Headspace Gas Chromatography: A Technique to Assess Ethanol Concentration in Zebrafish Embryos
Abstract
Source: Lovely, C. B. et al. Quantification of Ethanol Levels in Zebrafish Embryos Using Head Space Gas Chromatography. J. Vis. Exp. (2020).
This video demonstrates the use of headspace gas chromatography to determine the ethanol concentrations in zebrafish embryos. This method provides a useful tool for qualitative and quantitative analysis of volatile organic compounds.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Measuring the embryonic volume using water displacement
NOTE: In this protocol, 24 h postfertilization (hpf) embryos (Figure 1) are used. The embryos used in the volume measurements are not used in the ethanol analysis.
- Place 10 embryos and extraembryonic fluid in 1.5 mL microcentrifuge tube marked at a volume of 250 μL (Figure 2A). Add water to the 250 μL fill line (see sample with dyed water in Figure 2B).
- Repeat step 1.1 to set up receptacles for embryos that have had their chorions removed.
- To remove the chorion, place the embryos in their chorions in a 100 mm Petri dish with 2 mg/mL of protease cocktail in embryo media at room temperature (RT) for 10 min. Every few minutes, gently swirl the embryos to break the chorion.
- Once all the embryos are free of their chorion, remove the dechorionated embryos from protease cocktail/embryo media and place in a new 100 mm Petri dish with fresh embryo media to wash the embryos. Repeat this wash step 1 more time (for a total of 2 washes). Transfer the embryos to a fresh 100 mm Petri dish.
- Using the smallest tip possible and without damaging the embryos, carefully remove all liquid from around the embryos using a p200 micropipettor (Figure 2C) and weigh the water with a scale with <0.1 mg precision. To determine the volume of the sample of 10 embryos, subtract the weight/volume of the water (1 mL of water = 1 g of water.) removed from 250 μL. To determine the volume of a single embryo, divide the difference between 250 μL and the weight of the water removed by 10.
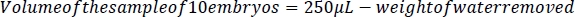
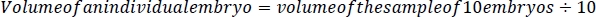
2. Treating embryos with ethanol
- Gather embryos from mating tanks and place in a standard 100 mm Petri dish. Count out no more than 100 embryos per single Petri dish and incubate at 28.5 °C.
NOTE: If the chorion is to be removed (step 1.2), it needs to be removed before adding ethanol. - At 6 hpf, add up to 100 embryos to a new standard 100 mm Petri dish either with embryo media or embryo media + 1% ethanol (v/v). Cover, but DO NOT seal the Petri dish. Place the embryos in a low temp incubator set at 28.5 °C for 18 h, or until the embryos reach the developmental time point of 24 hpf.
3. Preparing workflow before processing the embryos for head space gas chromatography
- Make a solution of 5 M NaCl in water (450 μL will be needed per sample tube) to denature all proteins and prevent ethanol metabolism. Make the protease cocktail at a concentration of 2 mg/mL in embryo media.
- Label a 1.5 mL microcentrifuge tube and a 2 mL gas chromatograph vial for each sample. Label additional 2 mL gas chromatograph vials for the ethanol standards described below as well as air, water, and the 5 M NaCl/protease cocktail blanks.
- Set up three p200 micropipettors two set to 50 μL, and the third set to 200 μL; a p1000 micropipettor set to 450 μL and a p2 micropipettor set to 2 μL. For the glass pipettes used to transfer embryos from the Petri dishes to the 1.5 mL microcentrifuge tubes, quickly pass the pipette tip through a flame to smooth the edges to not damage the embryos when drawing them into the pipette.
4. Processing embryos for head space gas chromatography
NOTE: Both embryos in their chorions and those previously removed from their chorions are treated the same for consistency in the calculation of dilution factors.
- Using the two p200 micropipettors set to 50 μL, draw 50 μL of the protease cocktail solution into one and draw 50 μL of water into the second.
- Using the glass pipette and micropipettor, quickly place 10 embryos (from step 2.2) in a 1.5 mL microcentrifuge tube and close the cap (as described in Figure 2A). Repeat for all samples to be tested, controls, and ethanol treated embryos.
- Using the p200 micropipettor set to 200 μL, quickly open the cap and remove all residual embryo media (as described in Figure 2C). Quickly place the pipette containing 50 μL of water in the tube and rapidly but gently (to not damage the embryos) add, then remove the water. Quickly add 50 μL of the protease cocktail solution from the waiting pipettor and close the tube cap (Figure 2D).
NOTE: Perform this process one sample at a time. - Let the sample sit at RT for 10 min to allow the protease cocktail to degrade the chorion. Then, quickly add 450 μL of 5 M NaCl and close the tube cap (Figure 2E). Vortex the samples for 10 min. To speed up the process, set up the mixer to vortex multiple samples at the same time.
NOTE: Add a small amount (~100 μL) of a silica bead mixture (2 sizes, 0.5 mm and 1 mm bead) to any tube with embryos older than 24 hpf. In older embryos, the notochord will remain intact regardless of how long they are homogenized. - After homogenizing for 10 min, quickly remove 2 μL of homogenized embryo supernatant and add to a gas chromatograph vial. Quickly seal the vial with the polytetrafluoroethylene cap.
5. Preparing media and ethanol standards
- To prepare the media standards, dilute the media by a factor of 10 with a 5 M NaCl/protease cocktail solution. Add 2 μL of each sample to a gas chromatograph vial and seal with a polytetrafluoroethylene cap.
- To prepare the ethanol standards, create a serial dilution of 100% ethanol in 5 M NaCl/protease cocktail solution to the following concentrations: 0.3125, 0.625, 1.25, 2.5, 5, 10, 20, and 40 mM. Add 2 μL of each standard to a gas chromatograph vial and seal with a polytetrafluoroethylene cap.
6. Preparing the head space gas chromatography
NOTE: Head space gas chromatography is used to quantify ethanol levels, not for separation.
- Set the heater for the autosampler to 58 °C and turn on. Allow the heater to reach 58 °C, and turn on the air and hydrogen gas lines feeding the gas chromatograph (for flame ionization used to quantify the ethanol).
NOTE: The psi should be set to properly operate the chromatograph according to the manufacturer's specifications. Make sure the helium line is on and set to the proper psi. - For the septum, make sure the number of samples injected is not >100. For the injection fiber, make sure the number of samples injected is not >500.
NOTE: If either is over the amount of injections, they will need to be changed before running the test samples. - Turn on the analysis software and make sure the workstation is set up properly. Store all data according to lab/department standards. Create a new sample list for the samples to be run.
- Initiate the startup method and wait for the flame ionization detector to stabilize before running the samples. Once stable, run the software startup method to clean the fiber.
7. Sample measurements using head space gas chromatography
- Once the startup method is complete, create 1 new line per sample in the sample list. From the methods menu in the analysis software, load 436 Current spme ethanol 2013 3min absorb 2_5min rg run.METH for each sample on the sample list.
- Fill out the sample list, starting with the air, water, 5 M NaCl/protease cocktail blanks. Then enter in the standards in order, from 0.3125 to 40 mM. Follow the standards with a second round of the air, water, and 5 M NaCl/protease cocktail blanks.
- Enter all homogenized embryo supernatant samples to be tested, from lowest to highest predicted ethanol concentration. End by entering a third and final round of the air, water, and 5 M NaCl/protease cocktail blanks.
- Add the gas chromatograph vials to the autosampler in the order in which the samples were entered. Allow samples to warm for 10–15 min. Start the sample runs in the software.
- After all the samples and the final blanks have run, activate the shutdown method in the software by adding a final sample in the sample list and running standby.METH. Back up all data acquired during the sample runs. Turn off the equipment in the following order: autosampler heater, hydrogen tank and, only after the chromatograph temperature reaches 30 °C, the air tank. Leave the helium tank on to preserve the wax column.
8. Sample ethanol peak integration and sample concentration analysis
NOTE: All values from 8.3 on were calculated in an excel file that all equations prefilled.
- Once the shutdown method is complete, click on Open chromatogram. Open the folder containing the results. Samples are automatically integrated in this program.
- In the results, make sure the correct peaks have been integrated (ethanol peaks between 2 and 2.5). Once all samples have been confirmed, print or export the results.
- Plot the peak height of the ethanol standards on a graph. Calculate the slope, Y intercept and R2 values for the ethanol standards (R2 should be >0.99).
NOTE: These values will be used to determine the ethanol concentration from the sample peak height. - For each sample, subtract the peak height of the sample from the Y intercept of the ethanol standards. Divide this value by the slope of the ethanol standards to obtain the ethanol value for each sample in the GC vials.
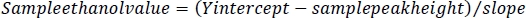
- To calculate the ethanol concentration in the embryos, first calculate the dilution factor for each sample. Take the volume measure calculated in step 1.3 of this protocol and divide it by the volume measure plus 500 μL. This represents the 5 M NaCl/protease cocktail solution added to each sample during embryo processing (steps 4.3 and 4.4).
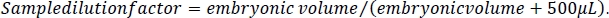
- Using this sample dilution factor, multiply by the sample ethanol value for each sample. The results will be in mM concentration. Calculate media reference samples by multiplying the media ethanol value by the dilution factor of 10.
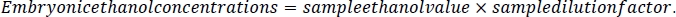
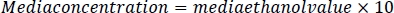
Representative Results
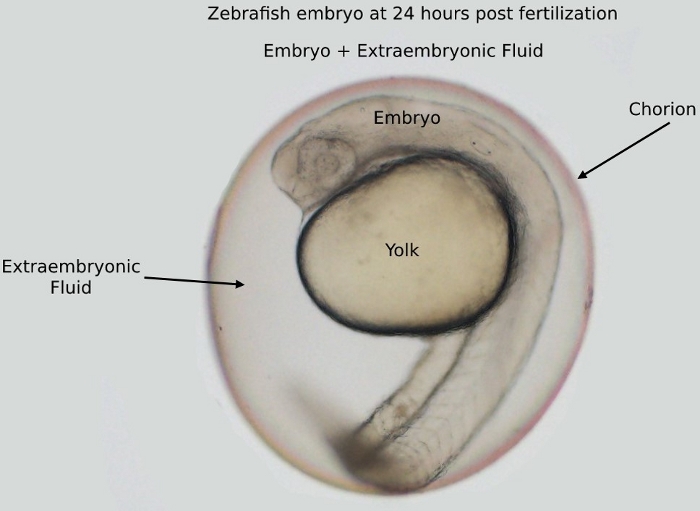
Figure 1: An image of an embryo at 24 h postfertilization (hpf) inside the chorion. The embryo and the yolk are surrounded by the extraembryonic fluid, all located inside the chorion.
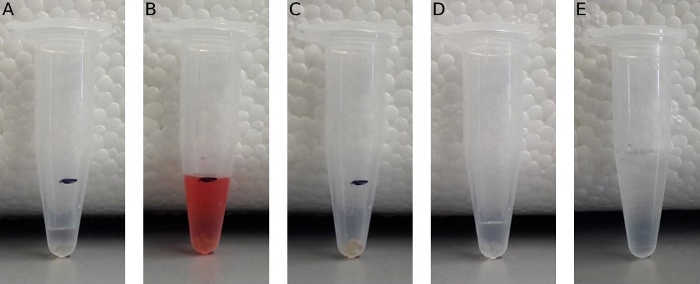
Figure 2: Protocol to measure embryonic volume and process the embryos for analysis. (A) Ten 24 hpf embryos transferred to a 1.5 mL microcentrifuge tube marked at a 250 μL volume. (B) The microcentrifuge tube with embryos filled with water (water is dyed so it is easier to see at the 250 μL mark). (C) All of the water is removed from the tube and weighed. (D) Ten embryos transferred to a 1.5 mL microcentrifuge tube. The water was removed as in (C) and replaced with 50 μL of protease cocktail. (E) After 10 min, 450 μL of 5 M NaCl was added to the tube from (D).
Disclosures
The authors have nothing to disclose.
Materials
| AutoSampler, CP-8400 | Varian | Gas Chromatograph Autosampler | |
| Ethanol | Decon Labs | 2701 | |
| Gas chromatograph vial with polytetrafluoroethylene/silicone septum and plastic cap 2 mL | Agilent | 8010-0198 | Can reuse the vials after cleaning, but not the caps/septa |
| Gas Chromatograph, CP-3800 | Varian | ||
| Helium | Provided by contract to the university | ||
| HP Innowax capillary column | Agilent | 19095N-123I | 30 m x 0.53 mm x 1.0 μm film thick |
| Hyrdogen | Provided by contract to the university | ||
| Microcentrifuge tube 1.5 mL | Fisher Scientific | 2682002 | |
| Micropipette tips 10 μL | Fisher Scientific | 13611106 | |
| Micropipette tips 1000 μL | Fisher Scientific | 13611127 | |
| Micropipette tips 200 μL | Fisher Scientific | 13611112 | |
| Pipetman L p1000L Micropipette | Gilson | FA10006M | |
| Pipetman L p200L Micropipette | Gilson | FA10005M | |
| Pipetman L p2L Micropipette | Gilson | FA10001M | |
| Polytetrafluoroethylene/silicone septum and plastic cap | Agilent | 5190-7021 | Replacement caps/septa for gas chromatograph vials |
| Solid-phase microextraction fiber assembly Carboxen/Polydimethylsiloxane | Millipore Sigma | 57343-U | Replacement fibers |
| Star Chromatography Workstation | Varian | Chromatography software | |
| Thermogreen Low Bleed (LB-2) Septa | Millipore Sigma | 23154 | Replacement inlet septa |

