyglactin sutures.
- Rinse the conjunctival sac with 5% povidone-iodine solution.
- Perform a 0.3 mL subconjunctival injection of 20 mg of gentamicin, 2 mg of dexamethasone, and 2% xylocaine.
- Check the condition of the fundus and lens using a microscopic view.
- Remove the suture(s) from the nictitating membrane using surgical forceps and Westcott conjunctival scissors (optional).
- Apply neomycin ointment or ofloxacin ophthalmic ointment into the conjunctival sac.
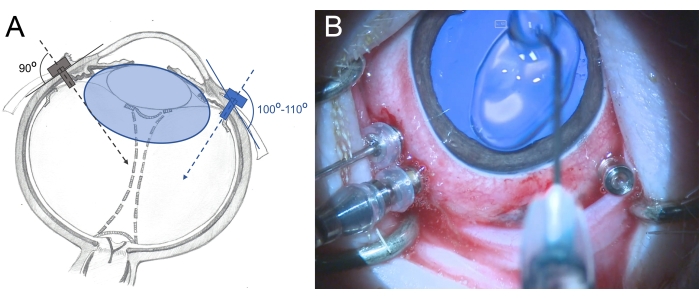
Figure 6: Insertion of the trocars in the eye of a minipig. (A) Schematic depiction of the trocars, which are inserted perpendicularly into the sclera toward the center of the vitreous cavity in the human eye (gray color) and in an oblique manner toward the posterior retina in the minipig eye (blue color) to avoid damage to the lens. The lens of the minipig (blue colored) is larger than that in humans and relative to the vitreous cavity size. (B) Intraoperative view of the inserted trocars in a three-port PPV. The cornea is covered with methylcellulose to prevent drying and swelling. Please click here to view a larger version of this figure.
4. Postoperative care
- Apply topical bacitracin zinc/hydrocortisone acetate/neomycin sulfate or 0.3% ofloxacin into the conjunctival sac of the animals five times per day.
- Postoperatively, check the following eye parameters: turgor of the soft tissues of the eye using palpation, inflammatory reaction on the eye surface, and squinting as a protective reaction of the eyelids using a hand-held slit lamp or indirect ophthalmoscope.
- For systemic postoperative care, use the following antibiotics:
- Inject intramuscularly ceftiofur hydrochloride at the dose of 3 mg/kg BW (1 mL/kg) on the second and third day of stability.
- Inject tulathromycin (1 mL/40 kg BW) after 72 h post-surgery for the prevention of secondary bacterial infection.
- Perform an intramuscular injection of flunixin (2 mL/45 kg of live weight) and tramadol hydrochloride (100 mg) every 24 h for 3 days after surgery to prevent pain.
- Keep the minipigs in a specialized air-conditioned facility with a temperature range of 18-22 °C and an artificial 13 h/11 h light/dark regime.
- Ensure they have free access to water and standard feeding (twice per day).
5. Postoperative procedures
- Postoperative ophthalmic examinations
- In the postoperative period, inspect the eyes with an indirect ophthalmoscope for the presence of inflammation (i.e., redness, tissue swelling, or mucus congestion in the conjunctival sac). Measure the intraocular pressure in the operated eye using the palpation method.
- Postoperative imaging
- Induce sedation in the minipig by the intramuscular injection of a TKX mixture before fundus photography and OCT examination. Instill 1% tropicamide and 10% phenylephrine hydrochloride eye drops into the conjunctival sac of the minipig in order to induce mydriasis.
- Employ a lid speculum to maintain open eyes. For moisturizing the eye surface and for obtaining a clear OCT image, wash the cornea of the animal with saline solution (0.9% NaCl) every 30-60 s.
- Position the minipig on the operating table in the same manner as during the operation (Figure 2B,C, Figure 3A). The main requirement is to place the head on the side and perpendicular to the scanning piece of the OCT device. Use styrofoam pads under the animal's snout to stabilize the head, bringing the eye surface into a horizontal position.
- Collect color fundus images with a color non-mydriatic fundus camera, as this allows for documenting the anterior segment, retina, and optic disc. Additionally, take a red-free image of the retina with the non-mydriatic fundus camera.
- Perform optical coherence tomography imaging using the spectral-domain OCT system. During the OCT or fundus imaging, tilt the minipig's head manually toward the OCT lenses or fundus camera lenses to optimize the view of the posterior retina and the area of implantation (Figure 2C). For optimal imaging of the implanted carrier on the fundus, apply the infrared reflectance light of the OCT device to focus on the implant (Figure 2C). Use the OCT crossline and retina map scanning modes.
- Apply ofloxacin ophthalmic ointment at the end of the examination under the lid of the animal's eye.
- Move the minipig to the indoor facility and observe for its general condition until the end of sedation (approximately 2 h to 5 h).
6. Enucleation of the eye post mortem after euthanasia
- Sedate the minipigs with an intramuscular injection of the TKX mixture followed by an intravenous (through a 22-G ear cannula) bolus application of 1% propofol (20 mL/animal) followed by exsanguination. Do not use general fixatives.
- Sacrifice the animals by exsanguination during deep general anesthesia 7 days, 14 days, 28 days, and 42 days after cell graft implantation.
- Use forceps and scissors to remove the upper and lower eye lids. Rremove the third eye lid and cut through the conjunctiva. Cut the eye muscles and the optic nerve.
- Enucleate the eyes post mortem using surgical scissors and surgical forceps. Ensure that the procedure is performed by an experienced person.
Representative Results
The results of the subretinal implantation of the cell carrier in Liběchov minipigs are presented in Table 2. Successful implantation was defined as obtaining sufficient data for histologic and immunohistochemical study. Failed cases were defined as eyes with severe intraoperative complications, which made further observation of the eye tissues impossible.
The application of the proposed technique with the use of silicone oil tamponade allows for controlling the condition of the subretinal transplant using imaging modalities starting from the next day after the surgery until the time of enucleation (Figure 7, Figure 8, and Figure 9).
Fundus imaging and SD-OCT
The minipigs were examined in the postoperative period using fundus imaging, red-free imaging, and spectral domain optical coherent tomography (Figure 7). High-quality fundus imaging was enabled by using clear optic media, including a clear lens and the use of silicone oil tamponade (Figure 7A). The site of the retinotomy showed no signs of a proliferative reaction (Figure 7A, yellow arrows), and the PTE frame of the cell carrier was clearly visible through the semi-transparent layers of the porcine retina. On the red-free imaging, the reflectivity of the cultivated hRPEs on the carrier did not differ from the reflectivity of the endogenous porcine RPE layer (Figure 7B). On the SD-OCT, the PTE frame caused only minor shadowing of the underlying anatomical structures and slight thickening of the retina (Figure 7C, red arrows). No atypical hypo- or hyper-reflective zones were noticed on the SD-OCT, and the Bruch's membrane appeared to remain undamaged as well. Figure 8 presents fundus and iOCT images of the scaffold cultivated with primary human RPE cells 1 month after the surgery (Figure 8). The cell carrier itself (without any cells) caused no significant increase in retinal thickness (Figure 9C). These findings suggest that the intraoperative iatrogenic impact of the implant was minimal and that the implanted cell carrier underwent sufficient adaptation of the implanted cells to the overlying photoreceptor cells and neuroretinal tissue.
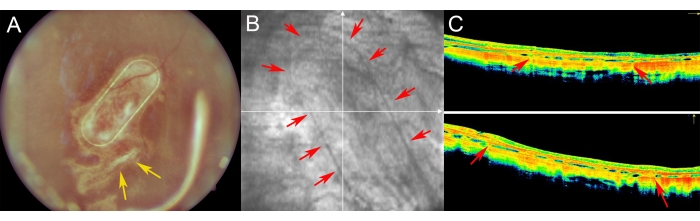
Figure 7: Postoperative imaging of the retina in minipigs. (A) Fundus imaging, (B) Red-free image, and (C) optical coherence tomography imaging of the nanofibrous carrier with primary human RPE cells in a 1 week follow-up after subretinal transplantation in a minipig eye. (A) The yellow arrows indicate the site of retinotomy. (B) Red arrows demonstrate the margins of the nanofibrous cell carrier. (C) The red arrows show the slight shadowing of the OCT signal caused by the supporting PET frame of the nanofibrous carrier, which was implanted into the subretinal space. Please click here to view a larger version of this figure.
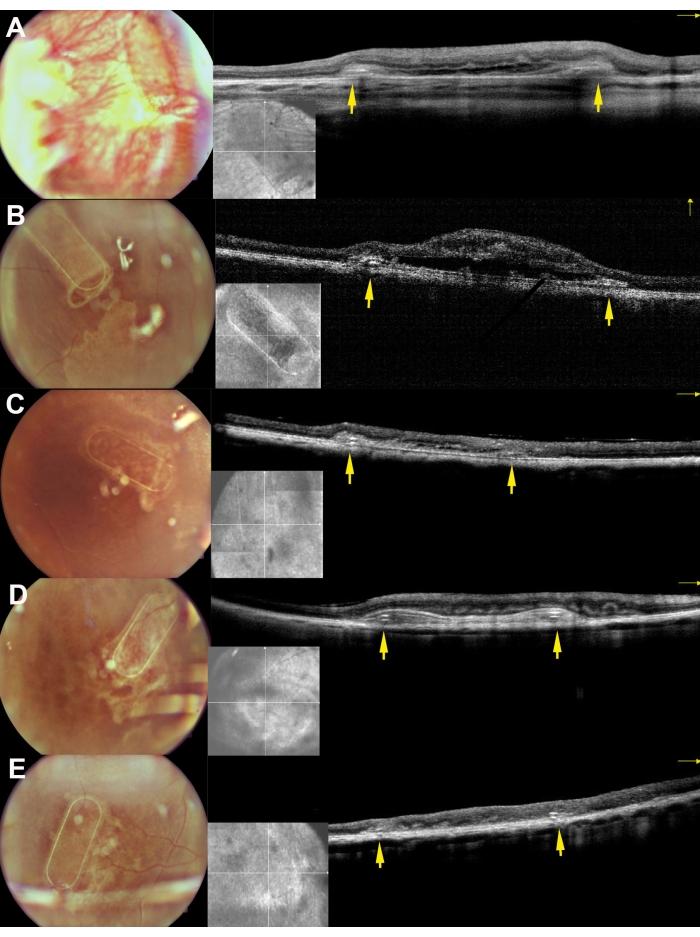
Figure 8: Fundus imaging and iOCT images of the scaffolds 30 days after subretinal implantation in the minipigs. A, B, C, D, and E correspond to pigs 169, 182, 179, 199, and 224, respectively. The yellow arrows depict the frame of the scaffold. Please click here to view a larger version of this figure.
Histological and immunohistochemical analysis
After the euthanasia of the animals, whole minipig eyes were removed and fixed in 4% paraformaldehyde (PFA) for 24 h. The anterior part of the eye was removed, and the implanted nanofibrous carrier was identified in the nasal central retina and isolated with the sclera attached. All the tissues were cryoprotected in graded sucrose solutions, and vertical frozen sections were cut, as described in detail43. Histology of the nanofibrous membrane without RPE cells after 4 weeks of implantation revealed retinas without inflammation and degenerative changes (Figure 9A). The presence of the nanofibrous membrane was detected in polarized light (Figure 9B).
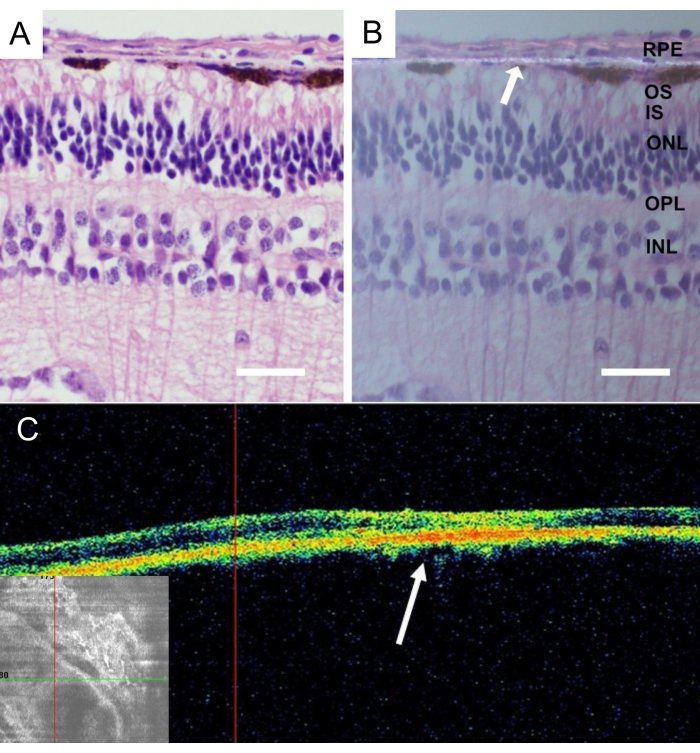
Figure 9: Histological analysis of the implanted acellular nanofibrous membrane. Hematoxylin-eosin staining of the acellular nanofibrous membrane 4 weeks after implantation (A) with standard illumination and (B) with polarized light microscopy. The white arrow indicates the nanofibrous membrane localization (scale bar: 50 µm). (C) In vivo optical coherence tomography pictures of the acellular nanofibrous membrane after 4 weeks after implantation depicts good acceptance and adherence of the nanofibrous membrane in the subretinal space. The white arrow indicates the location of the implant in the cross-sectional image of the retina. Please click here to view a larger version of this figure.
Figure 10 shows the hematoxylin-eosin (H&E) staining of the retinal area containing the implanted primary hRPE cells on a nanofibrous carrier (yellow arrow) in the minipig eye. The pigmented appearance of the implanted primary hRPEs formed a continuous yet irregular pigmented layer (Figure 10, red arrows). After longer observation periods (6 weeks), the neuroretina underneath the implants showed a rosette-like or hypertrophic reaction-like appearance around the retinotomy site, likely as a result of iatrogenic manipulation. These morphologic results are comparable to the SD-OCT findings and support the evidence for the minimal impact of carrier delivery on the retinal tissue.
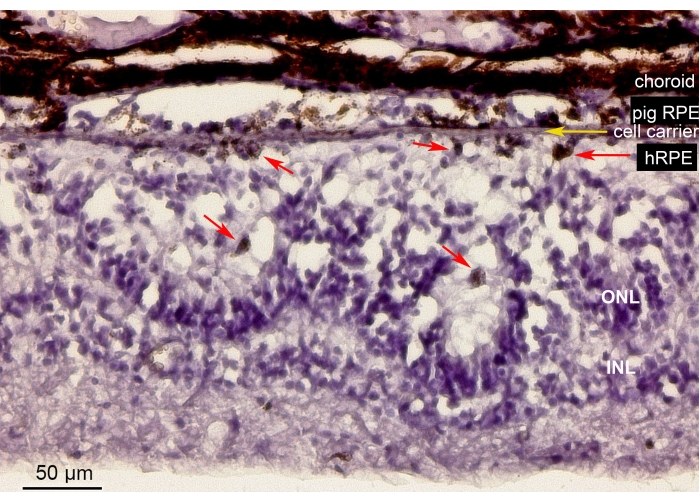
Figure 10: Histological analysis of the implanted nanofibrous membrane with the primary hRPEs. Hematoxylin-eosin staining of the retinal area containing the implanted nanofibrous carrier (yellow arrow) with the primary hRPEs in the minipig eye. The animal was euthanized and analyzed 6 weeks after implantation. The primary hRPEs were clearly distinguishable by their size, round shape, and pigmentation (red arrows) in the subretinal space opposite the photoreceptors. The photoreceptor nuclei in the ONL build rosette-like structures. The subretinal space appears hypertrophic. Abbreviations: hRPE = primary human retinal pigmented epithelium, ONL = outer nuclear layer, INL = inner nuclear layer. Please click here to view a larger version of this figure.
Immunostaining was performed employing a two-step indirect method. The sections were incubated at room temperature overnight in CRALBP, a monoclonal primary antibody, at a dilution of 1:100. Immunofluorescence was performed using Alexa Fluor 488-conjugated secondary antibody.
The implanted primary hRPEs were present in the area of implantation and expressed the typical RPE CRALBP marker similar to the endogenous minipig RPE cells (Figure 11A). In contrast, the morphology of the implanted cells appeared to not assume a monolayer shape after implantation yet remained localized within the defined subretinal space (Figure 11A,B, white arrows). The following RPE/retinal markers and morphological appearance remained positive after the 6 week post-implantation period: the presence of pigment/melanin granules, the end-stage retinal specific neuronal markers for the rod bipolar (PKC-alpha) and the cone photoreceptors (PNA), and the GFAP positivity-a sign of microglial activation.
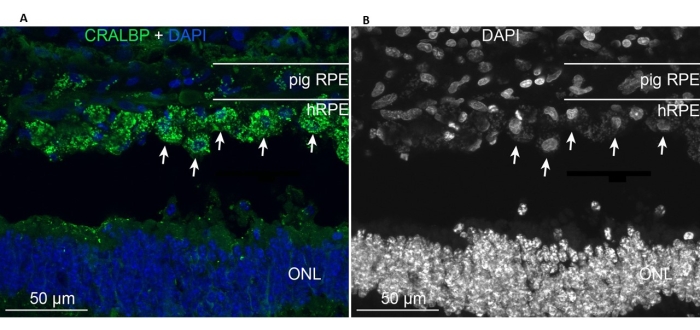
Figure 11: Immunolabeling with the RPE cell marker CRALBP (cellular retinaldehyde binding protein) in a minipig 6 months after implantation of primary hRPEs. (A) Vertical frozen sections of the treated pig eye were immunolabeled with CRALBP monoclonal antibody (green) and counterstained with DAPI (blue). (B) Single depiction of cell nuclei labeling with DAPI in black and white, as high contrast reveals the round shape of the individual hRPE cells (some shown with white arrows). Abbreviations: hRPE = human retinal pigment epithelium, ONL = outer nuclear layer. Please click here to view a larger version of this figure.
Ocular complications
In total, there were 27 of 29 (93.1%) successfully performed operations. The definition "successfully performed surgeries" was applied to those cases where the operated eye did not show any clinically significant postoperative complications until the time of enucleation that could influence the histological and immunohistochemical study. Reduced transparency of the optical media impacted the postoperative imaging in four cases (13.7%); nonetheless, these eyes were processed with further histologic and immunohistochemical analysis.
Intraoperative peripheral retinal detachment occurred in four cases (13.8%). In two cases, it was managed by aspiration of the subretinal fluid during fluid-gas exchange and the application of laser photocoagulation of the retina in the area of detachment. In the other two cases (6.9%), the retinal detachment was associated with massive retinal and subretinal bleeding, which made implantation of the cell carrier impossible and led to the termination of the surgery and immediate euthanasia of the minipig while on the operating table.
| No | Parameters | Standard used settings |
| 1 | Vitrectomy speed (cutting rate) | up to 20,000 cuts/min |
| 2 | Venturi pump | 50-180 mmHg |
| 3 | Rise time | 1 sec |
| 4 | Irrigation pressure | 18-25 mmHg |
| 5 | Air infusion pressure | 20-25 mmHg |
| 6 | Bipolar exodiathermy | 18-26% |
| 7 | Monopolar endodiathermy | 16-18% |
| 8 | Laser photocoagulation of the retina, 532 nm | Power 100-150 mW |
| Interval 100 ms | ||
| Duration 100 ms |
Table 1: Standard parameters used during vitrectomy and laser photocoagulation.
| Total animals, n | 18 |
| Total eyes, n | 36 |
| Operated eyes, n | 29 |
| Successful implantation, n | 27 |
| Failed cases, n | 2 |
| Mean surgery time, min | 57 |
| Success rate, % | 93.1 |
Table 2: Results of the standardized surgical technique with subretinal implantation of the cell carrier in Liběchov minipigs between 2016 and 2020.
Supplementary File 1: Summary of the studies dedicated to the subretinal implantation of RPE cells on the cell carrier. Please click here to download this File.
Discussion
The subretinal implantation of RPE cells with different origins is a very promising trend in eye research for the treatment of retinal degenerative disorders, such as AMD3,4,8,9,10,11,12,13,14,15,25. The main idea of this approach is to substitute the damaged RPEs with healthy RPEs cultured ex vivo (Supplementary File 1)44,45,46,47,48. The use of cell carriers to transplant the cultivated RPE cells represents the most reasonable approach, since the porous membranes maintain the polarized RPE cell layer in the correct orientation with regard to the photosensory layer.
Optimal animal model
A critical step in developing such treatment approaches is the use of the optimal animal model49. In the past, small and large animal models have been used, including rabbits, dogs, pigs, and non-human primates8,9,10,11,12,13,14,15,27,29. In this paper, we propose the use of the Liběchov minipig model and describe the preoperative, surgical, and postoperative steps that enable robust transplantation efficacies. The Liběchov minipig was originally bred about 20 years ago and has been frequently used in biomedical research in the field of neurodegenerative diseases, such as Parkinson's and Huntington's disease29,50. Since the pig possesses a relatively large brain with a blood supply and immunologic response similar to those in humans, it has been used as an animal model for allogeneic transplantation experiments as well51,52,53,54. Even though the retina of the minipigs does not possess a human-like macula and fovea, it contains the area centralis and visual streaks, which are regions of the retina with a high concentration of cone photoreceptors30. The similar size to the human eye, the presence of a cone-enriched central retina, the well-described immune system, and the presence of methods to assess the morphology and function post-surgery are important arguments for the use of this large animal model in the presented study.
Surgical procedure
To the best of our knowledge, there are no standardized and widely accepted surgical techniques for the vitreoretinal transplantation of RPE cells on carriers. One of the key issues of cell replacement therapy is the challenging surgical technique that has a risk of intraoperative and postoperative complications linked to retinal detachment, hypotony, episcleral, choroidal, and/or retinal bleeding, and high intraocular turbulence, which can lead to scaffold damage. Postoperatively, there is a risk of proliferative vitreoretinopathy, endophthalmitis, hypotony, retinal detachment, and cataract formation4,10,13,14,15.
The first studies on approaches using cell carriers were performed in chinchilla bastard rabbits13,16,25. Even though these animals represent a small animal model, the results focusing on the technical aspects of the surgery were crucial in the development of the procedures in large animal models and are, therefore, summarized below.
A custom-made 23 G infusion cannula was initially used with two side ports in order to redirect the jet stream, which helped to resolve the collapse of the bleb and consequent retinal detachment13. In the present study, we did not notice any such collapse of the bleb. The possible reason for that could be the bigger size of the eyeball and the performance of the core vitrectomy with spared vitreous on the periphery at the cannula infusion site, which could reduce the force of the directed jet stream.
Difficulties during the ejection of the cell carrier from the instrument were another intraoperative obstacle in the small animal models, which were categorized as "trapped with the instrument". Additionally, the authors suggested that the residual vitreous on the retinal surface could cause a backward "jump" of the carrier out of the retinotomy orifice after implantation. This problem can be solved with an enzyme-assisted vitrectomy, which enables a smooth, continuous ejection of the cell carrier into the subretinal space. In the majority of cases, the authors repositioned the carrier to obtain a more distant location of the implant away from retinotomy. In our case series, we also experienced a situation in which the cell carrier remained attached to the tip of the injector. However, that was managed by slow and gentle manipulation of the light pipe and the injector's tip. We did not observe any residual vitreous at the site of retinotomy in any of our cases. The use of TA-assisted PPV in the surgeries can be suggested as a method to reduce the risk of residually attached vitreous. Multiple staining with TA may be necessary to remove the overlying vitreous completely.
In a different study, the results of subretinal implantation of human RPE stem cells grown as a polarized cellular monolayer on a polyester membrane were reported24. During the experiments, the same surgical technique described previously was used13, but a two-port PPV approach was applied. Finally, a step-by-step protocol for the subretinal implantation of cell carrier surgery in rabbits was published subsequently25. This study presents a very detailed and easily repeatable description of the surgical procedure, including preoperative and postoperative care, which are based on previous experience as well.
During the use of large animal models in subsequent studies, not only technical questions were addressed but also questions regarding the immune reaction to the transplanted cells, as well as cell carrier size-related issues. A study using cynomolgus monkeys (Macaca fascicularis) described the results of the subretinal implantation of human stem cell-derived RPE monolayers15. All the animals underwent systemic immunosuppression, which consisted of sirolimus (loading dose of 2 mg, daily dose of 1 mg) and tetracycline (7.5 mg/kg– BW) starting 7 days prior to the surgery and lasting 3 months after the surgery. The surgical procedure was performed according to protocols described previously24,25. The authors used a 25 G three-port PPV approach with chandelier endo-illumination. Importantly, a TA-assisted PVD was used to exclude residual vitreoretinal adhesion on the posterior retina. As an addition to the originally described procedure, the authors removed the host RPE layer in the area of future implantation using a 20 G custom-made extendable loop instrument.
In our minipig study, we also used systemic immunosuppression. However, the type of immunosuppression differed from the one described above. We administered a subcutaneous injection of tacrolimus-eluting polymer microspheres as a depot at a dose of 0.25 mg/kg BW to hamper cell graft rejection and inflammatory reactions. We did not remove the host RPE cell layer during surgery, as our primary aim was to analyze the safety of the procedure and the viability of the implanted cells but not their integration into the host retina.
Previously, the safety and feasibility of the subretinal implantation of a monolayer of hESC-derived RPEs on a foldable non-degradable mesh-supported submicron parylene-C membrane (6.25 mm x 3.5 mm, 0.4 µm thick) was assessed in 14 female Yucatán minipigs10. After cultivation, the cells were seeded onto a mesh-supported membrane. Immunosuppression was performed using the systemic administration of tacrolimus (no regime and dose indicated) and intravitreal injections of 0.7 mg of a dexamethasone implant at the end of surgery. PPV was performed with a 20 G approach. The authors used an intravitreal injection of triamcinolone acetonid for better visualization of the vitreous body. The large sclerotomy was 2 mm to 3 mm in size. After the subretinal injection, the retina was flattened with a temporary injection of perfluorocarbon liquid. After the fluid-air exchange, a silicone oil tamponade (1,000/5,000 cSt) was performed. Postoperative care included the ocular application of dexamethasone/neomycin/polymyxin B ointment 1 week after the surgery. The authors reported a success rate of 91% (i.e., efficient subretinal implantation and sufficient postoperative imaging data). In our study, the intravitreal injection of TA crystals was used intraoperatively and mainly to visualize the vitreous body. However, the local immunosuppressive action of this drug remains unclear. The nanofibrous cell carriers used in our study were 5.2 mm x 2.1 mm and 3.7 µm thick, with pore sizes of 0.4 µm. During the surgery, we performed direct FAX instead of injecting perfluorocarbon liquid. Our surgical success rate (93.1%) was consistent with and slightly better than that of Koss et al.10.
The subretinal transplantation of fully degradable cell carriers (scaffold) for subretinal implantation was first studied in 2019 in Yorkshire pigs14. The study was mainly focused on the biodegradable characteristics of fibrin hydrogel implants. The authors noted that the aggressive immunosuppression used on the domestic pigs could inhibit a local inflammatory reaction potentially caused during biodegradation of the fibrin hydrogel implants. However, they did not specify the immunosuppressive therapy used in the pigs. During PPV, they performed a 3.6 mm long sclerotomy for insertion of the subretinal implantation device parallel to and approximately 3.5 mm posterior to the limbus. Additionally, they used a pneumatic-driven injection system aiming to reduce the hand-placement instability caused by finger manipulation. In our case series, all the sclerotomies were 2.5 mm to 3.0 mm from the limbus. The large sclerotomy for the insertion of the injector was 3 mm long. The implantation injector used in our study was operated by hand. Thorough cautery of the pars plana of the ciliary body and a sufficient cut inside the large sclerotomy appear to be crucial for avoiding intraoperative complications such as iatrogenic peripheral retinal detachment, bleeding, and loss of the implant.
In summary, we describe the use of the Liběchov minipig model for the transplantation of RPE cells on biodegradable carriers as a treatment option for inherited and acquired retinal diseases. Similarities in eye anatomy and physiology, as well as with regard to the immune system, allow us to develop and improve on the surgical techniques and instrumentation for the subretinal implantation of cells, which can be easily transferred to the treatment of human eye disorders. It is important to assure that surgeries on minipigs are performed using the same instrumentation (including implantation delivery tools) when utilized in human surgeries, thus facilitating the application of gained experience and know-how to humans. Alternative large-eye animal models with the presence of a macular area, such as non-human primates, could be useful for the follow-up and analysis of the anatomical and functional changes after subretinal implantation in the central retinal area. The detailed description of the preoperative, surgical, and postoperative care procedures will be useful for future studies by increasing efficient and standardized data generation.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The project was supported by The Czech Science Foundation (Project Number 18-04393S) and Norway Grants and Technology Agency of the Czech Republic (KAPPA Programme, Project Number TO01000107).
Materials
| Technical equipment | |||
| Wato EX-65 with a Mindray iMEC10 | Mindray, Shenzhen, China | Wato Ex-65 | anesthesia machine |
| R-Evolution CR | Optikon, Rome, Italy | R-Evolution CR | phacoemulsifier/vitrectome |
| Green laser Merilas 532α | Meridian, Thun, Switzerland | Merilas 532α | ophthalmic green laser |
| Microscope Hi-R NEO 900A | Haag-Streit Surgical, Wedel, Germany) | Hi-R NEO 900A | ophthalmic surgery microscope |
| Camera Sony PMW-10MD | Sony, Tokyo, Japan | PMW-10MD | full HD medical 2-piece |
| Non-contact vitreoretinal surgical system MERLIN BIOM | Volk, Mentor, OH, USA | MERLIN BIOM | BIOM |
| Steam sterilizer | Tuttnauer Europe B.V., Breda, NL | 3870 HSG | sterilizer |
| iCam100 | Optovue, Fremont, CA, USA | iCAM100 | funduscamera |
| iVue OCT100-2 | Optovue, Fremont, CA, USA | iVue OCT100-2 | OCT |
| Microsurgical instruments and devices | |||
| Cook Eye Speculum | Katena, New Jersey, US | K1-5403 | 15mm blades |
| Ophthalmology surgical drape | Hylyard, Alpharetta, Georgie, USA | 79304 | 132 x 142cm |
| Disposable Two step vitrectomy system. (23 gauge/ 0.6 mm) | DORC, Zuidland, Netherlands | 1272.ED06 | |
| Infusion line for 23G / 0.6 mm infusion cannula | DORC, Zuidland, Netherlands | 1279.P | |
| knife 2.75mm, IQ Geometry Tm Slit Knife Angled, Bevel Up | Surgical Specialties Corporation, Reading, USA | 72-2761G | |
| Extendible 41G subretinal injection needle. (23 gauge / 0.6 mm) | DORC, Zuidland, Netherlands | 1270.EXT | |
| Omnifix 3ml Luer Lock Solo siringe | BBraun, Melsungen, Germany | 4617022V | 3ml |
| 1ml soft-inject Tuberculin | Henke Sass Wolf, Tuttlingen, Germany | 5010.200V0 | 1ml |
| 8-0 Coated Vicryl | Ethicon, Puerto Rico, USA | J409G | |
| Purified Silicone Oil (in syringe) 10 ml | (FCI, Paris, France) | S5.7170 | 1000cSt |
| Pinnacle 360 Morris Vertical Scissors 23Ga | Synergetics, O'Fallon, USA | 10.24.23PIN | 23Ga |
| Revolution DSP 23Ga ILM forceps | Alcon, Geneva, Switzerland | 706.44 | Griesharber revolution |
| 23ga Straight Laser Probe | Synergetics, O’Fallon, USA | 55.21.23 | |
| FCI Protect 2.0% | FCI Ophthalmics, Paris, France | S5.9100 | viscoelastic |
| DK Westcott style Stitch Scissors, Curve | Duckworth & Kent, Hertfordshire, England | 1-501 | Curve |
| Pierse Notched Forceps, 0,3mm Straigh | Duckworth & Kent, Hertfordshire, England | 2-100-1E | 0,3mm straigh |
| DK Harms-Tubingen Straight Tying Forceps | Duckworth & Kent, Hertfordshire, England | 2-504E | 6mm |
| DK Needle Holder, Straigh | Duckworth & Kent, Hertfordshire, England | 3-201 | 9mm straigh |
| Medications and solutions | |||
| Unitropic 1% gtt. | UNIMED PHARMA spol. s r.o., Bratislava, Slovak republic | tropicamidum 10 mg/ml | eye drops |
| Diprophos | Merck Sharp & Dohme B.V., Haarlem, Netherlands | betamethasonum 7 mg/ml | 1ml |
| Alcon BSS Irrigation Solution | Alcon, Geneva, Switzerland | balance salt solution (BSS) | 500ml |
| Betaisodona | Mundipharma, Cambridge, United Kingdom | povidon-Iodine 1g/10ml | 30ml |
| Depo-medrol 120mg | Pfizer, New Yourk, USA | methylprednisolon | 5ml/200mg |
| Shotapen | Virbac Carros Cedex, France | benzylpenicillin, dihydrostreptomycin | 250ml |
| Flunixin a.u.v. | Norbrook, Newry, Northern Ireland | flunixinum 50,0 mg | 250ml |
| Tramal 100MG/2ML | Stada Arzneimittel AG, Bad Vilbel, Deutschland | tramadol | 2ml |
| Zoletil 100 | Virbac Carros Cedex, France | tiletamine, zolazepam | 100mg |
| Narkeran 10 | Vetoquinol, Magny-Vernois, France | ketamin | 2ml |
| Rometar 20mg/ml | Spofa pharmaceutica, Prague, Czech republic | xylazinum | 20mg |
| Braunol 75mg/g | B.Braun medical, Prague, Czech republic | povidone iodine | 75mg/g |
| Propofol 1% MCT/LCT | Fresenius Kabi, Bad Homburg, Deutschland | propofol | 10mg/1ml |
| Isoflurane 100% Inhalation vapour, liquid | Piramal Critical Care Limited, West Drayton, United Kingdom | isoflurane | 100% |
| Benoxi gtt. 4mg/1ml | Unimed pharma, Bratislava, Slovakia | oxybuprakaine | 10ml |
| Neosynephrin POS 10% gtt. | Ursapharm , Saarbrücken, Deutschland | fenylefrin chloride | 10ml |
| Ophthalmo-framykoin 1X5GM | Zentiva a.s., Prague, Czech republic | bacitracin zinc/hydrocortisone acetate/hydrocortisoneacetate/neomycin sulfate | 5mg |
| Floxal ung. | Dr. Gerhard Mann Chem.-Pharm. Fabrik, Berlin, Germany | ofloxacin | 0.30% |
| Eficur inj. | Hipra, Amer, Spain | ceftiofurum hydrochloridum | 50mg / 1ml |
| Draxxin | Zoetis Inc., New Jersey, USA | tulathromycinum | 100mg / 1ml |
| Tramal | Stada Arzneimittel AG, Bad Vilbel, Deutschland | tramadoli hydrochloridum | 100mg / 2ml |
| Xylapan | Vetoquinol, Magny-Vernois, France | xylazinum | 0.4 mg/kg |
| Proparacaine hydrochlorid ophthalmic solution 0,5% | Bausch&Lomb Incorporated Tampa, FL, USA | Proparacaine hydrochlorid | 0.50% |
| Prograf | Astellas Pharma, Deerfield, Illinois, USA | Tacrolimus powder | 1mg |
| Cell carrier, cultivated cells cultures, and implantation injector | |||
| Falcon Cell Culture Inserts | Corning Inc., Kenneburg, ME, USA | 353103 | |
| TrypLE Express Enzyme (1X) | Thermo Fisher Scientific, MA, USA | 12604021 | |
| DMEM/F-12 | Thermo Fisher Scientific, MA, USA | 11320033 | |
| Biolaminin 521 LN (LN521) | BioLamina, Sundbyberg, Sweden | LN521-02 | |
| GlutaMAX Supplement | Thermo Fisher Scientific, MA, USA | 35050061 | |
| 2-Mercaptoethanol | Thermo Fisher Scientific, MA, USA | J66742.0B | |
| Penicillin-Streptomycin | Sigma-Aldrich, San Luis, Mi, USA | P4333 | |
| CRALBP | Novus Biologicals, Abingdon, UK | NB100-74392 | |
| Alexa Fluor 488 | Thermo Fisher Scientific, Germany | 21202 |
References
- Pascolini, D., Mariotti, S. P. Global estimates of visual impairment: 2010. British Journal of Ophthalmology. 96 (5), 614-618 (2011).
- Bressler, N. M., Bressler, S. B., Fine, S. L. Age-related macular degeneration. Survey of Ophthalmology. 32 (6), 375-413 (1988).
- Binder, S., Stanzel, B. V., Krebs, I., Glittenberg, C. Transplantation of the RPE in AMD. Progress in Retinal and Eye Research. 26 (5), 516-554 (2007).
- Mano, F., et al. Methodological approach to improve surgical outcomes of a pig subretinal implantation model. Translational Vision Science & Technology. 11 (4), 24 (2022).
- Ramsden, C. M., et al. Stem cells in retinal regeneration: Past, present and future. Development. 140 (12), 2576-2585 (2013).
- Carr, A. -. J. F., et al. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends in Neurosciences. 36 (7), 385-395 (2013).
- Tezel, T. H., Kaplan, H. J., Del Priore, L. V. Fate of human retinal pigment epithelial cells seeded onto layers of human Bruch’s membrane. Investigative Ophthalmology & Visual Science. 40 (2), 467-476 (1999).
- Popelka, S., et al. A frame-supported ultrathin electrospun polymer membrane for transplantation of retinal pigment epithelial cells. Biomedical Materials. 10 (4), 045022 (2015).
- Kozak, I., et al. Safety and feasibility of new nanofiber subretinal delivery system with injector for RPE cell transplantation. Investigative Ophthalmology & Visual Science. 59 (9), 5670 (2018).
- Koss, M. J., et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatan minipigs. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv fur Klinische und Experimentelle Ophthalmologie. 254 (8), 1553-1565 (2016).
- Ghosh, F., Wong, F., Johansson, K., Bruun, A., Petters, R. M. Transplantation of full-thickness retina in the rhodopsin transgenic pig. Retina. 24 (1), 98-109 (2004).
- Christiansen, A. T., et al. Subretinal implantation of electrospun, short nanowire, and smooth poly(epsilon-caprolactone) scaffolds to the subretinal space of porcine eyes. Stem Cells International. 2012, 454295 (2012).
- Stanzel, B. V., et al. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Investigative Ophthalmology & Visual Science. 53 (1), 490-500 (2012).
- Gandhi, J. K., et al. Fibrin hydrogels are safe, degradable scaffolds for sub-retinal implantation. PloS One. 15 (1), 0227641 (2020).
- Liu, Z., et al. Surgical transplantation of human RPE stem cell-derived RPE monolayers into non-human primates with immunosuppression. Stem Cell Reports. 16 (2), 237-251 (2021).
- Mandai, M., et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. New England Journal of Medicine. 376 (11), 1038-1046 (2017).
- Schwartz, S. D., et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 385 (9967), 509-516 (2015).
- Kashani, A. H., et al. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: Experience from a phase 1/2a study. Ophthalmology Retina. 4 (3), 264-273 (2020).
- Kashani, A. H., et al. Subretinal implantation of a human embryonic stem cell-derived retinal pigment epithelium monolayer in a porcine model. Advances in Experimental Medicine and Biology. 1185, 569-574 (2019).
- Kashani, A. H., et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Science Translational Medicine. 10 (435), 4097 (2018).
- da Cruz, L., et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nature Biotechnology. 36 (4), 328-337 (2018).
- Diniz, B., et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: Improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 54 (7), 5087-5096 (2013).
- Sharma, R., et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Science Translational Medicine. 11 (475), 7624 (2019).
- Stanzel, B., et al. Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Reports. 2 (1), 64-77 (2014).
- Al-Nawaiseh, S., et al. A step by step protocol for subretinal surgery in rabbits. Journal of Visualized Experiments. (115), e53927 (2016).
- Stanzel, B., et al. Surgical approaches for cell therapeutics delivery to the retinal pigment epithelium and retina. Advances in Experimental Medicine and Biology. 1186, 141-170 (2019).
- Yaji, N., Yamato, M., Yang, J., Okano, T., Hori, S. Transplantation of tissue-engineered retinal pigment epithelial cell sheets in a rabbit model. Biomaterials. 30 (5), 797-803 (2009).
- Hruban, V., et al. Inheritance of malignant melanoma in the MeLiM strain of miniature pigs. Veterinarni Medicina. 49 (12), 453-459 (2004).
- Vodicka, P., et al. The miniature pig as an animal model in biomedical research. Annals of the New York Academy of Sciences. 1049, 161-171 (2005).
- Vízina, M., Wier, A. B., Collins, M. Comparative Ocular Anatomy in Commonly Used Laboratory Animals. Ocular Toxicology in Laboratory Animals. , 9-12 (2013).
- Chandler, M. J., Smith, P. J., Samuelson, D. A., MacKay, E. O. Photoreceptor density of the domestic pig retina. Veterinary Ophthalmology. 2 (3), 179-184 (1999).
- Wang, Q. X., et al. Biodegradable microsphere-loaded tacrolimus enhanced the effect on mice islet allograft and reduced the adverse effect on insulin secretion. American Journal of Transplantation. 4 (5), 721-727 (2004).
- Sevc, J., et al. Effective long-term immunosuppression in rats by subcutaneously implanted sustained-release tacrolimus pellet: Effect on spinally grafted human neural precursor survival. Experimental Neurology. 248, 85-99 (2013).
- Juhásová, J., et al. Osteogenic differentiation of miniature pig mesenchymal stem cells in 2D and 3D environment. Physiological Research. 60 (3), 559-571 (2011).
- Planka, L., et al. Nanotechnology and mesenchymal stem cells with chondrocytes in prevention of partial growth plate arrest in pigs. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czech Republic. 156 (2), 128-134 (2012).
- Soerensen, D. D., Pedersen, L. J. Infrared skin temperature measurements for monitoring health in pigs: a review. Acta Veterinaria Scandinavica. 57 (1), 5 (2015).
- Eichhorn, S. J., Sampson, W. W. Statistical geometry of pores and statistics of porous nanofibrous assemblies. Journal of the Royal Society Interface. 2 (4), 309-318 (2005).
- Szatmári-Tóth, M., et al. Clearance of autophagy-associated dying retinal pigment epithelial cells – a possible source for inflammation in age-related macular degeneration. Cell Death & Disease. 7 (9), 2367 (2016).
- Lukovic, D., et al. Human iPSC derived disease model of MERTK-associated retinitis pigmentosa. Scientific Reports. 5, 12910 (2015).
- Artero Castro, A., et al. Generation of gene-corrected human induced pluripotent stem cell lines derived from retinitis pigmentosa patient with Ser331Cysfs*5 mutation in MERTK. Stem Cell Research. 34, 101341 (2019).
- Artero Castro, A., León, M., Del Buey Furió, V., Erceg, S., Lukovic, D. Generation of a human iPSC line by mRNA reprogramming. Stem Cell Research. 28, 157-160 (2018).
- Artero-Castro, A., et al. correction recovers phagocytosis in retinal pigment epithelium derived from retinitis pigmentosa-human-induced pluripotent stem cells. International Journal of Molecular Sciences. 22 (4), 2092 (2021).
- Müller, B., Wagner, F., Lorenz, B., Stieger, K. Organotypic cultures of adult mouse retina: Morphologic changes and gene expression. Investigative Ophthalmology & Visual Science. 58 (4), 1930-1940 (2017).
- Sheridan, C. M., et al. Replacement of the RPE monolayer. Eye. 23 (10), 1910-1915 (2009).
- Radtke, N. D., et al. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. American Journal of Ophthalmology. 146 (2), 172-182 (2008).
- Maaijwee, K. J. M., et al. Histological evidence for revascularisation of an autologous retinal pigment epithelium–choroid graft in the pig. The British Journal of Ophthalmology. 91 (4), 546-550 (2007).
- Ghosh, F., Engelsberg, K., English, R. V., Petters, R. M. Long-term neuroretinal full-thickness transplants in a large animal model of severe retinitis pigmentosa. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv fur Klinische und Experimentelle Ophthalmologie. 245 (6), 835-846 (2007).
- Radtke, N. D., Seiler, M. J., Aramant, R. B., Petry, H. M., Pidwell, D. J. Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. American Journal of Ophthalmology. 133 (4), 544-550 (2002).
- Slijkerman, R. W., et al. The pros and cons of vertebrate animal models for functional and therapeutic research on inherited retinal dystrophies. Progress in Retinal and Eye Research. 48, 137-159 (2015).
- Schramke, S., et al. The Libechov minipig as a large animal model for preclinical research in Huntington’s disease – Thoughts and perspectives. Czech and Slovak Neurology and Neurosurgery. 78/111, 55-60 (2015).
- Tohyama, S., Kobayashi, E. Age-appropriateness of porcine models used for cell transplantation. Cell Transplantation. 28 (2), 224-228 (2019).
- Dall, A. M., et al. Quantitative [18F] fluorodopa/PET and histology of fetal mesencephalic dopaminergic grafts to the striatum of MPTP-poisoned minipigs. Cell Transplantation. 11 (8), 733-746 (2002).
- Shrader, S. M., Greentree, W. F. Göttingen minipigs in ocular research. Toxicologic Pathology. 46 (4), 403-407 (2018).
- Duarri, A., et al. Transplantation of human induced pluripotent stem cell-derived retinal pigment epithelium in a swine model of geographic atrophy. International Journal of Molecular Sciences. 22 (19), 10497 (2021).

