16.9:
Titration of a Polyprotic Acid
A polyprotic acid contains multiple ionizable protons, and each dissociates in a distinct step. The loss of each proton has a different Ka, and each subsequent acid dissociation constant is weaker than the previous one.
For example, sulfurous acid has two ionizable protons. Ka1 for the dissociation of the first proton is 1.6 × 10−2, whereas Ka2 for the dissociation of the second proton is 6.4 × 10−8.
If sulfurous acid is titrated with a strong base, such as sodium hydroxide, the first ionizable proton is removed initially, generating hydrogen sulfite ions. This part of the titration curve is similar to that of a weak monoprotic acid and a strong base. It has an equivalence point, and the pH of the solution at the half-equivalence point is equal to pKa1.
As more base is added, it neutralizes the second ionizable proton. As the concentration of hydrogen sulfite ion is equal to the initial sulfurous acid, the same amount of base is needed to neutralize it. Therefore, two moles of a base is necessary to neutralize one mole of a diprotic acid completely.
The titration curve for the second neutralization step also has a second half-equivalence point, where the pH of the solution equals pKa2, and a second equivalence point, which lies in the basic region.
Similarly, the curve for the titration of triprotic phosphoric acid with a strong base has three equivalence points.
Therefore, during the titration of a weak polyprotic acid, the number of equivalence points generated in the titration curve is equal to the number of ionizable protons present as long as the difference between the Ka values of the ionizable protons is more than ten thousand fold.
16.9:
Titration of a Polyprotic Acid
A polyprotic acid contains more than one ionizable hydrogen and undergoes a stepwise ionization process. If the acid dissociation constants of the ionizable protons differ sufficiently from each other, then the titration curve for such polyprotic acid generates a distinct equivalence point for each of its ionizable hydrogens. Therefore, titration of a diprotic acid results in the formation of two equivalence points, whereas the titration of a triprotic acid results in the formation of three equivalence points on the titration curve.
Carbonic acid, H2CO3, is an example of a weak diprotic acid. The first ionization of carbonic acid yields hydronium ions and bicarbonate ions in small amounts.
First ionization:
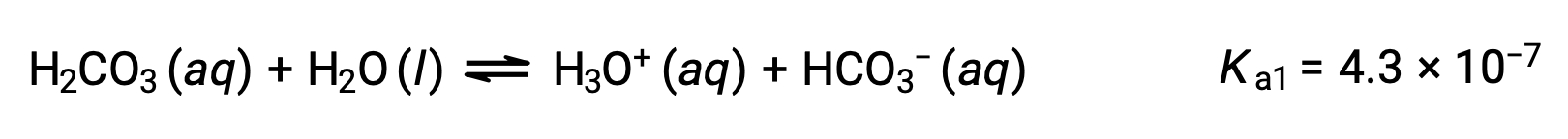
The bicarbonate ion can also act as an acid. It ionizes and forms hydronium ions and carbonate ions in even smaller quantities.
Second ionization:
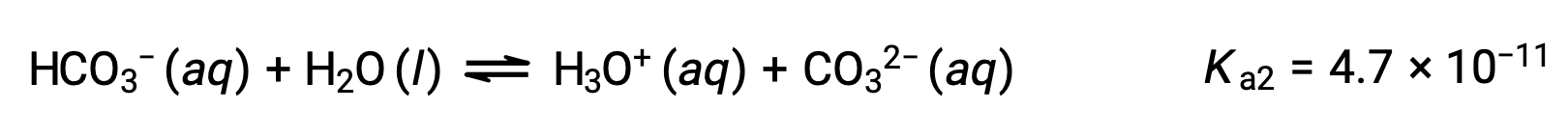
The Ka1 is larger than the Ka2 by a factor of 104. Therefore, when H2CO3 is titrated with a strong base like NaOH, it produces two distinct equivalence points for each ionizable hydrogen.
Phosphoric acid, a triprotic acid, ionizes in three steps:
First ionization:
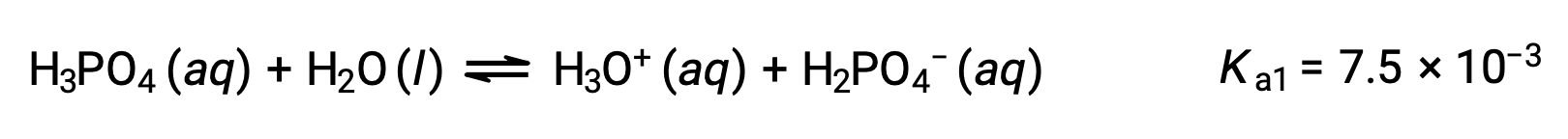
Second ionization:
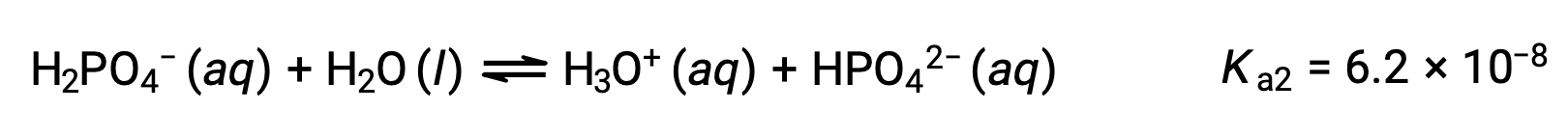
Third ionization:
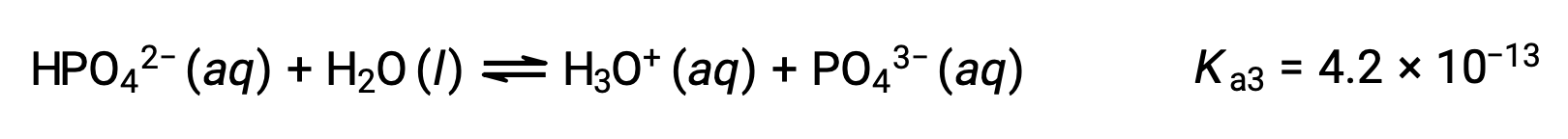
When H3PO4 is titrated with a strong base like KOH, it produces three equivalence points for each ionizable hydrogen. However, as HPO42− is a very weak acid, the third equivalence point is not easily discernible on the titration curve.
This text is adapted from Openstax, Chemistry 2e, Section 14.5: Polyprotic Acids.
