Intracerebroventricular Delivery of Gut-Derived Microbial Metabolites in Freely Moving Mice
Summary
Gut-derived microbial metabolites have multifaceted effects leading to complex behavior in animals. We aim to provide a step-by-step method to delineate the effects of gut-derived microbial metabolites in the brain via intracerebroventricular delivery via a guide cannula.
Abstract
The impact of gut microbiota and their metabolites on host physiology and behavior has been extensively investigated in this decade. Numerous studies have revealed that gut microbiota-derived metabolites modulate brain-mediated physiological functions through intricate gut-brain pathways in the host. Short-chain fatty acids (SCFAs) are the major bacteria-derived metabolites produced during dietary fiber fermentation by the gut microbiome. Secreted SCFAs from the gut can act at multiple sites in the periphery, affecting the immune, endocrine, and neural responses due to the vast distribution of SCFAs receptors. Therefore, it is challenging to differentiate the central and peripheral effects of SCFAs through oral and intraperitoneal administration of SCFAs. This paper presents a video-based method to interrogate the functional role of SCFAs in the brain via a guide cannula in freely moving mice. The amount and type of SCFAs in the brain can be adjusted by controlling the infusion volume and rate. This method can provide scientists with a way to appreciate the role of gut-derived metabolites in the brain.
Introduction
The human gastrointestinal tract harbors diverse microorganisms impacting the host1,2,3. These gut bacteria can secrete gut-derived metabolites during their utilization of dietary components consumed by the host4,5. Interestingly, the gut metabolites not metabolized in the periphery can be transported to other organs via circulation6. Of note, these secreted metabolites can serve as mediators for the gut-brain axis, defined as the bidirectional communication between the central nervous system and the gut7. Previous studies have shown that gut-derived metabolites can modulate complex behavior and emotion in animals8,9,10,11.
Short-chain fatty acids (SCFAs) are the main metabolites produced by gut microbiota during the fermentation of dietary fiber and indigestible carbohydrates6. Acetate, propionate, and butyrate are the most abundant SCFAs in the gut12. SCFAs serve as the energy source for cells in the gastrointestinal tract. Unmetabolized SCFAs in the gut can be transported to the brain through the portal vein, thus modulating brain and behavior6,12. Previous studies have suggested that SCFAs might play a critical role in neuropsychiatric disorders6,12. For example, intraperitoneal injection of butyrate in BTBR T+ Itpr3tf/J (BTBR) mice, an animal model of autism spectrum disorder (ASD), rescued their social deficits13. Antibiotic-treated rats receiving microbiota from depressive subjects showed an increase in anxiety-like behaviors and fecal SCFAs14. Clinically, alterations in fecal SCFAs levels were observed in people with ASD compared to typically developing controls15,16. People with depression have lower fecal SCFAs levels than healthy subjects17,18. These studies suggested that SCFAs can alter behavior in animals and humans through various routes.
Microbial metabolites exert diverse effects on multiple sites in the body, impacting host physiology and behaviors4,19, including the gastrointestinal tract, vagus nerve, and sympathetic nerve. It is difficult to pinpoint the precise role of gut-derived metabolites in the brain when administering the metabolites via peripheral routes. This paper presents a video-based protocol to investigate the effects of gut-derived metabolites in the brain of a freely moving mouse (Figure 1). We showed that SCFAs could be acutely given through the guide cannula during behavioral tests. The type, volume, and infusion rate of metabolites can be modified depending on the purpose. The site of cannulization can be adjusted to explore the impact of gut metabolites in a specific brain region. We aim to provide scientists with a method to explore the potential impact of gut-derived microbial metabolites on the brain and behavior.
Protocol
All the experimental protocols and the animals' care were approved by the National Cheng Kung University (NCKU) Institutional Animal Care and Use Committee (IACUC).
1. Preparation for the experimental animal
- Obtain 6-8-week-old wild-type C57BL/6JNarl male mice from a vendor.
- House the mice in a standard mouse cage with standard mouse chow and sterilized water ad libitum.
NOTE: The housing conditions for the Laboratory Animal Center of NCKU are 22 ± 1 °C temperature, 55% ± 10% humidity, and a 13 h/11 h light/dark cycle.
2. Stereotaxic surgery
- Prepare and sterilize the stereotaxic instrument, surgical instruments, and related items.
NOTE: All items that will directly contact the surgical site should be sterilized to avoid infection. - Anesthetize the mouse by placing it in the Plexiglas cage with 1%-5% isoflurane in oxygen.
NOTE: Closely observe the mouse to ensure the breathing rate is maintained at around one breath per second. - Take the mouse out of the anesthesia chamber. Shave the surgical site (mouse head) with a pet trimmer. Place the mouse onto the stereotaxic frame by fixing the mouse incisors on the incisor bar in the stereotaxic incisor holder. Cover the nose with the nosecone mask.
- Anesthetize the mouse with 1%-2.5% isoflurane in oxygen throughout the stereotaxic surgery. Evaluate the nociception of the mouse via the toe pinch reflex and ensure a constant breathing rate before incising the surgical site.
- Place a heating pad (37.0 °C) underneath the mouse on the stereotaxic frame to maintain body temperature during surgery. Alternatively, maintain the core temperature with the aid of a rectal thermal probe connecting the programmed warmer and the heating pad.
- Remove the trimmed fur on the head using adhesive tape. Inject the mouse with analgesic Ketoprofen (5 mg/kg) subcutaneously to relieve pain. Apply eye ointment to avoid dry eyes.
- Insert the pointed ear bar in the ear canal to fix the head.
- Center the head by adjusting the scale of the ear bar.
- Tighten the nose clamp on the incisor holder to avoid vertical movement. Press the head gently to check that the head is fixed and avoid the head becoming loose during subsequent surgery.
- Disinfect the scalp with three alternating scrubs of chlorhexidine using a cotton swab. Start each scrub from the middle toward the outer side (from the most disinfected central area to the least disinfected area).
NOTE: The usage of scrubs from the middle to the outside could help scientists minimize infection and contamination from the fur. The outer area is close to the unshaved head region, which still has a great amount of fur and is not easy to disinfect thoroughly. - Incise the scalp in an anterior/posterior manner (<1 cm) using a surgical blade. Open the incision and wipe the skull with a cotton swab held by microdissecting forceps.
NOTE: The microdissecting forceps, surgical blade, and cotton swabs must be sterilized by autoclaving before surgery. During the surgical process, sterilize all the surgical equipment using a glass bead sterilizer (150 °C) for at least 5 s before and after each animal. Prepare a sterilized beaker to hold the surgical blade and forceps during the surgical process and between animals. - Identify the Bregma and Lambda on the skull. Use the Bregma as the reference to locate the region of interest.
- Optional: Mount the stereotaxic drill on the stereotaxic drill holder, and use the tip of the drill to point Bregma as the reference. Sterilize the stereotaxic drill using the glass bead sterilizer (150 °C) for at least 5 s.
- Calibrate and align the flat skull horizontal plane in left/right and anterior/posterior planes by Bregma/Lambda.
NOTE: If not in a correct horizontal plane, remount the mouse on the stereotaxic instrument.
3. Commercial customized guide cannula implantation
- Identify and label the position of the right lateral ventricle, based on the stereotaxic coordinates: Distance to Bregma, anterior/posterior (A/P): 0.26 mm, medial/lateral (M/L): -1.0 mm, dorsal/ventral (D/V): -2.0 mm20.
NOTE: The coordinates can be modified based on the region of interest. The coordinates for the lateral ventricle were based on adult C57BL/6J mice with a weight range of 26-30 g. If younger mice are used, refer to the discussion. - Drill a hole (diameter = 1.5 mm) through the skull at the labeled site using a stereotaxic drill for the implantation of a commercial guide cannula.
- Drill two to four more holes (diameter = 1.5 mm) on the skull using a stereotaxic drill for the mounting of stainless steel screws.
NOTE: Sterilize the stereotaxic drill using a glass bead sterilizer (150 °C) for at least 5 s before and after each animal. - Wipe the bone scraps and stop bleeding with a cotton swab.
- Wipe the skull with Lidocaine (1 mg/kg) using a cotton swab for local anesthetic, antipruritic, and pain-relieving effects. If bleeding does not stop after drilling, gently place a cotton swab on the hole for hemostasis.
- Mount two to four stainless screws on the holes to provide anchors for dental acrylic.
- Place the commercial guide cannula on the stereotaxic cannula holder and disinfect with the glass bead sterilizer (150 °C).
NOTE: The commercial guide cannula, commercial dummy, and commercial injector (Figure 2A) were customized with the specifications shown in the Table of Materials. - Move the stereotaxic cannula holder to the hole drilled for the lateral ventricle and slowly insert the commercial guide cannula into the hole until the desired depth (2.5 mm).
NOTE: The dorsal/ventral coordinate can be defined by setting the tip of the commercial guide cannula as the reference when the tip is barely inserted into the hole. - Apply 10 µL of n-butyl cyanoacrylate adhesive (tissue adhesive glue) to fix the commercial guide cannula in the drilled hole and wait for 3-4 min. Release the commercial guide cannula gently from the stereotaxic cannula holder and move the holder away.
- Apply the dental acrylic to the incised scalp to fix the commercial guide cannula and wait for at least 5 min. Implant the commercial dummy into the commercial guide cannula to avoid cannula clogging by blood or body fluid (Figure 2B).
- Release the ear bar from the ear canal and remove the mouse from the stereotaxic frame.
- Place the mouse into a new cage with a heating pad underneath for recovery from anesthesia and continuously observe until the mouse fully awakens.
NOTE: Do not leave the animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. An animal that has undergone surgery should not return to the company of other animals until fully recovered. - Return the mouse to single housing or group housing, depending on the institutional IACUC protocol and the experimental design. For group housing, ensure that fewer mice are housed in a cage to minimize unwanted injuries or the detachment of the cannula.
- Administer Ibuprofen (0.2 mg/mL) in the drinking water for at least 3 days for postoperative care, and monitor twice a day for signs of pain and distress for at least 3 days.
- During postoperative care, apply roxithromycin ointment around the skin to prevent inflammation and infection in the mice.
- Keep monitoring the animal's state and give a timely intraperitoneal injection of 5% glucose and/or 0.9% sodium chloride to provide enough energy.
- If the state of pain, distress, or infection steadily deteriorates, euthanize the mouse by CO2 inhalation.
- Wait for 1 week post operation for the mouse to be ready for the intracerebroventricular delivery of SCFAs and behavioral testing.
4. Preparation of SCFAs
- Dissolve the sodium acetate, sodium butyrate, and sodium propionate in artificial cerebrospinal fluid (ACSF) (see the Table of Materials).
- Ensure the chemicals are fully dissolved, then adjust the pH to 7.4, and filter the SCFAs mixture through a 0.22 μm filter for sterilization.
5. Set up the infusion system for intracerebroventricular delivery of SCFAs during behavioral testing
- Mount a ceiling camera to record the behavior. Connect the camera with a computer to control the video recording software (Figure 3).
- Fill a 10 μL syringe with distilled water.
NOTE: Avoid air bubbles in the microliter syringe. - Connect a microinjection pump with the microinjection controller.
- Mount the microliter syringe on the microinjection pump. To install the syringe, press the button to loosen the clamp and install the syringe onto the corresponding position. Close the clamp and tighten the plunger-retaining screw on the microinjection pump (Figure 4A).
- Insert the commercial injector into the polyethylene tube (Figure 2A).
- Hang the polyethylene tube on the ceiling camera above the testing arena.
- Fill the polyethylene tube with distilled water using an insulin syringe. Connect the microliter syringe to the hanging polyethylene tube.
NOTE: Ensure the polyethylene tube is long enough to allow the mouse to move freely throughout the testing arena.
6. System settings of the microinjection controller
- Turn on the microinjection controller and press Display All Channels to access the Command screen (Figure 4C). Press Configuration and set Volume Target 到 9,800 nL with Delivery Rate 到 100 nL/s. Infuse 9,800 nL of distilled water from the polyethylene tube connected to the microliter syringe (press Direction to switch to the Infuse mode and press RUN) (see red squares in the Command screen of Figure 4C).
- Press Configuration and set Volume Target 到 3,000 nL with Delivery Rate 到 100 nL/s. Withdraw 3,000 nL of mineral oil (press Direction to switch to the Withdraw mode and press RUN) (see red squares in the Command screen of Figure 4C).
NOTE: A clear oil-water phase separation should be observed on the polyethylene tube. - Disassemble the polyethylene tube from the microliter syringe needle. Spit out 3,000 nL of distilled water from the microliter syringe needle (press Direction to switch to the Infuse mode and press RUN).
- Insert the microliter syringe back into the polyethylene tube. Press Configuration and set Volume Target 到 9,500 nL with Delivery Rate 到 100 nL/s. Withdraw 9,500 nL of SCFAs (press Direction to switch to the Withdraw mode and press RUN). Label the oil-SCFAs phase to validate whether the SCFAs are successfully infused.
- Press Configuration and set the desired Volume Target with Delivery Rate 到 7 nL/s. Press Direction to switch to the Infuse mode (see red squares in the Command screen of Figure 4C).
NOTE: Determine the volume based on the infusion time. For example, if the infusion time is 3 min for the delivery rate of 7 nL/s, target volume = 1,260 nL. - Press RUN to infuse the microliter syringe forward until the liquid comes out at the front end of the commercial injector before inserting the injector into the cannula for SCFAs injection.
7. Infusion of SCFAs into lateral ventricle through the commercial guide cannula in freely moving mouse
- Anesthetize the mouse by placing it in the Plexiglas cage with 1%-5% isoflurane in oxygen.
NOTE: Closely observe the mouse to ensure the breathing rate is maintained at around one breath per second. - Scruff the mouse and insert the commercial injector into the commercial guide cannula (Figure 4B).
NOTE: If the commercial guide cannula is plugged by blood or body fluid, gently unclog it with tweezers. - Allow the mouse to recover from anesthesia for 15 min in a cage prior to behavioral testing.
- For the basic locomotion test, place the mouse in a novel cage and allow it to freely explore for 35 min. Infuse SCFAs using a Delivery Rate of 7 nL/s for a target volume of 2,100 nL in the first 5 min (press Direction to the Infuse mode and press RUN).
NOTE: The locomotion in the novel cage can be analyzed using animal behavior video tracking software21,22. - Anesthetize the mouse (repeating step 7.1) and remove the commercial injector from the commercial guide cannula.
NOTE: The mouse can be repeatedly injected with different controls/metabolites after giving the appropriate length of time to wash out the previous injection. As long as the cannula is fixed on the mouse head, the mouse can be repeatedly tested with different metabolites.
8. Restoration of the microinjection system
- Disassemble the polyethylene tube from the microliter syringe.
- Inject air into the tube using the insulin syringe to discard the distilled water in the polyethylene tube. Empty the microliter syringe.
- Press Reset Pos on the Configuration screen to open the Syringe Stop Definition screen (Figure 4C).
- Press Withdraw until a beeping sound occurs to reset the microinjection pump to the fully withdrawn position (Figure 4C).
- Return to the Command screen and turn off the microinjection controller if there is an **END REACHED** sign on this screen (Figure 4C).
9. Optional: Validation of intracerebroventricular injection by neural tracer
- Infuse 2,100 nL of the neural tracer with the Delivery Rate of 7 nL/s to verify the infusion site.
NOTE: Leave the injector in the guide cannula for 5 min to prevent backflow. - Anesthetize the mouse by an overdose of isoflurane (5%) 30 min after the infusion of neural tracer.
- Check the breathing rate and tail/paw pinch reflex in the anesthetized mouse.
NOTE: Mice must be unresponsive before the next step. - Make a 4-5 cm incision through the skin, muscle, and abdominal wall below the rib cage.
- Slightly move the liver away from the diaphragm carefully.
- Incise the diaphragm to expose the heart of the mouse.
- Perfuse the mouse through the heart with phosphate-buffered saline (PBS) and ice-cold 4% paraformaldehyde in PBS.
- Decapitate the mouse and dissect the brain carefully with microdissecting forceps and microdissecting scissors to take out the whole brain23. Place the brain samples in ice-cold 4% paraformaldehyde in PBS for 3-4 days and wash them 3 x 5 min with PBS.
- Cut the brain into two parts in the mouse brain slice holder at the eighth cut of the mouse brain slice holder (1 mm/section) from the anterior to posterior direction. Place the brain in the embedding mold and embed the brain samples in low-melting point agarose (4% in PBS).
- Glue the brain embedded in agarose onto the stage of the vibratome using superglue. Section the brain coronally into 50 µm brain slices using the vibratome.
- Incubate the brain slices in the antibody targeting the neural tracer diluted in blocking buffer (1:1,000 dilution) overnight at room temperature.
NOTE: The blocking buffer contained 10% horse serum, 0.1% Triton X-100, and 0.02% sodium azide. - Wash the slices 3 x 5 min with PBST (PBS with 0.1% triton X-100).
- Incubate the brain slices in fluorescence-dye conjugated secondary antibody diluted in blocking buffer (1:500 dilution) for 2 h at room temperature.
- Wash the slices 3 x 5 min with PBS.
- Mount the brain slices on the slide with a mounting medium containing 4',6-diamidino-2-phenylindole (DAPI).
- Cover the slide with a microscope coverslip.
- Apply nail polish on the slide edge to avoid leakage of the mounting medium.
- After overnight incubation at room temperature, protected from light, image the fluorescence signal in the infusion site using a fluorescence microscope.
10. Optional: Infusion of metabolites through a customized stainless steel guide cannula in the lateral ventricle in mice
- Follow protocol sections 1-8 and replace the commercial guide cannula with a stainless steel guide cannula to infuse chemicals through a stainless steel injector in the mouse.
NOTE: The pros and cons of the different commercial and stainless steel cannulas are elaborated upon in the discussion section. - Perform the surgical protocol in the same way as described in protocol sections 2 and 3, except remember to replace the commercial guide cannula with a stainless steel guide cannula (Figure 5B).
NOTE: The stainless steel guide cannula, stainless steel dummy, and stainless steel injector (Figure 5A) were customized with the specifications shown in the Table of Materials. Insert the customized stainless steel guide cannula into the hole until the desired depth (2.0 mm). - Infuse SCFAs via the stainless steel guide cannula using the microinjection system consisting of the microinjection controller and microinjection pump (Figure 4B) (same as protocol sections 4-7). For the stainless steel dummy of the stainless steel guide cannula, bend one side of the stainless steel injector gently until the tip of the other side is 1 mm longer than the stainless steel guide cannula.
- Infuse 2,100 nL of the neural tracer through the stainless steel guide cannula into the lateral ventricle in mice (same as protocol section 9).
- Collect the sample for 30 min after neural tracer infusion (same as protocol section 9).
- Perform image acquisition in the neural tracer infused-brain slices (same as protocol section 9).
Representative Results
The mouse was infused with SCFAs 1 week after recovery from the guide cannula implantation to evaluate locomotor activity in a novel cage. The mouse was placed in a novel cage and infused with 2,100 nL of SCFAs or ACSF in the first 5 min (delivery rate of 7 nL/s) into the brain through the commercial guide cannula implanted in the lateral ventricle of the brain. The locomotor activity in a novel cage was recorded for an additional 30 min after infusion. No difference was observed in the locomotor activity in the novel cage between infusion of SCFAs and ACSF (Figure 6) (n = 2 mice per group; data are shown as mean ± s.e.m. and analyzed by two-way ANOVA).
To validate the accuracy of implantation of the guide cannula in the brain regions, a fluorescent neural tracer was infused into the mice via the guide cannula at the same volume and delivery rate as the SCFAs (2,100 nL in 5 min; 7 nL/s). The brains were collected for histology 30 min later. The fluorescent dye was detected in the lateral ventricle and the surrounding regions of the mouse brain (Figure 7A). A stainless steel guide cannula was implanted into the lateral ventricle of the brain for the infusion of neural tracer under the same conditions. Similar to the guide cannula, the results showed that a fluorescent dye signal was detected in the lateral ventricle and the surrounding regions of the mouse brain even after infusion through the stainless steel guide cannula (Figure 7B).
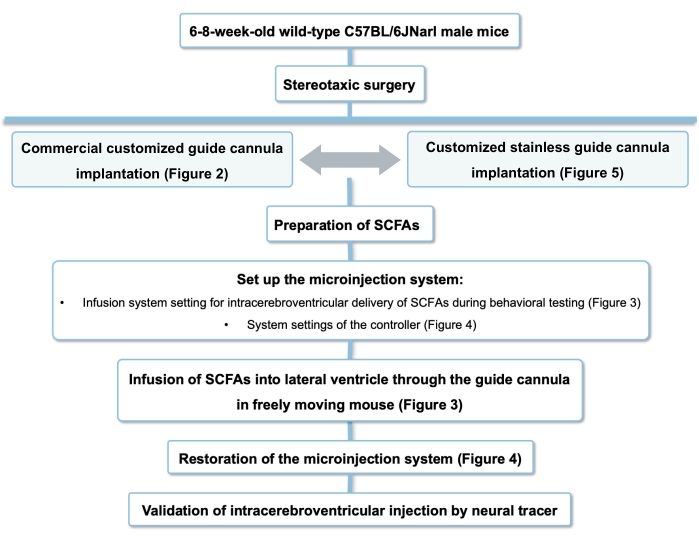
Figure 1: The procedure for intracerebroventricular infusion in freely moving mice. The flow chart for the intracerebroventricular delivery of gut-derived microbial metabolites in freely moving mice. Abbreviation: SCFAs = short-chain fatty acids. Please click here to view a larger version of this figure.
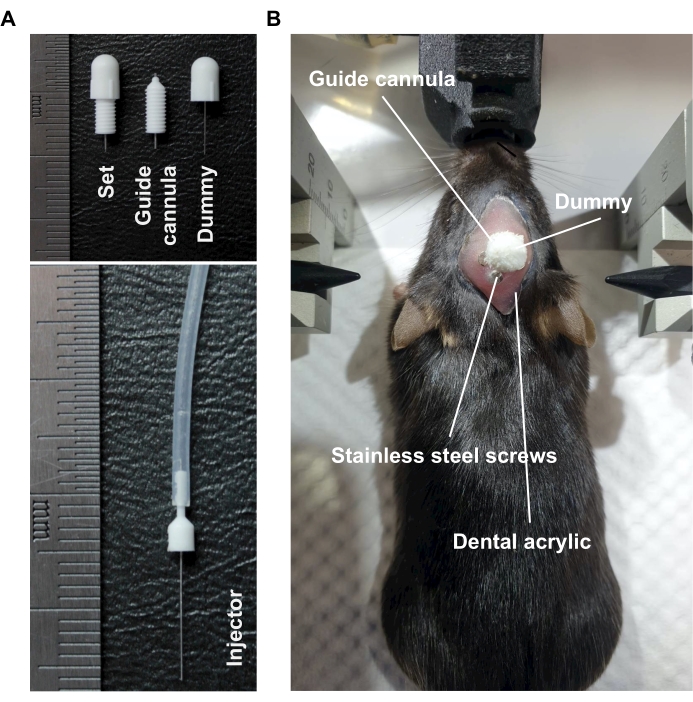
Figure 2: Implantation of the commercial guide cannula in mice. (A) The representative image of commercial guide cannula, dummy, and injector. (B) The representative image of commercial guide cannula and dummy implanted into the brain of mice by fixation with dental acrylic. Please click here to view a larger version of this figure.
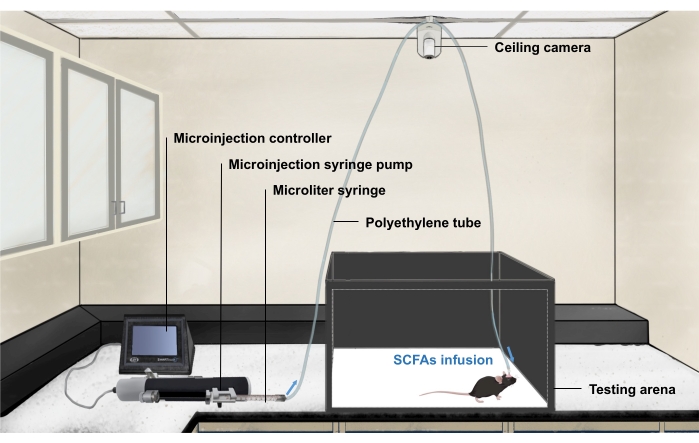
Figure 3: The intracerebroventricular infusion rig for behavior testing. The diagram of microinjection system, video recording system, and behavioral apparatus. Abbreviation: SCFAs = short-chain fatty acids. Please click here to view a larger version of this figure.
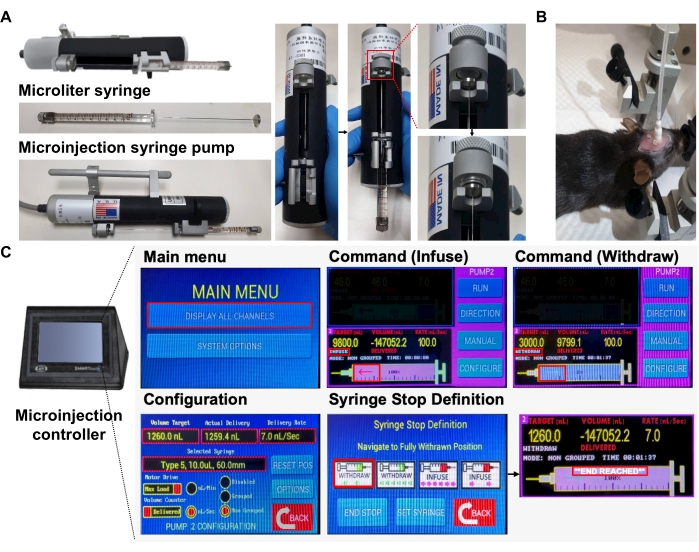
Figure 4: The microinjection system. (A) The installation of the microliter syringe using a microinjection pump. (B) The insertion of the commercial injector connected with polyethylene tube into the commercial guide cannula (C) The touch screen of the microinjection controller. Please click here to view a larger version of this figure.
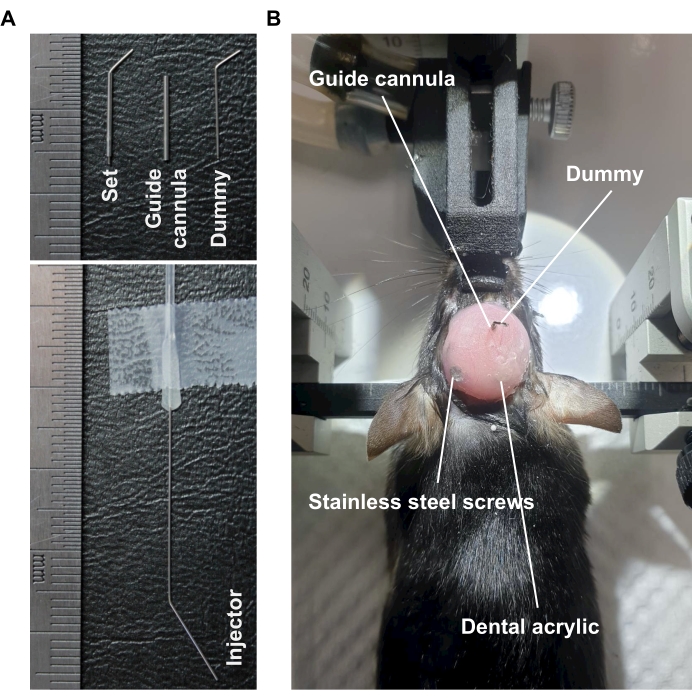
Figure 5: Implantation of stainless steel guide cannula in the lateral ventricle of mice. (A) The representative image of stainless steel guide cannula, dummy, and injector. (B) The representative image of stainless steel guide cannula and dummy implanted into the brain of mice by fixation with dental acrylic. Please click here to view a larger version of this figure.
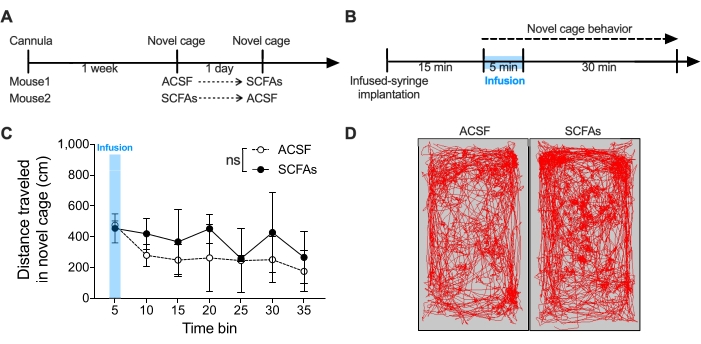
Figure 6: Locomotor activity of SCFAs-infused mice in the novel cage. (A) Timeline schematic of guide cannula implantation and novel cage locomotion upon ACSF or SCFAs infusion. (B) Timeline schematic for placement of infusion syringe, infusion window (blue shadow), and the novel cage behavior testing. (C) Total distance moved by ACSF- and SCFAs-infused mice in a novel cage for 35 min. The time window for infusion is indicated with blue shadow (0-5 min). (D) Representative images of trajectories for ACSF- and SCFAs-infused mice in a novel cage. n = 2 mice per group. Data shown as mean ± s.e.m. and analyzed by two-way ANOVA. ns: not significant. Abbreviations: SCFAs = short-chain fatty acids; ACSF = artificial cerebrospinal fluid. Please click here to view a larger version of this figure.
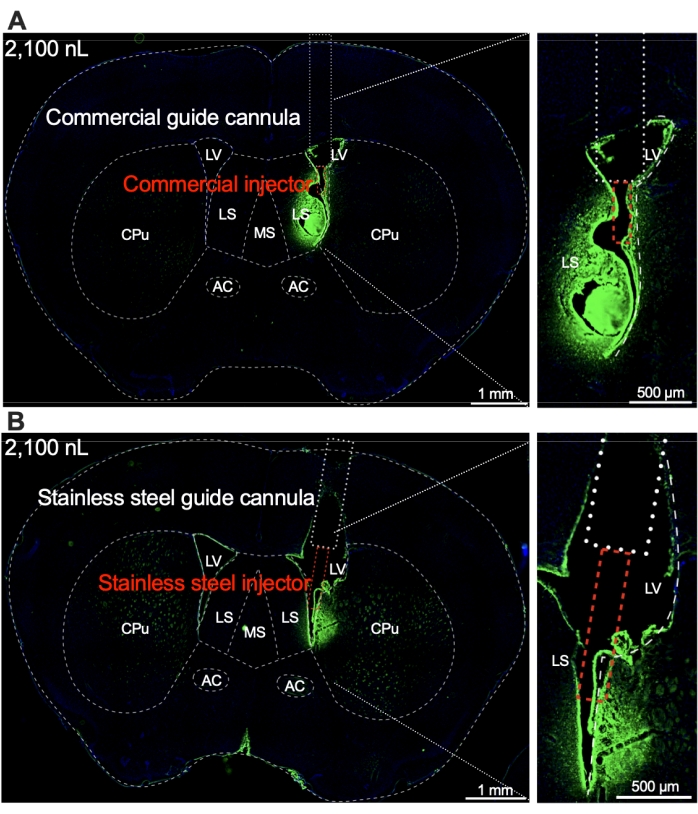
Figure 7: The histology of brain-infused fluorescent dye. Infusion through the customized (A) commercial and (B) stainless steel guide cannula implanted in the lateral ventricle (LV) of mice. Scale bars = 1 mm (left) and 500 µm (right). Blue: DAPI staining; Green: Anti-Fluorescent Gold labeling. Abbreviations: LV = lateral ventricle; AC = anterior commissure; CPu = caudate putamen; LS = lateral septum; MS = medial septum. Please click here to view a larger version of this figure.
Discussion
Gut-derived metabolites have been associated with brain-mediated diseases without much precise mechanism, partially due to their multiple binding sites in the body6,12,24. Previous reports indicated that SCFAs could serve as ligands for G protein-coupled receptors, epigenetic regulators, and sources for energy production at multiple sites in the body6,12. To bypass the confounding factors that originate from the periphery (such as immune cells, hormones, and autonomic nervous system), a method was developed by adopting intracerebroventricular injection of SCFAs into the brain through a guide cannula in freely moving mice. In addition, the cannulization and infusion sites were validated to explore the brain areas potentially affected by SCFAs. Altogether, this paper presents a precise, meticulous, and validated method to deliver gut-derived metabolites into the brain for gut-brain axis research.
The intracerebroventricular delivery of drugs and chemicals through a guide cannula into animals has been well-established25,26,27,28,29 for various drug tests29, disease modeling26,28, and specific design of behavioral tests27. Most importantly, several studies have delivered gut microbial metabolites into the brain through intracerebroventricular injection and tested their effects on physiological functions30,31,32,33. This protocol provides an add-on attribute in administering gut metabolites in an acute manner to the brain in real time, which would allow scientists to comprehend the dynamic effects of gut metabolites on the brain and behavior.
The delivery rate for the metabolite infusion is crucial to simulate the flow of cerebrospinal fluid in the brain ventricles. One study indicated that the cerebrospinal fluid production rate in mice is 5.3 nL/s34. To control the flow rate of SCFAs naturally in freely moving mice, we have evaluated the flow rate for this microinjection system (Figure 3 and Figure 4). The SCFAs could be infused smoothly without much resistance even when the 350 cm long polyethylene tube was used to infuse SCFAs in a freely moving mouse. With the fill-up of distilled water and mineral oil to decrease the liquid resistance in the polyethylene tube (see protocol sections 5 and 6), the flow rate could be lowered to 7 nL/s from 100 nL/s. Infusing the SCFAs or ACSF at a flow rate of 7 nL/s did not result in any abnormal behavior of the mice.
The amount and dosage of gut-derived metabolites for intracerebroventricular injection are critical. It remains challenging to comprehensively analyze the absolute levels of gut-derived metabolites in various brain regions. For example, very few studies have shown the detection of the physiological levels of SCFAs in the brain35,36. One study showed that the concentrations of acetate, propionate, and butyrate in the brains of mice are approximately 1640.6-3281.2 µg/g, 288.18-384.24 µg/g, and 44.036-66.054 µg/g, respectively36. However, another study reported lower levels of SCFAs in the brain (acetate 128.1 µg/g, propionate 0.3883 µg/g, and butyrate 0.1640 µg/g)35. The amounts of infused acetate, propionate, and butyrate adopted in this protocol were 5.81385 µg/g, 4.62378 µg/g, and 2.6124 µg/g, respectively. Therefore, the concentrations of the SCFAs infused in this protocol are comparable to physiological concentrations. However, we could not exclude the possibility that the concentrations of SCFAs may vary in distinct brain regions. Several studies showed that the delivery of propionate into the brain ventricle by intracerebroventricular injection (4 µL of 0.26 M propionic acid in 1 min; approximately 249.756 µg/g) impaired social behavior and cognition in rats30,31. The dosage and flow rate of the infused propionate were relatively high, as reported in mice35 and rats37. Therefore, advances in metabolite analysis and metabolomic profiling in the brain and the periphery will help researchers better understand the spatial and temporal dynamic changes in gut-derived metabolites.
Two types of guide cannulas have been presented in this protocol for intracerebroventricular injection in mice-a commercial guide cannula and a stainless steel guide cannula. The commercial guide cannula and dummy are well-designed for implantation. It is not easy for mice to remove the customized dummy due to the cap. On the contrary, the stainless steel dummy can be easily removed from the stainless steel guide cannula by mice during the 1-week recovery, causing the clotting of blood/cerebrospinal fluid in the cannula. However, the commercial customized cannula is 64 mg, and the dummy for this cannula is 86 mg. Thus, the total weight of the commercial customized cannula and dummy is 150 mg. In contrast, the customized stainless steel guide cannula is 18.3 mg, and the dummy for this cannula is 8.5 mg. Thus, the total weight of the customized stainless steel guide cannula is 26.8 mg. Theoretically, the low weight of the stainless steel guide cannula set would minimize the impact of the cannula on the animal's movement and the brain. Moreover, the cost of the commercial guide cannula is higher than the stainless steel guide cannula and injector. Hence, we recommend the stainless steel guide cannula for inexperienced researchers performing stereotaxic surgery in mice.
The implanted cannula can be clogged by blood and cerebrospinal fluid due to brain damage. This clogging of the cannula could occur more frequently in the stainless steel cannula over the commercial cannula. The dummy can be threaded to tighten the commercial cannula securely (Figure 2A) but not for the stainless steel cannula (Figure 5A). Therefore, ensuring the attachment of the dummy during the recovery period would minimize the clogging of the cannula. A new dummy must be inserted if detachment occurs during the 1-week recovery. Moreover, a disposable sterilized injector must be inserted several times to unclog the cannula before mounting the injector connecting the polyethylene tube.
The choice of anesthetic drugs will highly influence the surgery and behavior testing. Here, the inhalation anesthetic isoflurane was chosen over injected anesthetics because the recovery time is shorter and it is less harmful to the animals for humane reasons. However, the dosage for isoflurane inhalation can be varied depending on the mouse's status and body weight. Therefore, the status of anesthesia must be observed closely during the entire surgical procedure, and the isoflurane vaporizer adjusted accordingly. The optimized breathing rate should be one breath per second during all procedures. Moreover, the gas filter canister filled with activated carbon should be connected to the anesthesia chamber and the nosecone mask to void the isoflurane and exhaust gas pollution in the operating area. This will protect the surgeon from the toxic effect of isoflurane.
The representative results demonstrated that SCFAs infusion did not produce any dramatic effect on locomotion in a novel cage. This could be because a small number of animals was used to obtain this result (Figure 6). Moreover, we only infused the SCFAs for the first 5 min of the novel cage locomotion behavior test but not the entire testing because most of the behavior was tested within a short time window (5-15 min). The time window for the gut-derived microbial metabolites should be adjusted based on the investigators' hypothesis.
The time for anesthesia and regaining consciousness from anesthesia are critical for behavioral tests. Here, the mice were anesthetized briefly for the injector implantation and SCFAs infusion, and behavior testing was performed after 15 min. The time to recover after the cessation of inhalation anesthesia was determined based on a previous study38. A pilot study ensured the mice were active, freely moving, and not uncomfortable 15 min after anesthesia. We introduced the anesthesia step for the mounting of the injector for the following reasons. First, the injector gauge is 33 G; it is very challenging to insert such a delicate injector into the cannula when the animal is actively moving. In addition, the scruffing of conscious mice may produce stress on the mice39, confounding the behavioral outcomes. Second, the cannula is occasionally clogged due to the clotting of the blood/cerebrospinal fluid. Unclogging the cannula gently before mounting the injector would be ideal for metabolite infusion. Based on these two reasons, it is recommended to briefly anesthetize the mice for mounting the injector. Investigators can wait longer (30 min) if there is any concern about the animal's recovery from the inhalation anesthesia. Moreover, a separate set of infusion-injector connecting the polyethylene tube can be set up to accelerate the experiments.
This protocol has several limitations. First, the implantation of the guide cannula and the connecting polyethylene tube would limit the surgical area for implanting optic fibers for optogenetic and fiber photometry into the same coordinate, the surrounding area, or even the same hemisphere of the brain. It will be even more challenging to implant the lens for microendoscopy on the mouse head along with the implanted guide cannula. Second, the implantation of the guide cannula may generate a significant weight on the mouse head. The total weight of a customized cannula set is 26.8-150 mg, a screw is ~48 mg, and the mounted dental acrylic is 450-500 mg. The entire installation set may restrict the movements of the mice. However, recent studies have implanted a miniscope for monitoring calcium signals in freely moving mice40,41. The weight of the miniscope ranges from 1.6 g to 4.5 g, excluding the screws and dental acrylic. Therefore, the weight of the guide cannula may be considered relatively acceptable for mouse behavior testing. Third, the connecting polyethylene tube for the infusion is ~350 cm long, which may limit the movement of mice during the behavior test. To address this concern, pretesting for locomotion in an open-field test may be required to evaluate the impact of the connecting polyethylene tube on mouse motor function. Fourth, dental acryl may produce a neurotoxic effect on the mice. However, it is required to attach the guide cannula to the mouse head during the testing period. To decrease the potential neurotoxic effect of dental acryl on mice, the operators must be familiar with the usage of dental acryl. Dental acryl is better applied when the dental acryl mixture (powder and liquid) is slightly solidified to reduce the penetration of dental acryl liquid into the brain.
Microbiota and their metabolites are associated with behavior function at distinct development milestones. Although this protocol is based on adult C57BL/6 mice with body weights ranging from 26 g to 30 g, it can also be applied to mice of different ages and sizes. Previous studies have adopted stereotaxic surgery in young mice42,43,44,45. However, the coordinates for brain regions may vary depending on the size and age of mice. We recommend referencing the age-corresponding atlas or online resource (http://mouse.brain-map.org/static/atlas). Moreover, the coordinates must be validated by injecting trypan blue or fluorescence dye into the young cull mice, thus optimizing the method before executing it on the experimental mice. The guide cannulas used in this protocol are all customized and can be adjusted after validating the coordinates for different sizes of mice.
This protocol will be compatible with most rodent behavior testing with an open arena on top of the apparatus without much modification. Rodent behavior tests can be conducted with a polyethylene tube wiring on top of the mouse head without any obstacle, including open-field test, elevated plus/zero maze, step down test, direct social interaction, adult ultrasonic vocalization, forced swim test, tail suspension, water maze, T or Y maze, novel object recognition, marble burying, self-grooming test, beam crossing, pole test, and water avoidance stress exposure. Tests using enclosed chambers or tubes will need to be modified to allow the polyethylene tube to freely move along with the mice, such as three-chamber social test, light-dark box, fear conditioning, sucrose preference, restraint stress, startle test, and prepulse-pulse inhibition. We suggest pretesting and estimating the length needed for the polyethylene tube on the cull mice before testing.
Gut microbial metabolites have been shown to impact host behaviors8,46,47,48,49. Scientists can adopt this method to directly investigate the temporal and spatial effects of gut microbiota-derived metabolites on the brain and the behaviors in mice. For example, the microbial metabolite 4-ethylphenyl sulfate (4EPS) was increased in preclinical mouse models of ASD and people with ASD46,48,49. Injection of 4EPS and colonization of the bacteria producing 4EPS increased anxiety-like behavior and impaired oligodendrocyte maturation in the paraventricular nucleus of the thalamus (PVT) in mice46,48. It would be fascinating to evaluate the direct effect of 4EPS on the PVT of mice during anxiety-like behavior testing. However, the absolute concentration of 4EPS in the PVT is still unknown. Therefore, a dose-response test might be critical to determine the adequate levels of 4EPS in the PVT. A similar concept can be adopted for the effects of other microbial metabolites on the brain and behavior.
Circuit-based neurotechnologies, such as optogenetics, chemogenetics, and in vivo calcium imaging, are critical methods allowing scientists to understand the neural circuit in the control of behavior50,51,52. The gut-brain axis is a complicated connection critical for the gut microbes and their metabolites to mediate host behavior. A growing number of studies have employed circuit-based neurotechnologies to understand the intriguing crosstalk between the gut and the brain33,53,54,55,56,57,58,59. This protocol will provide an alternative way to understand brain region-based control of behavior caused by gut metabolites. Combining this protocol with circuit-based neurotechnologies will allow researchers to gain insight into the circuit-based control of behavior and brain activity contributed by gut metabolites.
In conclusion, the concept of the gut-brain axis is well accepted in the scientific community and promotes the possibility of the involvement of gut-derived metabolites in neuropsychiatric disorders11,13,60,61,62,63,64,65. To interrogate how and what gut-derived metabolites impact the brain and behavior of mice, a comprehensive and physiological-based methodology will be needed in the field. This article provides a step-by-step protocol to deliver gut-derived metabolites into the brain directly, most importantly, in a freely moving mouse. This design can be further adapted to investigate the region-specific effects by delivery of the gut-derived metabolites into various brain regions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We acknowledge the Laboratory Animal Center staff at National Cheng Kung University (NCKU) for caring for the animals. This work was supported by the scholarship from Prof. Kun-Yen Huang Education Fund of CHENG-HSING Medical Foundation to C.-W.L.; the funds from the Ministry of Science and Technology (MOST) in Taiwan: (Undergraduate Research MOST 109-2813-C-006-095-B) to T.-H.Y.; (MOST 107-2320-B-006-072-MY3; 109-2314-B-006-046; 110-2314-B-006-114; 110-2320-B-006-018-MY3) to W.-L.W.; and the Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at NCKU to W.-L.W.
Materials
| Material | |||
| Advil Liqui-Gels Solubilized Ibuprofen A2:D41 | Pfizer | n/a | |
| Alexa Fluor 488 donkey anti-rabbit | ThermoFisher Scientific | A-21206 | |
| Anti-Fluorescent Gold (rabbit polyclonal) | Millipore | AB153-I | |
| Bottle Top Vacuum Filter, 500 mL, 0.22 μm, PES, Sterile | NEST | 121921LA01 | |
| CaCl2 | Sigma-Aldrich | C1016 | ACSF: 0.14 g/L |
| Chlorhexidine scrub 2% | Phoenix | NDC 57319-611-09 | |
| Chlorhexidine solution | Phoenix | NDC 57319-599-09 | |
| Commercial dummy | RWD Life Science | 62004 | Single_OD 0.20 mm/ M3.5/G = 0.5 mm |
| Commercial guide cannul | RWD Life Science | 62104 | Single_OD 0.41 mm-27G/ M3.5/C = 2.5 mm |
| Commercial injector | RWD Life Science | 62204 | Single_OD 0.21 mm-33G/ Mates with M3.5/C = 3.5 mm/G = 0.5 mm |
| D-(+)-Glucose | Sigma-Aldrich | G8270 | ACSF: 0.61 g/L |
| Dental acrylic | HYGENIC | n/a | |
| Fixing screws | RWD Life Science | 62521 | |
| Fluoroshield mounting medium with DAPI | Abcam | AB104139 | |
| Horse serum | ThermoFisher Scientific | 16050130 | |
| Insulin syringes | BBraun | XG-LBB-9151133S-1BX | 1 mL |
| Isoflurane | Panion & BF biotech | DG-4900-250D | |
| KCl | Sigma-Aldrich | P3911 | ACSF: 0.19 g/L |
| Ketoprofen | Swiss Pharmaceutical | n/a | |
| Lidocaine | AstraZeneca | n/a | |
| Low melting point agarose | Invitrogen | 16520 | |
| MgCl2 | Sigma-Aldrich | M8266 | ACSF: 0.19 g/L |
| Microscope cover slips | MARIENFELD | 101242 | |
| Microscope slides | ThermoFisher Scientific | 4951PLUS-001E | |
| Mineral oil light, white NF | Macron Fine Chemicals | MA-6358-04 | |
| NaCl | Sigma-Aldrich | S9888 | ACSF: 7.46 g/L |
| NaH2PO4 | Sigma-Aldrich | S8282 | ACSF: 0.18 g/L |
| NaHCO3 | Sigma-Aldrich | S5761 | ACSF: 1.76 g/L |
| n-butyl cyanoacrylate adhesive (tissue adhesive glue) | 3M | 1469SB | 3M Vetbond |
| Neural tracer | Santa Cruz | SC-358883 | FluoroGold |
| Paraformaldehyde | Sigma-Aldrich | P6148 | |
| Polyethylene tube | RWD Life Science | 62329 | OD 1.50, I.D 0.50 mm and OD 1.09, I.D 0.38 mm |
| Puralube Vet (eye) Ointment | Dechra | 12920060 | |
| Sodium acetate | Sigma-Aldrich | S2889 | SCFAs: 13.5 mM |
| Sodium azide | Sigma-Aldrich | S2002 | |
| Sodium butyrate | Sigma-Aldrich | B5887 | SCFAs: 8 mM |
| Sodium propionate | Sigma-Aldrich | P1880 | SCFAs: 5.18 mM |
| Stainless guide cannula | Chun Ta stainless steel enterprise CO., LTD. | n/a | OD 0.63 mm; Local vendor |
| Stainless injector | Chun Ta stainless steel enterprise CO., LTD. | n/a | OD 0.3 mm; dummy is made from injector; local vendor |
| Superglue | Krazy Glue | KG94548R | |
| Triton X-100 | Merck | 1.08603.1000 | |
| Equipment | |||
| Cannula holder | RWD Life Science | B485-68217 | |
| Ceiling camera | FOSCAM | R2 | |
| Digital stereotaxic instruments | Stoelting | 51730D | |
| Dissecting microscope | INNOVIEW | SEM-HT/TW | |
| Glass Bead Sterilizer | RWD Life Science | RS1501 | |
| Heating pad | Stoelting | 53800M | |
| Leica microscope | Leica | DM2500 | |
| Micro Dissecting Forceps | ROBOZ | RS-5136 | Serrated, Slight Curve; Extra Delicate; 0.5mm Tip Width; 4" Length |
| Micro Dissecting Scissors | ROBOZ | RS-5918 | 4.5" Angled Sharp |
| Microinjection controller | World Precision Instruments (WPI) | MICRO2T | SMARTouch Controller |
| Microinjection syringe pump | World Precision Instruments (WPI) | UMP3T-1 | UltraMicroPump3 |
| Microliter syringe | Hamilton | 80014 | 10 µL |
| Optical Fiber Cold Light with double Fiber | Step | LGY-150 | Local vendor |
| Pet trimmer | WAHL | 09962-2018 | |
| Vaporiser for Isoflurane | Step | AS-01 | Local vendor |
| Vibratome | Leica | VT1000S | |
| Software | |||
| Animal behavior video tracking software | Noldus | EthoVision | Version: 15.0.1416 |
| Leica Application Suite X software | Leica | LASX | Version: 3.7.2.22383 |
References
- Lynch, J. B., Hsiao, E. Y. Microbiomes as sources of emergent host phenotypes. Science. 365 (6460), 1405-1409 (2019).
- Dinan, T. G., Cryan, J. F. The microbiome-gut-brain axis in health and disease. Gastroenterology Clinics of North America. 46 (1), 77-89 (2017).
- Sharon, G., Sampson, T. R., Geschwind, D. H., Mazmanian, S. K. The central nervous system and the gut microbiome. Cell. 167 (4), 915-932 (2016).
- Krautkramer, K. A., Fan, J., Backhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nature Reviews: Microbiology. 19 (2), 77-94 (2021).
- Lavelle, A., Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nature Reviews: Gastroenterology & Hepatology. 17 (4), 223-237 (2020).
- Dalile, B., Van Oudenhove, L., Vervliet, B., Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nature Reviews: Gastroenterology & Hepatology. 16 (8), 461-478 (2019).
- Morais, L. H., Schreiber, H. L. T., Mazmanian, S. K. The gut microbiota-brain axis in behaviour and brain disorders. Nature Reviews: Microbiology. 19 (4), 241-255 (2021).
- Sharon, G., et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 177 (6), 1600-1618 (2019).
- St Laurent, R., O’Brien, L. M., Ahmad, S. T. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. 神经科学. 246, 382-390 (2013).
- Govindarajan, N., Agis-Balboa, R. C., Walter, J., Sananbenesi, F., Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. Journal of Alzheimer’s Disease. 26 (1), 187-197 (2011).
- Needham, B. D., et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 602 (7898), 647-653 (2022).
- Silva, Y. P., Bernardi, A., Frozza, R. L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Frontiers in Endocrinology. 11, 25 (2020).
- Kratsman, N., Getselter, D., Elliott, E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology. 102, 136-145 (2016).
- Kelly, J. R., et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research. 82, 109-118 (2016).
- Wang, L., et al. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Digestive Diseases and Sciences. 57 (8), 2096-2102 (2012).
- Adams, J. B., Johansen, L. J., Powell, L. D., Quig, D., Rubin, R. A. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterology. 11, 22 (2011).
- Skonieczna-Zydecka, K., et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients. 10 (12), 1939 (2018).
- Szczesniak, O., Hestad, K. A., Hanssen, J. F., Rudi, K. Isovaleric acid in stool correlates with human depression. Nutritional Neuroscience. 19 (7), 279-283 (2016).
- Martin, A. M., Sun, E. W., Rogers, G. B., Keating, D. J. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Frontiers in Physiology. 10, 428 (2019).
- Franklin, K. B. J., Paxinos, G. . Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates. , (2013).
- York, J. M., Blevins, N. A., McNeil, L. K., Freund, G. G. Mouse short- and long-term locomotor activity analyzed by video tracking software. Journal of Visualized Experiments. (76), e50252 (2013).
- Berg, L., Gerdey, J., Masseck, O. A. Optogenetic manipulation of neuronal activity to modulate behavior in freely moving mice. Journal of Visualized Experiments. (164), e61023 (2020).
- Meyerhoff, J., et al. Microdissection of mouse brain into functionally and anatomically different regions. Journal of Visualized Experiments. (168), e61941 (2021).
- Needham, B. D., Kaddurah-Daouk, R., Mazmanian, S. K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nature Reviews: Neuroscience. 21 (12), 717-731 (2020).
- Geiger, B. M., Frank, L. E., Caldera-Siu, A. D., Pothos, E. N. Survivable stereotaxic surgery in rodents. Journal of Visualized Experiments. (20), e880 (2008).
- Xiaoguang, W., et al. Establishment of a valuable mimic of Alzheimer’s disease in rat animal model by intracerebroventricular injection of composited amyloid beta protein. Journal of Visualized Experiments. (137), e56157 (2018).
- Venniro, M., Shaham, Y. An operant social self-administration and choice model in rats. Nature Protocols. 15 (4), 1542-1559 (2020).
- Ucal, M., et al. Rat model of widespread cerebral cortical demyelination induced by an intracerebral injection of pro-inflammatory cytokines. Journal of Visualized Experiments. (175), e57879 (2021).
- Oberrauch, S., et al. Intraventricular drug delivery and sampling for pharmacokinetics and pharmacodynamics study. Journal of Visualized Experiments. (181), e63540 (2022).
- Shultz, S. R., et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behavioural Brain Research. 200 (1), 33-41 (2009).
- Shultz, S. R., et al. Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (FAST) and seizure-resistant (SLOW) rats. Behavioural Brain Research. 278, 542-548 (2015).
- Perry, R. J., et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 534 (7606), 213-217 (2016).
- Muller, P. A., et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 583 (7816), 441-446 (2020).
- Pardridge, W. M. CSF, blood-brain barrier, and brain drug delivery. Expert Opinion on Drug Delivery. 13 (7), 963-975 (2016).
- Wu, J. -. T., et al. Oral short-chain fatty acids administration regulates innate anxiety in adult microbiome-depleted mice. Neuropharmacology. , (2022).
- Lee, J., et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circulation Research. 127 (4), 453-465 (2020).
- Chiu, C., et al. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS. 9 (1), 3 (2012).
- Schuler, B., Rettich, A., Vogel, J., Gassmann, M., Arras, M. Optimized surgical techniques and postoperative care improve survival rates and permit accurate telemetric recording in exercising mice. BMC Veterinary Research. 5, 28 (2009).
- Hurst, J. L., West, R. S. Taming anxiety in laboratory mice. Nature Methods. 7 (10), 825-826 (2010).
- Shuman, T., et al. Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nature Neuroscience. 23 (2), 229-238 (2020).
- de Groot, A., et al. NINscope, a versatile miniscope for multi-region circuit investigations. Elife. 9, 49987 (2020).
- Kim, J. Y., Grunke, S. D., Levites, Y., Golde, T. E., Jankowsky, J. L. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. Journal of Visualized Experiments. (91), e51863 (2014).
- Wolter, J. M., et al. Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA. Nature. 587 (7833), 281-284 (2020).
- Graybuck, L. T., et al. Enhancer viruses for combinatorial cell-subclass-specific labeling. Neuron. 109 (9), 1449-1464 (2021).
- Xie, M., et al. TREM2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration. Nature Neuroscience. 25 (1), 26-38 (2022).
- Hsiao, E. Y., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 155 (7), 1451-1463 (2013).
- Bermudez-Martin, P., et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. 9 (1), 157 (2021).
- Needham, B. D., et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 602 (7898), 647-653 (2022).
- Stewart Campbell, A., et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial. Nature Medicine. 28 (3), 528-534 (2022).
- Grienberger, C., Konnerth, A. Imaging calcium in neurons. Neuron. 73 (5), 862-885 (2012).
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nature Neuroscience. 18 (9), 1213-1225 (2015).
- Roth, B. L. DREADDs for neuroscientists. Neuron. 89 (4), 683-694 (2016).
- Kaelberer, M. M., et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 361 (6408), (2018).
- Needham, B. D., Tang, W., Wu, W. L. Searching for the gut microbial contributing factors to social behavior in rodent models of autism spectrum disorder. Developmental Neurobiology. 78 (5), 474-499 (2018).
- Schretter, C. E., et al. A gut microbial factor modulates locomotor behaviour in Drosophila. Nature. 563 (7731), 402-406 (2018).
- Chu, C., et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 574 (7779), 543-548 (2019).
- Wu, W. L., et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature. 595 (7867), 409-414 (2021).
- Buchanan, K. L., et al. The preference for sugar over sweetener depends on a gut sensor cell. Nature Neuroscience. 25 (2), 191-200 (2022).
- Han, W., et al. A neural circuit for gut-induced reward. Cell. 175 (3), 665-678 (2018).
- Yamawaki, Y., et al. Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World Journal of Biological Psychiatry. 13 (6), 458-467 (2012).
- Ho, L., et al. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Review of Neurotherapeutics. 18 (1), 83-90 (2018).
- Liu, J., et al. Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer’s disease via upregulating GPR41 and inhibiting ERK/JNK/NF-kappaB. Journal of Agricultural and Food Chemistry. 68 (27), 7152-7161 (2020).
- van de Wouw, M., et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. Jounal of Physiology. 596 (20), 4923-4944 (2018).
- Olson, C. A., et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 173 (7), 1728-1741 (2018).
- Stewart Campbell, A., et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial. Nature Medicine. 28 (3), 528-534 (2022).

