Laser Cell Ablation in Intact Drosophila Larvae Reveals Synaptic Competition
Summary
This protocol demonstrates the laser cell ablation of individual neurons in intact Drosophila larvae. The method enables the study of the effect of reducing competition between neurons in the developing nervous system.
Abstract
The protocol describes single-neuron ablation with a 2-photon laser system in the central nervous system (CNS) of intact Drosophila melanogaster larvae. Using this non-invasive method, the developing nervous system can be manipulated in a cell-specific manner. Disrupting the development of individual neurons in a network can be used to study how the nervous system can compensate for the loss of synaptic input. Individual neurons were specifically ablated in the giant fiber system of Drosophila, with a focus on two neurons: the presynaptic giant fiber (GF) and the postsynaptic tergotrochanteral motor neuron (TTMn). The GF synapses with the ipsilateral TTMn, which is crucial to the escape response. Ablating one of the GFs in the 3rd instar brain, just after the GF starts axonal growth, permanently removes the cell during the development of the CNS. The remaining GF reacts to the absent neighbor and forms an ectopic synaptic terminal to the contralateral TTMn. This atypical, bilaterally symmetric terminal innervates both TTMns, as demonstrated by dye coupling, and drives both motor neurons, as demonstrated by electrophysiological assays. In summary, the ablation of a single interneuron demonstrates synaptic competition between a bilateral pair of neurons that can compensate for the loss of one neuron and restore normal responses to the escape circuit.
Introduction
Laser ablation is a preferred tool for dissecting neural circuits in a wide variety of organisms. Developed in model genetic systems like worms and flies, it has been applied across the animal kingdom to study the structure, function, and development of the nervous system1,2,3. Here, single-neuron ablation was employed to investigate how neurons interact during circuit assembly in Drosophila. The escape system of the fly is a favorite circuit for analysis because it contains the largest neurons and the largest synapses in the adult fly, and the circuit has been well-characterized in the past decades4. The role neuron-neuron interactions play in the assembly of the Giant Fiber circuit is a focal point of this research.
One type of interaction that has been a focal point in neuroscience since the work of Hubel and Wiesel in the 1960s is "synaptic competition"5,6. In this protocol, laser ablation was used to revisit the role of competition through single-cell ablation in the giant fiber system (GFS) of Drosophila, where the molecular underpinnings of the phenomena might be discovered.
Ablation of neurons in the developing fly has been difficult for a variety of reasons, including visualizing the target neurons, the precision of the ablation method, and the survival of the specimen. To overcome these problems in the GFS, the UAS/Gal4 system7 was used to label neurons of interest, and a two-photon microscope was used to remove the presynaptic giant fiber or the postsynaptic jump motor neuron (TTMn).
In this study, to determine the role that neighboring bilateral neurons play in adjusting synaptic connectivity and synaptic strength in the GFS, one of the bilateral pairs of neurons (either presynaptic GF or postsynaptic motor neuron) was deleted just before pupal development. At this developmental stage, GF axonogenesis has not been completed8. The GF structure and function of the synaptic circuit in the adult were then examined, with particular attention given to the output of the remaining GF.
Protocol
All animals used for the protocol were of the species Drosophila melanogaster. There are no ethical issues surrounding the use of this species. Ethical clearance was not necessary to carry out this work. The details of the Drosophila species, reagents, and equipment used in the study are listed in the Table of Materials.
1. Breeding Drosophila and selecting the correct larval stage
- Choose a Gal4 driver line that drives expression in the cells that are to be ablated and recombine it with or cross it to a UAS-GFP reporter line. Raise flies on standard fly food at 25 °C.
NOTE: For giant fiber (GF) ablation, R91H05-Gal4 or A307-Gal4 was recombined with UAS-GFP. ShakB(lethal)-GAL4 recombined with UAS-GFP was used for TTMn ablations (see Table of Materials). - Select larvae that have started to emerge from the food and crawl up the side of the food vial. Those larvae are in the wandering stage, which is the preferred stage for performing GF cell ablation.
NOTE: Ablation can be performed at any larval stage. The last larval stage was important for the manipulation of the development of the GFS because the GF cell bodies can be easily identified in the brain, but the GF axons have not made connections to their targets at this stage.
2. Preparing the 3rd instar larva
NOTE: Larvae were anesthetized using a method similar to Burra et al.9. To keep procedure times short, only prepare one larva at a time. Short exposure to anesthetic will enhance the survival of experimental animals10.
- Prepare a glass dish with a tightly fitting lid. Place a cotton ball in the dish, and under a fume hood, add about 3-5 ml of ethyl ether (dangerous, flammable liquid, lab coat, gloves, goggles). Cover the dish with the lid.
- Collect individual 3rd instar larvae from fly vials. At the 3rd larval stage, larvae leave the food medium and crawl up along the side of the food vial11.
- Ensure that larvae are still moving and haven't started puparium formation. Larvae that are stationary and show a shortening of the body are starting pupation and are unsuitable for ablation. Pick larvae up with a small paintbrush.
NOTE: The 3rd larval stage starts after the second molt at 72 h of development (after egg laying), and lasts until puparium formation at 120 h for flies raised at 25 °C.
- Ensure that larvae are still moving and haven't started puparium formation. Larvae that are stationary and show a shortening of the body are starting pupation and are unsuitable for ablation. Pick larvae up with a small paintbrush.
- Transfer one larva into a small, open container. Place the container in the glass dish with ethyl ether and close the dish tightly. When opening the glass dish, place it under a snorkel or in a fume hood to prevent fumes from escaping.
- In intervals of 30 s, remove the lid with the larva from the dish and check the larva for mobility under a dissection microscope. The larva will generally be immobilized within 1 min. Once the mouth hooks stop twitching, the larva is ready for mounting. Discard any larvae that are not fully immobilized after 3 min.
NOTE: The top of an upside-down lid of a microcentrifuge tube (lid removed from the tube) can be used as a container.
- In intervals of 30 s, remove the lid with the larva from the dish and check the larva for mobility under a dissection microscope. The larva will generally be immobilized within 1 min. Once the mouth hooks stop twitching, the larva is ready for mounting. Discard any larvae that are not fully immobilized after 3 min.
3. Mounting larvae on slides for ablation
- Place the anesthetized larva on a glass microscope slide. Submerge the larva in a drop of insect saline; this will wash away some of the food debris that might stick to the larva.
- Under the dissection microscope, remove most of the saline with a paper tissue. Position the larva dorsal side up for GF ablation or the ventral side up for TTMn ablation. Slowly lower a glass coverslip onto the larva. Add saline to the side of the coverslip to fill in the space between the glass slide and the cover slip.
- For GF ablation, check the positioning of the brain under high magnification on the dissection microscope. Ensure that the brain is lying level and is visible through the cuticle. Often, the brain will be covered with fat tissue, making visualization and cell ablation impossible.
- To displace any fat tissue covering the brain, apply slight pressure to the coverslip with forceps and move the coverslip from side to side. If the fat tissue can't be moved away from the brain this way, use a different larva instead.
4. Locating the target cells
NOTE: The multi-photon system used for this study was mounted on an upright microscope. System-specific software was used to control acquisition and laser stimulation settings. The system was equipped with an epifluorescence light source to locate the samples. The objective lens was a water immersion lens with 25x magnification, a long 2 mm working distance, and an NA of 1.10.
- Place the sample on the multi-photon microscope's stage and use the GFP filter to locate the sample in epifluorescence mode. For GF ablation, the cell bodies can be identified in the brain, as seen in Figure 1A,C,D. For TTMn ablation, a cluster of four cells can be identified from the ventral side in the ventral nerve cord (VNC), as shown in Figure 1A,B. When done focusing, center the cells in the field of view and switch to 2-photon mode.
5. Setting up the ablation parameters
- Adjust laser settings and detector gain to view the GFP-expressing cells with the Galvano scanner. A wavelength of 870 nm works best for visualizing the GFP signal. Center the cell in the field of view.
- Use a circular region of interest (ROI) to define the area for ablation. Ensure that the ROI covers most of the surface of the cell (Figure 1E,G).
- Set up the ablation protocol in the software. Set one frame of acquisition, then stimulation, followed by another acquisition frame.
- Start the stimulation laser power setting at a lower value (10%-20 %) and run the stimulation protocol. A successful ablation can be recognized by a clear circle in the center of the cell soma at the location of the ROI and an increase in fluorescence surrounding the ROI (Figure 1F).
- If the ablation was not successful and merely bleached the cell (Figure 1H), increase the laser power by increments of 5% or the number of loops one at a time and run the protocol again. The applied laser power was between 10% and 40% for the system used in the protocol.
NOTE: Laser power settings for ablation depend greatly on how deep the cells are located in the tissue and other factors such as cuticle folding. If cells are bright and clearly visible, laser power will be on the lower end of the range. Laser power will be different for each system depending on the laser model and the age of the laser.
- If the ablation was not successful and merely bleached the cell (Figure 1H), increase the laser power by increments of 5% or the number of loops one at a time and run the protocol again. The applied laser power was between 10% and 40% for the system used in the protocol.
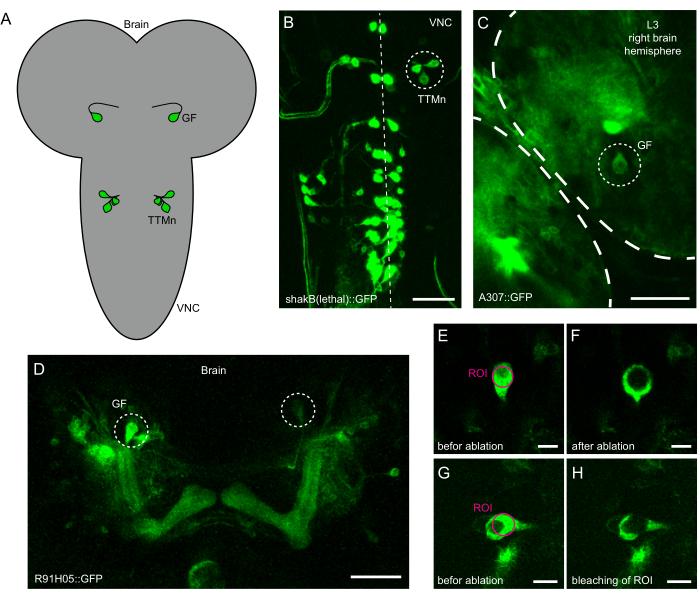
Figure 1: Identification of neurons for laser ablation in intact Drosophila L3 larvae. (A) Schematic of the location of giant fiber (GF) soma in the brain and cluster of motor neurons containing the tergotrochanteral motorneuron (TTMn) in the ventral nerve cord (VNC). (B) Maximum intensity projection of shakB(lethal)-Gal4 driving expression of GFP in the larval VNC. The midline is indicated by the dotted line. The circle indicates the cluster of neurons containing the TTMn that are targeted for laser ablation. Scale bar: 50 µm. (C) Partial projection view of the larval brain expressing GFP under the control of A307-Gal4. The circle indicates the GF soma targeted for laser ablation. Scale bar: 50 µm. (D) Maximum intensity projection of the brain with R91H05-Gal4 driving UAS-GFP. Both GFs (circles) are identifiable by location, shape, and size. Scale bar: 50 µm. (E) GF soma enlarged view before laser ablation. The magenta circle indicates the target region for laser ablation. Scale bar: 10 µm. (F) GF soma enlarged view after delivery of localized laser power and successful ablation. Scale bar: 10 µm. (G) GF soma enlarged view before laser ablation. The magenta circle indicates the target region for laser ablation. Scale bar: 10 µm. (H) GF soma enlarged view after delivery of localized laser power and unsuccessful ablation (bleaching). Scale bar: 10 µm. Please click here to view a larger version of this figure.
6. Recovering the larvae
- After successfully ablating the cell, remove the coverslip and gently pick up the larvae from the slide with a paintbrush. Place the larvae in a food vial. Larvae will start crawling around again within 30 min.
NOTE: Keeping procedure time short improves the survival rates of animals. With experience, the experiment can be performed in just under 10 min. - Continue raising larvae at standard conditions until they eclose from the pupal casing.
7. Testing the functionality of the GFS in adult flies
NOTE: The following steps are explained in detail in Allan and Godenschwege12, and Augustin et al.13.
- Test adult flies at age 2-5 days after eclosion. Anesthetize flies with CO2 and mount them on dental wax by pushing the legs into the wax. Spread the wings at a 90° angle and fix them in place with the wax. Secure the head by pushing the proboscis into the wax.
- Place stimulation wires into both eyes, a ground wire into the abdomen, and glass recording electrodes into the jump muscles (TTMs) on each side.
- Stimulate the GF circuit with single stimuli to measure response latency for both muscles. Stimulate the circuit with 100 Hz and 200 Hz trains of stimuli, respectively, to measure the following frequency for both muscles.
8. Dissection and labeling of the giant fiber system for confocal imaging
NOTE: Fly CNS dissection and dye injection are detailed in Boerner and Godenschwege14.
- Gently remove the fly from the dental wax with forceps and transfer it to a silicone elastomer-lined Petri dish under a dissection scope.
- Remove the legs, wings, and proboscis with scissors and use insect pins to secure the fly dorsal side up in the elastomer coating.
- Make a lateral incision along the midline from the middle abdomen, through the entire thorax, and up the neck, connecting the thorax to the head. Open the fly with insect pins. Remove organs and glands from the fly without damaging the nervous system.
NOTE: At this point, GF axons can be injected with dyes to label the GFs and electrically coupled neurons13. - Remove the head capsule from the head to expose the brain.
9. Immunohistochemistry of the nervous system
- After CNS dissection, fix the fly in 4% PFA in PBS for 45 min at room temperature (RT). Wash for 20 min in PBS at RT (3 times).
- Block the sample for 1 h at RT in 3% BSA in PBS with 0.5% detergent solution.
- Incubate for 2-3 nights at 4 °C in 3% BSA in PBS with 0.3% detergent solution and rabbit anti-GFP (1:500). Wash for 20 min in PBS at RT (6 times).
- Incubate overnight at 4 °C in PBS with anti-rabbit Alexa 488. Wash for 20 min in PBS at RT (6 times).
- Dehydrate samples with an ascending ethanol series (50%, 70%, 90%, 100% ethanol) for 10 min each step. Remove the pins and trim off the abdomen and the flight muscles. Transfer the rest of the thorax containing the nervous system onto a slide and cover it with a mounting medium like methyl salicylate. Place a coverslip on the sample and seal it with nail polish.
- Image the nervous system on a confocal microscope to analyze the anatomy of the GF terminal.
Representative Results
This method can be used to manipulate the development of specific neuronal networks in the nervous system of Drosophila. The primary research question here was the formation of synaptic connections. Removing either the presynaptic GF or the postsynaptic TTMn enabled the investigation of reactive synaptogenesis at this central synapse and the molecular mechanisms crucial for synaptic function and development. As described in the protocol, laser cell ablation of one of the GFs or one of the TTMns was performed, and the effects on the synapse after pupal development in the adult fly were analyzed.
To ensure that the procedure doesn't have a negative effect on animal survival, mock ablations were performed in parallel. For mock ablations, animals were treated the same way as experimental animals, but laser pulses were delivered with a laser power of 0%. In 82.4% of cases, animals from mock ablations developed into adult flies. GFs of mock ablated flies were able to respond with a latency of under 1 ms and follow a 100 Hz stimulation for 79.3% of the stimuli. GF anatomy was indistinguishable from untreated flies.
For each experimental animal, the ablation was verified anatomically. In GF-ablated animals, the unablated GF was dye-injected and imaged (see below). If only one GF axon was visible in the cervical connective (CvC), ablation of the other GF was assumed. Ablation could also be verified through the absence of a GFP-labelled soma on the ablated side of the brain (Figure 2A, circle). To assess TTMn ablation, the GFP signal in the adult VNC was analyzed where the TTMn soma and dendrite can be easily identified. The absence of the TTMn on one side was easily recognized due to missing soma and dendrites (Figure 2B, circle).
To test the function of the GFS in treated flies, synaptic potentials from the jump muscles12,13, which are innervated by the TTMn, were recorded (Figure 3A). The GFs form a mixed electrical-chemical synapse with the TTMn medial dendrite15. The GF terminal is unbranched and shows a typical lateral bend that is opposed to the TTMn dendrite at the synaptic region (Figure 3D, Figure 4A arrowhead). In control flies, each GF innervates its ipsilateral motoneuron exclusively. In recordings from the TTM fibers, the strength of the connection was assessed by analyzing response latency at 1 Hz stimulation and following frequency at 100 Hz stimulation (Figure 3B,C). Control flies showed an average response latency of less than 1 ms and could follow a 100 Hz stimulation for 78.1% of the stimuli (Figure 3B). After physiological recordings were performed, the specimen was dissected to reveal the CNS and GFs, which were dye-injected with a mix of TRITC-Dextran and Neurobiotin. The large Dextran molecule labels the GF axon and terminal but does not pass through gap junctions. Neurobiotin can cross the gap junctions between GF and TTMn, and the Neurobiotin signal was detected in the postsynaptic dendrite (Figure 3E,F, arrowheads). This Neurobiotin dye-coupling confirmed electric coupling between the two neurons. Neurobiotin can also be detected in many other neurons in the VNC that are connected through gap junctions to the GF16 (Figure 3E,F).
The same physiological and anatomical tests were performed after one GF was ablated in L3. The axon of most (13/15) of the remaining GFs in ablation flies exhibit a split of the terminal into two branches: one in the usual ipsilateral location and one crossing the midline to a homologous position contralateral to the intact GF (Figure 3G, arrow). Each branch was dye-coupled to one of the TTMn dendrites (Figure 3H,I, arrowheads). Physiological recordings from the TTMs confirmed this synaptic connection of one GF to both TTMns, although more weakly than normal. TTM response latencies were greater than 1 ms for both sides, and the following frequencies at 100 Hz were 37.1% for the TTMn ipsilateral to the intact GF and 28% for the contralateral TTM (Figure 3C).
Since ablation of one GF caused the remaining GF to innervate both target neurons, the next experiment aimed to observe the response of neighboring GFs when one lacked a normal ipsilateral target motor neuron. For this experiment, one of the postsynaptic neurons was deleted, and the anatomy and function of the remaining TTMn as well as the morphology of the GF terminals, were assessed. When visualizing both TTMns and the GFs in adult control animals, the region of presumed synaptic contact was clearly visible (Figure 4A, arrowheads). Each TTMn medial dendrite was apposed to the ipsilateral GF presynaptic terminal. Small projections of TTMn dendrites from both sides were contacting the midline (Figure 4C; inset). Dye coupling with Neurobiotin was noticeably visible in both TTMn (Figure 4D, arrowheads).
When one TTMn was ablated, the morphology of the remaining TTMn medial dendrite was changed. The medial dendrite in these specimens extended a long, thick branch across the midline (Figure 4I,K; yellow arrows). Additionally, thinner projections towards both GF terminals extended anteriorly (Figure 4E,G; yellow arrowheads). The GF, ipsilateral to the TTMn, showed its usual anatomy with a terminal projecting along the TTMn dendrite.
The response of the "orphaned" GF lacking a target motor neuron was of primary interest. In most cases (61.5% of animals), the orphaned GF exhibited a split terminal with one terminal on the ipsilateral side and the other terminal crossing the midline and projecting towards the contralateral TTMn dendrite (Figure 4J, arrows). These crossed branches of the GF were always smaller than those seen in the complimentary experiment when the neighboring GF was ablated (Figure 3F). The ipsilateral terminal contacted the TTMn branch that extended across the midline (Figure 4I, yellow arrow). In 23% of the specimens, the orphaned GF crossed the midline (Figure 4F, arrow) and projected along one of the TTMn anterior branches (Figure 4E, yellow arrowheads) and was dye-coupled to the remaining TTMn (Figure 4H, arrowhead).
Two more phenotypes were observed in TTMn ablation flies (Figure 4M). In one out of 13 flies, both GFs made the normal bend and stayed on their respective sides of the nervous system. In one other case, the orphaned GF exhibited the bendless phenotype, where the orphaned GF did not make a terminal and stopped before the area where it usually bends. For all four phenotypes, the TTM recordings on the side of the intact TTMn were comparable to wildtype (WT) physiology with response latency below 1 ms and following frequency at 100 Hz at 100% or close to 100%. Removing one target can allow for both GFs to share the remaining target TTMn. In most cases, both GFs will make a connection with the remaining TTMn. Generally, the orphaned GF will exhibit a bilateral terminal. In control flies, GFs will never cross the midline; however, in about 85% of TTMn ablation flies, one GF crossed the midline to contact the contralateral TTMn. The innervation of one TTMn by two GF did not influence synaptic function negatively.
It is noteworthy that the absence of the neighboring GF has a larger effect than the absence of the target motor neuron. The bilateral terminal formed in the absence of the contralateral GF is much larger and more obviously functional than the changes when the target is removed.
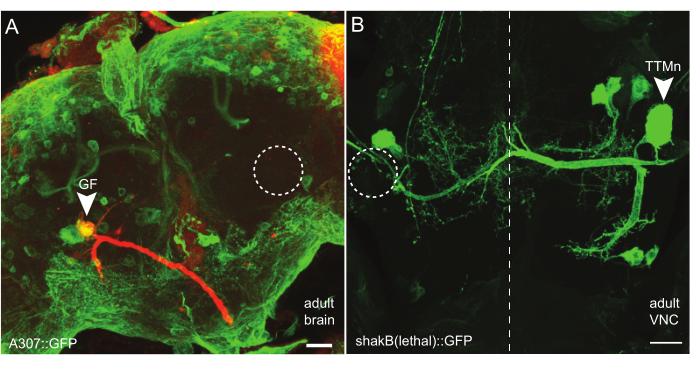
Figure 2: Examples of successful ablations in the adult CNS. (A) Maximum intensity projection of an adult fly brain after larval GF ablation. GFP (green) is driven by A307-GAL4. The circle indicates the area where the ablated GF is missing. The arrowhead points at the remaining GF expressing GFP on the other side of the brain. The GF was filled with TRITC-Dextran (red). The axon is projecting posteriorly towards the VNC. Scale bar: 20 µm. (B) Maximum intensity projection of an adult VNC where one TTMn was ablated in L3. The remaining TTMn (arrowhead) is expressing GFP under the shakB(lethal) driver. The area of the missing TTMn is indicated by the circle. Scale bar: 20 µm. Please click here to view a larger version of this figure.
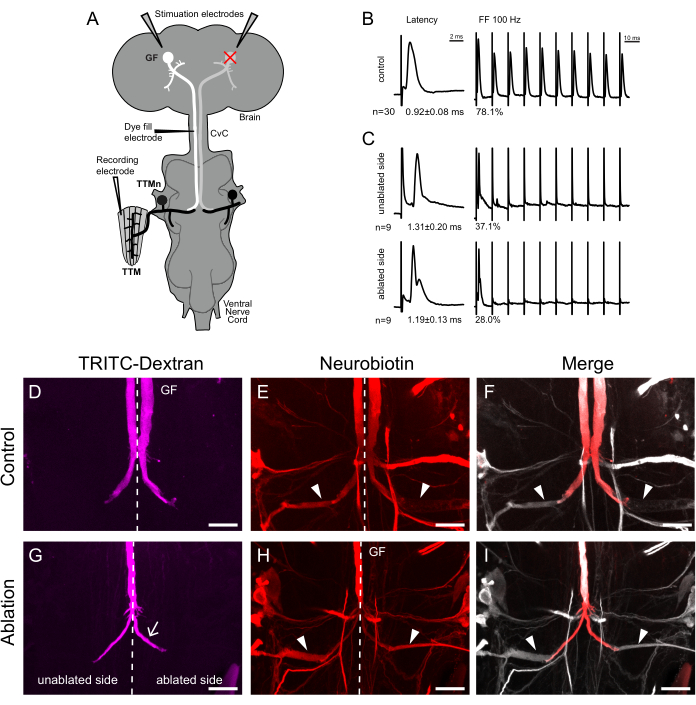
Figure 3: Larval ablation of one giant fiber (GF) causes bilateral branching of presynaptic terminal of the remaining GF. (A) Schematic of the experimental setup for testing adult giant fiber system (GFS) structure and function. The recording electrode measures latency and the following frequency of tergotrochanteral motorneuron (TTMn) outputs at the tergotrochanteral muscles (TTM) upon stimulation of the brain in the intact animal. Following nervous system dissection, the dye was injected into the GF axons at the cervical connective (CvC). (B,C) Sample traces for TTM response latency and following frequency at 100 Hz (n represents the number of TTM recorded from each situation with mean latency and following frequency shown). (B) Control animals with two intact GFs show TTM latencies below 1 ms and following frequencies close to 100% at 100 Hz. (C) The single remaining GF in ablated animals innervates both TTMns. Latencies for both TTMns are longer than in controls, and following at 100 Hz, the frequency is significantly decreased. (D–I) Maximum intensity projection of confocal image stacks for dye-filled GFs. TRITC-Dextran (D,G,F,I in red) and Neurobiotin (E,H,F,I in grey) were co-injected. Neurobiotin can cross gap junctions between the GF and the TTMn, labeling the postsynaptic neurons. The dotted lines indicate the midline. (D) Typical GF anatomy of control animals with the terminal bend. (E,F) Neurobiotin labels many postsynaptic neurons electrically coupled to the GFs. The trans synaptically labeled TTMn dendrites are indicated by arrowheads. (G) After ablation, the remaining GF grows an extra terminal (arrow), which crosses the midline to innervate the contralateral TTMn. (H,I) Both TTMn (arrowheads) are dye-coupled to the same GF. Scale bars: 20 µm. Please click here to view a larger version of this figure.
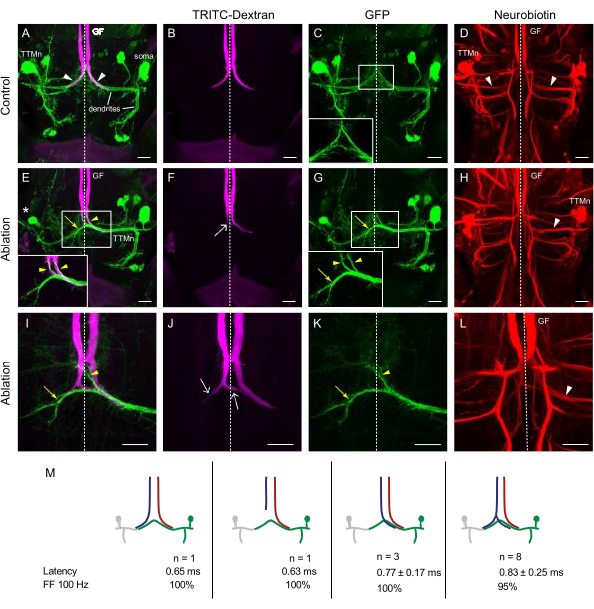
Figure 4. Larval ablation of one tergotrochanteral motor neuron (TTMn) causes innervation of the remaining TTMn by both giant fibers (GF). (A–L) Maximum intensity projections of confocal image stacks of TTMns labeled with GFP (green) under the control of shakB(lethal)-Gal4 and the GFs were dye filled with TRITC-Dextran (magenta) and Neurobiotin (red). The dotted line indicates the midline of the nervous system. (A–D) Control specimens with both TTMns intact. The GFs make their typical terminal bend away from the midline to contact the TTMn (A,B), and each GF contacts one TTMn. TTMn dendrites meet at the midline (C). Each GF is dye coupled to its TTMn (D, white arrowheads). (E–H) The left TTMn was ablated. The medial dendrite of the remaining TTMn projects across the midline (G, yellow arrow). The orphaned GF crosses the midline (white arrow) at the synaptic region and contacts the contralateral TTMn dendrite (E,F). The GFs are dye coupled to the remaining TTMn (H, arrowhead). (I–L) Example of another GF phenotype where the left TTMn was ablated. The medial dendrite of the remaining TTMn projects across the midline (I and K, yellow arrow). The left orphaned GF makes a bilateral terminal; one branch projects contralaterally, and the other stays ipsilateral (J, arrows) to connect to the TTMn. Scale bars: 20 µm. (M) Schematic of the observed four different GF phenotypes in TTMn ablation animals. n indicates the number of animals in each class. Given are the averages with standard error for TTM response latency and following frequency (FF) at 100 Hz for the side of the intact TTMn. Please click here to view a larger version of this figure.
Discussion
Cell ablation with a 2-photon microscope proved to be a highly successful method to manipulate neuronal circuit development in Drosophila. Since this method is non-invasive, it causes minimal damage to the animal. The data support the usefulness of this cell-specific manipulation of known circuits.
Crucial for the success of the ablation was selecting the most appropriate Gal4 driver. Since the GFS is well studied, many specific Gal4 driver lines have been described7. Most Gal4 drivers are very restricted but still expressed in many other neurons. One challenge was to reliably identify the target cells in the intact animal. For animals with a clearly visible GFP label, ablation could be performed at low laser power and often required only one trial. In animals where fat tissue was covering the brain or GFP expression was weak, deletion of target cells required higher laser power and multiple loops of laser exposure. The GF cell bodies were located about 100 µm deep from the dorsal side of the brain. For experiments where target cells are located at a deeper depth, it might be useful to perform ablation at earlier larval stages when the nervous system is smaller.
In previous experiments in the lab, different methods for cell ablation were explored. Using the UAS/Gal4 system to drive the expression of toxins also led to the deletion of specific neurons. However, it is much harder to control the deletion of single cells and avoid off-target effects. The other difficulty is controlling the exact timing of the cell deletion event. Laser cell ablation enabled precise control over the target location and the timing of deletion, but there were still some challenges to overcome before the method was perfected and success rates of over 80% (animal survival and successful cell ablation) were reached. The survival rate of the animals was negatively affected by long exposure to ethyl ether and long procedure times. Studies have shown that high survival rates to pupation (78%) and eclosion to adults (67%) can be achieved for 3.5 min exposure of L3 Drosophila larvae to Ethyl Ether10. Exposure for 3.5 min will ensure the animal is immobile for up to 8 h. For laser cell ablation, animals need to be immobile for 30 min or less. Therefore, exposure time to ethyl ether can be kept well below 3.5 min. In most cases, larvae were fully immobile within 1 min.
The laser ablation results demonstrate that the GF can be cleanly ablated, and the effect on the contralateral neighboring GF is easily assessed. The ablation result was clear and consistent. In 13 of 15 single-cell ablations, the remaining GF partners exhibit a bilateral terminal that is never seen in control animals. This bilaterally symmetric terminal is easily recognized, and the unusual contralateral branch is similar in size to a normal terminal and is dye-coupled to the contralateral target motor neuron. The bilateral terminal is dye-coupled to both target motor neurons and functions nearly wildtype in most specimens, driving both the normal-ipsilateral target as well as the contralateral target motor neuron with latencies and following frequencies near the control values.
The complementary experiment, a unilateral ablation of a target motor neuron, provides an interesting comparison. The remaining motor neuron often exhibits a reactive dendritic growth, extending a process across the midline into space normally occupied by the deleted neighbor. Although this dendrite is not as large as the deleted one, it demonstrates a reaction similar to the GFs missing their bilateral mate. In contrast, the now target-less GF responds only modestly to the absent target, extending minor processes across the midline and along the remaining motor neuron dendrites. This modest response contrasts sharply with the GF's dramatic response to a missing bilaterally homologous GF, where a large contralateral terminal is formed to complement the normal terminal and form a bilateral terminal.
A parallel ablation experiment has recently been done on the presynaptic inputs to the GF in the brain, where a similar type of "competition" for synaptic space was revealed17. Visual inputs to the GF tile the glomerulus where the visual projection neurons (VPN) reach the GF dendrites. Deleting one group of the VPNs (in this case genetically) demonstrates that the remaining VPNs take over and innervate some of the absent neighbor's synaptic space and produce a compensatory physiological recovery of the visual response to looming stimuli. When the first arriving afferents are deleted, the later arrivals occupy space and are no longer occupied. The major phenomenological similarity to the present work is the apparent role physical occupancy plays in determining the balance between the various inputs. The output terminals of the two GFs appear to compete with each other for physical synaptic space.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Experiments on the 2-photon microscope were performed in the FAU Stiles-Nicholson Brain Institute Advanced Cell Imaging Core. We would like to thank the Jupiter Life Science Initiative for financial support.
Materials
| Alexa Fluor 488 AffiniPure Goat Anti-Rabbit IgG (H+L) | Jaxkson ImmunoResearch | 111-545-003 | |
| Anti-green fluorescent protein, rabbit | Fisher Scientific | A11122 | 1:500 concentration |
| Apo LWD 25x/1.10W Objective | Nikon | MRD77220 | water immersion long working distance |
| Bovine Serum Albumin (BSA) | Sigma | B4287-25G | |
| Chameleon Ti:Sapphire Vision II Laser | Coherent | ||
| Cotton Ball | Genesee Scientific | 51-101 | |
| Dextra, Tetramethylrhodamine, 10,000 MW, Lysine Fixable (fluoro-Ruby) | Fisher Scientific | D1817 | |
| Drosophila saline | recipe from Gu and O'Dowd, 2006 | ||
| Ethyl Ether | Fisher Scientific | E134-1 | Danger, Flammable liquid |
| Fly food B (Bloomington recipe) | LabExpress | 7001-NV | |
| Methyl salicylate | Fisher Scientific | O3695-500 | |
| Microcentrifuge tube 1.5 mL | Eppendorf | 22363204 | |
| Microscope cover-slip 18×18 #1.5 | Fisher Scientific | 12-541A | |
| Neurobiotin Tracer | Vector Laboratories | SP-1120 | |
| Nikon A1R multi-photon microscope | Nikon | on an upright FN1 microsope stand | |
| NIS Elements Advanced Research | Nikon | Acquisition and data analysis software | |
| Paraformaldehyde (PFA) | Fisher Scientific | T353-500 | |
| PBS (Phosphate Buffered Salin) | Fisher BioReagents | BP2944-100 | Tablets |
| R91H05-Gal4 | Bloomington Drosophila Stock Center | 40594 | |
| shakB(lethal)-GAl4 | Bloomington Drosophila Stock Center | 51633 | |
| Superfrost microscope glass slide | Fisher Scientific | 12-550-143 | |
| Triton X-100 | Fisher Scientific | 422355000 | detergent solution |
| UAS-10xGFP | Bloomington Drosophila Stock Center | 32185 |
References
- Chung, S. H., Mazur, E. Femtosecond laser ablation of neurons in C. elegans for behavioral studies. Appl Phys A Mater Sci Process. 96 (2), 335-341 (2009).
- Bower, D. V., et al. Airway branching has conserved needs for local parasympathetic innervation but not neurotransmission. BMC Biol. 12, 92 (2014).
- Angelo, J. R., Tremblay, K. D. Laser-mediated cell ablation during post-implantation mouse development. Dev Dyn. 242 (10), 1202-1209 (2013).
- Allen, M. J., Godenschwege, T. A., Tanouye, M. A., Phelan, P. Making an escape: Development and function of the Drosophila giant fibre system. Semin Cell Dev Biol. 17 (1), 31-41 (2006).
- Hubel, D. H., Wiesel, T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 28 (6), 1041-1059 (1965).
- Wiesel, T. N., Hubel, D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 28 (6), 1029-1040 (1965).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118 (2), 401-415 (1993).
- Allen, M. J., Drummond, J. A., Moffat, K. G. Development of the giant fiber neuron of Drosophila melanogaster. J Comp Neurol. 397 (4), 519-531 (1998).
- Burra, S., Wang, Y., Brock, A. R., Galko, M. J. Using Drosophila larvae to study epidermal wound closure and inflammation. Methods Mol Biol. 1037, 449-461 (2013).
- Kakanj, P., Eming, S. A., Partridge, L., Leptin, M. Long-term in vivo imaging of Drosophila larvae. Nat Protoc. 15 (3), 1158-1187 (2020).
- Bainbridge, S. P., Bownes, M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 66, 57-80 (1981).
- Allen, M. J., Godenschwege, T. A. Electrophysiological recordings from the Drosophila giant fiber system (GFs). Cold Spring Harb Protoc. 2010 (7), (2010).
- Augustin, H., Allen, M. J., Partridge, L. Electrophysiological recordings from the giant fiber pathway of d. Melanogaster. J Vis Exp. 47, e2412 (2011).
- Boerner, J., Godenschwege, T. A. Whole mount preparation of the adult Drosophila ventral nerve cord for giant fiber dye injection. J Vis Exp. 52, e3080 (2011).
- Blagburn, J. M., Alexopoulos, H., Davies, J. A., Bacon, J. P. Null mutation in shaking-b eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: A structural study. J Comp Neurol. 404 (4), 449-458 (1999).
- Kennedy, T., Broadie, K. Newly identified electrically coupled neurons support development of the Drosophila giant fiber model circuit. eNeuro. 5 (6), (2018).
- Mcfarland, B. W., et al. Axon arrival times and physical occupancy establish visual projection neuron integration on developing dendrites in the Drosophila optic glomeruli. bioRxiv. , (2024).

.
