Systemic Injection of Neural Stem/Progenitor Cells in Mice with Chronic EAE
Summary
The transplantation of neural stem/progenitor cells (NPCs) holds great promises in regenerative neurology. The systemic delivery of NPCs has turned into effective, low invasive, and therapeutically very efficacious protocol to deliver stem cells in the brain and spinal cord of rodents and nonhuman primates affected by experimental chronic inflammatory damage of the central nervous system.
Abstract
Neural stem/precursor cells (NPCs) are a promising stem cell source for transplantation approaches aiming at brain repair or restoration in regenerative neurology. This directive has arisen from the extensive evidence that brain repair is achieved after focal or systemic NPC transplantation in several preclinical models of neurological diseases.
These experimental data have identified the cell delivery route as one of the main hurdles of restorative stem cell therapies for brain diseases that requires urgent assessment. Intraparenchymal stem cell grafting represents a logical approach to those pathologies characterized by isolated and accessible brain lesions such as spinal cord injuries and Parkinson’s disease. Unfortunately, this principle is poorly applicable to conditions characterized by a multifocal, inflammatory and disseminated (both in time and space) nature, including multiple sclerosis (MS). As such, brain targeting by systemic NPC delivery has become a low invasive and therapeutically efficacious protocol to deliver cells to the brain and spinal cord of rodents and nonhuman primates affected by experimental chronic inflammatory damage of the central nervous system (CNS).
This alternative method of cell delivery relies on the NPC pathotropism, specifically their innate capacity to (i) sense the environment via functional cell adhesion molecules and inflammatory cytokine and chemokine receptors; (ii) cross the leaking anatomical barriers after intravenous (i.v.) or intracerebroventricular (i.c.v.) injection; (iii) accumulate at the level of multiple perivascular site(s) of inflammatory brain and spinal cord damage; and (i.v.) exert remarkable tissue trophic and immune regulatory effects onto different host target cells in vivo.
Here we describe the methods that we have developed for the i.v. and i.c.v. delivery of syngeneic NPCs in mice with experimental autoimmune encephalomyelitis (EAE), as model of chronic CNS inflammatory demyelination, and envisage the systemic stem cell delivery as a valuable technique for the selective targeting of the inflamed brain in regenerative neurology.
Introduction
Strong evidence has arisen from in vivo studies attesting to the therapeutic efficacy of the transplantation of somatic neural stem/precursor cells (NPCs) in animal models of CNS disorders1-8. Nevertheless, a number of issues relating to the delivery of stem cells into the host require careful consideration before these experimental results can be translated into clinical applications. A particularly substantial hurdle towards the development of (nonhematopoietic) restorative stem cell therapies for multifocal, chronic inflammatory brain diseases is the identification of the ideal route of NPC injection. A firm understanding of the pathophysiology of the targeted disease (focal or multifocal; primary inflammatory or primary degenerative), and a cautious analysis of feasibility and risk issues associated with the delivery techniques are in identifying the optimal protocol for stem cell delivery.
While the focal (e.g. into the nervous system parenchyma) stem cell transplantation is a logical approach to the treatment of CNS diseases characterized by spatially confined areas of damage (e.g. Parkinson’s and Huntington’s disease, brain and spinal cord traumatic injuries, and stroke), the very same approach may prove to be practically not feasible in conditions such as MS, where a multifocal, chronic, and spatially disseminated CNS damage accumulates over time. In this latter case, targeting focal cell injections to individual lesions is also hindered by the limited capacity of transplanted NPCs to migrate over long distances within the CNS parenchyma, thus prompting the identification of alternative, more suitable methods of CNS targeting with less invasive NPC transplants.
Great promise emerged from the observations that NPCs target an intracranial tumor (e.g. glioma) in mice when injected intravascularly outside the CNS9. Following this seminal in vivo evidence of the stem cell pathotrophism10, extensive data have been accumulated pertaining to the feasibility and therapeutic efficacy of the systemic transplantation of NPCs in laboratory animals with experimental autoimmune encephalomyelitis (EAE), as a model of inflammatory CNS damage, via either intravenous (i.v.) or intracerebroventricular (i.c.v.) NPC injection1,2,5,6,8.. We have first shown that this is dependent on the capability of transplanted NPCs to target and enter the inflamed CNS, and to subsequently engage multiple intercellular communications programs within specific microenvironments in vivo11. In order to specifically target the CNS, NPCs are delivered directly into the cerebrospinal fluid (CSF) circulation by i.c.v. injection, or into the bloodstream via i.v. injection. Once entering either the bloodstream or CSF, transplanted NPCs actively interact with the blood brain (BBB) or blood cerebrospinal fluid (BCSFB) barriers and enter the CNS parenchyma. This interaction between the NPC graft and the BBB (or BCSFB) is regulated by specific set of NPC surface cell adhesion molecules (CAMs) and facilitated by the expression of high levels of CAM counter-ligands on activated endothelial/ependymal cells12-14. Examples of these CAMs include the receptor for hyaluronate, CD44, and the intercellular adhesion molecule (ICAM)-1 ligand very late antigen (VLA)-45,15,16 (that, in leukocytes, are responsible of the interaction with activated ependymal and endothelial cells), and to a much lower extent Lymphocyte function-associated antigen (LFA)-1 and P-selectin glycoprotein ligand (PSGL)-1. NPCs also express a wide range of chemokine receptors, including CCR1, CCR2, CCR5, CXCR3, and CXCR4 (but do not express CCR3 and CCR7), which are functionally active, both in vitro and in vivo5,16. Thus, systemically injected NPCs use these CAMs, along with G-protein coupled receptor (GPCRs), to accumulate at the level of the inflamed CNS. Conversely, NPCs injected systemically into healthy mice do not enter the CNS via vascular or cerebrospinal fluid space routes2. CNS inflammation, or endothelial/ependymal cell activation following systemic cytokine or lypopolisaccharide (LPS) injection as a model of chemically induced encephalitis, is therefore necessary for the accumulation of systemically injected NPCs into the brain and spinal cord2. Thus, successful targeting of the CNS with systemic NPC therapies is dependent on the identification of a disease specific window of Opportunity (WoO) in which the brain and spinal cord environment are conducive to the accumulation and transendothelial migration of NPCs. Such conditions generally arise in the context of acute and subacute inflammation17. Once having entered the CNS, transplanted undifferentiated NPCs have been shown to ameliorate the clinico-pathological features of mice as well as larger, nonhuman primates with EAE. This has been described to be dependent from minimal cell replacement2 and remarkable secretion of immune regulatory and neuroprotective paracrine factors within perivascular CNS2,5,6,18 vs non-CNS inflamed areas19,20 (e.g. lymph nodes) in response to the inflammatory cell signaling elicited by infiltrating immune cells5.
Herein we describe the key methodological aspects of the systemic injection of somatic NPCs into a mouse model of chronic EAE. More specifically, we define the protocols that we have established to (i) derive, expand and prepare for transplantation somatic NPCs from the subventricular zone (SVZ) of adult C57BL/6 mice; (ii) induce chronic EAE in such mice and (iii) perform therapeutically efficacious systemic (i.v. or i.c.v) NPC transplantation into EAE mice.
Protocol
All procedures involving animals are performed according to the principles of laboratory animal care approved by the UK Home Office under the animals (scientific procedures) act 1986 (PPL No. 80/2457 to SP).
1. Derivation of Somatic Neural Stem/Progenitor Cells (NPCs) from the Subventricular Zone (SVZ) of the Brain of Adult Mice
- Preparation of dissection instruments and media
NB: These two solutions must be prepared 1-2 hr before dissections instruments are ready and prewarmed at 37 ºC at least 20-30 min before use (it helps to dilute papain and optimizes enzyme activity).
- Autoclave one straight sharp scissor, one small surgical scissor and two small curved serrated forceps.
- ‘Digestion’ solution: Prepare two separate 50 ml tubes with:
Solution #1: 25 ml Earle’s Balanced Salt Solution (EBSS) + 10 mg ethylenediaminetetraacetic acid (EDTA) + 10 mg L-cysteine; and
Solution #2: 25 ml EBSS + 50 mg papain. - Prepare ‘Complete Growth Medium’ (CGM): complete mouse basal medium with mouse proliferation supplements, heparin 0.002%, basic fibroblast growth factor (bFGF; 10 ng/ml), epidermal growth factor (EGF; 20 ng/ml), and antibiotics (Pen/Strep).
- Brain dissection
NB: The removal of the temporal bones can easily damage the tissue because of their sharpness. To avoid this, firmly secure the bones with the forceps and pull out until the entire bone has been removed.
- For every preparation of NPCs, cull by cervical dislocation n= 5-7, 4-8 week old C57BL/6 mice.
- Cleanse with 70% ethanol (in water) the hair of the culled mice and cut behind the ears with the straight sharp scissor to remove the head;
- Make a midline incision using a small surgical scissor to cut the skin over the skull. Using the forceps, fold up the two patches of skin to expose the skull.
- With the small surgical scissor perform two little cuts at the base of the skull and remove the bones under the pons/medulla (ventral skull). Proceed by pulling out the temporal bones. With the same scissor perform a cut all along the skull, starting from the base to the bulbs, without damaging the surface of the brain. Also, perform a cut between the frontal bones and the eyes, to facilitate the removal of the parietal bones. With two forceps start removing the skull by pulling each of the parietal bones towards the outside. While pulling, pay attention to the meninges, which can damage the brain when the bones are being removed.
- Once the skull has been removed, slide the forceps between the brain and the skull to completely detach the meninges left. Gently lift up the brain from the skull. Cut the optic nerves to release the brain and finally collect the brain in a tube with cold phosphate buffered saline (PBS).
- Keep the tube on ice and repeat the same procedure for all the mice.
- Dissection of the SVZ, neurosphere formation and maintenance assays
Average number of cells x dilution factor x 104
Where 104 represents the conversion of the total volume of the hematocytometer chamber (total volume = 0.1 mm3 -> 10-4 cm3 -> 10-4 ml). When considering the dilution factor, both the one done with CGM and with the vital stain have to be taken into consideration. As an example, if 160 cells have been counted in the 4 corner squares, the final density (cells/ml) of the cell suspension will be:
160/4 x 5 (dilution with CGM) x 2 (dilution with vital stain) x 104
- Turn on a dissection hood equipped with a dissecting microscope.
- Clean all the surfaces with 70% ethanol (in water), and spray with an anti Mycoplasma solution to lower the risk of contamination.
- Cover the bottom of a beaker with sterile gauze (to preserve sharpened instruments) and fill it with 70% ethanol (in water). Soak in the beaker two pairs of forceps, one pair of micro scissors and a surgical blade.
- Place the whole brain on the brain-slicer matrix.
- Using two razor blades perform a coronal sectioning 2 mm from the anterior pole of the brain, excluding the optic tracts, and 3 mm posterior to the previous cut; and place the tissue on a clean Petri dish.
- Under the dissecting microscope isolate the SVZ tissue and cut into 1 mm3 pieces using iridectomy scissors. Repeat for all the brains.
- Mix well the two solutions (section 1.1.2) above and filter through a 0.22 μm filter immediately after the brains are dissected and the SVZs are ready to be digested.
- Transfer the sections into 30 ml of ‘Digestion’ solution, gently resuspend with a10 ml pipette and incubate 30 min at 37 °C in 5% CO2. Every 15 min gently shake the tube 2-3x.
- Centrifuge at 300 x g for 10 min.
- Remove the supernatant and resuspend the pellet in 200 μl of CGM by pipetting first with a P1000 and then with a P200 pipette (10-20x each), until obtaining a single cell suspension.
- Take the suspension up to 5 ml with CGM and transfer to a T25 cm2 flask.
- After 5-7 days in vitro (DIV) neurospheres will form. Collect the suspension into a 15 ml tube and centrifuge 10 min at 150 x g.
- Remove the supernatant and resuspend the pellet into 200 μl of CGM.
Dissociate the neurospheres mechanically by passaging the cells 100-150x with a P200 pipette, until obtaining a single cell suspension and take it up to 1 ml with CGM. - Dilute the cell suspension, e.g. 10 μl of cell suspension in 40 μl of CGM to obtain a 1:5 dilution, and mix well. Mix 10 μl of the dilute cell suspension with 10 μl of a vital stain and mix well.
- Fill a hemocytometer counting chamber with 10 μl of a 1:1 cell suspension:vital stain solution. Separately count the number of live (white) and dead (blue) cells in the 4 corner squares. To determine the number of cells/ml, apply the following calculation:
- Resuspend 2 x 105 cells in 5 ml of CGM and transfer to a T25 cm2 flask;
- Repeat sections 1.3.11-1.3.12 every 4-5 DIV. From the second passage of expansion onwards, assess the cellular viability by vital stain exclusion and start building up a continuous growth curve by plating NPCs at clonal density (8,000 cells/cm2), as described21. Keep the cell numbers and repeat the procedure every 4-5 DIV. To generate a continuous growth curve, proceed as follows:
NB: To have a good cell preparation, after 4-5 DIV spheres should have reached a diameter of 150-200 μm. To have a good estimate of the cell density, it is important to find an optimal dilution factor in order to have well distributed, nonoverlapping cells throughout the hematocytometer. Ideally, there should be between 50-200 total cells. When counting, only the cells comprised in the square without touching the borders have to be considered. Also, the viability of the cells is a crucial factor to have a healthy preparation. Importantly, it should be greater of 90%. A linear growth curve is another indicator of a healthy cell preparation.
- Count the number of live and dead cells (e.g. 1.3 x 106).
- Define the growth rate dividing the number of live cells by the number of plated cells (e.g. 1.3 x 106/2 x 105= 6.5).
- Calculate the total number of cells by multiplying the growth rate for the total number of cells present at the previous time point (at the beginning= 2 x 105).
- Report the mean ± standard deviation to build a linear trend line.
- Starting from passage 6, mechanical dissociation is replaced by enzymatic dissociation. After centrifugation at 150 x g for 10 min, remove the supernatant, resuspend the pellet in 200 μl of cell aggregate dissociation solution, resuspend 7-8x with a P200 and incubate 10 min at 37 °C, 5% CO2.
- Resuspend 7-8x with a P200 to obtain a single cell suspension and add 800 μl of CGM. Count the cells (both live and dead) and establish their viability. Resuspend 2 x 105 cells in 5 ml of CGM and transfer to a T25 cm2 flask.
- Viral transduction of NPCs for in vivo cell tracking
NB: To verify the efficiency of viral transduction, build a parallel growth curve with transduced and nontransduced NPCs. In addition, the clonal efficiency, that is the absolute number of neurosphere forming cells present within a cell preparation or a cell cycle analysis by DNA content (with propidium iodide, PI), may be used as further indications of the cell status. If the percentage of infected cells should not be good, the protocol of viral transduction can be repeated a second time with the same population of cells. Once the level of infection is satisfactory and the growth curve is similar to that obtained with wild type cells, transduced NPCs can be expanded and/or transplanted.
- Harvest the neurospheres and obtain a single cell suspension. Plate the cells at high density (1.5 x 106 cells in a T75 cm2 flask) in 10 ml CGM.
- After 12 hr add 3 x 106 T.U./ml of a third generation lentiviral vector pRRLsin. PPT-hCMV engineered with the E. coli-derived β-galactosidase (lacZ) or with green fluorescent protein (GFP).
- 48 hr later, harvest the cells, centrifuge at 300 x g for 10 min and replate the cells at a 1:1 ratio.
- After 3 passages in vitro verify the efficiency of the infection. Flow cytometry is commonly used to test infection efficacy, and the satisfactory level of infection is commonly accepted to be in the range of 75-95% of positive cells. Check again the efficiency of the infection after 3 further passages of expansion to verify the maintenance of the marker expression.
- Neurosphere freezing
- Harvest the neurospheres from a T25 cm2 flask, centrifuge at 300 x g for 10 min, and discard the supernatant.
- Resuspend the pellet with 1 ml of freezing medium (CGM with 10% dimethyl sulfoxide (DMSO).
- Place the cryovials at -80 °C within a freezing container.
- After at least 24 hr move the cells to a cryobox and store at -80 °C for months, or bring to liquid nitrogen (N2) for longer storage.
- Neurosphere thawing
NB: To avoid the toxic effects of prolonged contact of NPCs with DMSO, the thawing process must be as fast as possible.
- Remove the cryovial from the -80 °C/N2 and keep it on dry ice.
- Quickly thaw the vial on a water bath, until almost all the cell suspension is defrosted and only a small piece of iced suspension remains.
- Resuspend the cell suspension with 5 ml of prewarmed fresh CGM.
- Centrifuge at 300 x g for 10 min.
- Remove the supernatant, resuspend gently with 5 ml of fresh CGM and plate the cell suspension in a clean, untreated T25 cm2 flask.
- 1.7) NPC characterization
NB: The last wash PBS can be replaced with 0.05% sodium azide-PBS to prevent fungal growth or contaminations, and coverslides are stored at 4 °C for 2-3 weeks.
NB: To stain for intracellular markers add a permeabilizing agent to PBS in the blocking solution. If the source of the primary antibody is goat, bovine serum albumin (BSA) or serum of other than goat should be used.
NB: To stain for intracellular markers use a permeabilizing agent in the primary antibody solution.
- Place a 13 mm glass coverslide on the bottom of a 24 well plate. Add 150 μl of coating solution on the top of each coverslide to create a small drop. Incubate for at least 30 min at 37 °C, 5% CO2.
- Harvest the neurospheres and dissociate to obtain a single cell suspension.
- Count live cells and dilute to obtain 80,000 cells/35 μl. Remove the excess of coating solution from the coverslide by gentle aspiration, and plate the 35 μl of cell suspension.
- Incubate 25 min at 37 °C, 5% CO2. Quickly check at the contrast phase microscope if the cells have started to adhere.
- Prepare the differentiation medium by mixing basal mouse medium with the appropriate supplements (see Table 1) and antibiotics (Pen/Strep).
- Add 400 μl of differentiation medium and incubate at 37 °C, 5% CO2.
- After 3 DIV, change half of the medium with fresh differentiation medium.
- After 6 DIV, remove the medium and wash once with PBS. Under a chemical hood remove the PBS and add 300 μl of prewarmed 4% paraformaldehyde (PFA) 4% sucrose. Incubate for 5 min at room temperature (RT). Remove the PFA and wash 3x with PBS.
- To proceed with immunocytochemistry, remove the PBS and block with PBS 10% normal goat serum (NGS) (blocking solution), and incubate 1 hr at RT.
- Remove the blocking solution and add the desired primary antibody diluted with PBS 1% NGS. Incubate at 4 °C O/N or, alternatively, 120 min at RT.
- After the incubation, wash 2x with PBS. Incubate with the appropriate secondary antibody diluted with PBS 1%NGS. Incubate 60 min at RT.
- Wash 2x with PBS and counter-stain the nuclei with 4′,6-diamidino-2-phenylindole (DAPI) diluted 1:20,000 with PBS permeabilizing agent for 3 min at RT in the dark. Wash twice with PBS and once with distilled water.
- Put a drop of mounting medium on a glass tissue slide and with forceps mount the coverslide with the cells facing the mounting medium. Gently press the coverslide to squeeze out the excess of mounting medium. Leave the coverslide in the dark at RT until the mounting medium is dried and store at 4 °C.
2. Myelin Oligodendrocyte Glycoprotein (MOG)-induced Experimental Autoimmunity in C57Bl/6 Mice
- Preparation of emulsion
- On a glass beaker prepare an emulsion composed of Incomplete Freund’s adjuvant containing 4 mg/ml of Mycobacterium tuberculosis [otherwise defined as Complete Freund’s Adjuvant (CFA)] and 200 μg of MOG (peptide 35-55), considering that the total amount of emulsion per mouse will be 300 μl.
NB: Appropriate controls of MOG-immunized mice in CFA are mice immunized with CFA only. The expected variance of disease onset in MOG-immunized mice is 11.3 (±1.8) dpi.
NB: When calculating the total volume of emulsion needed, consider twice as much volume as the number of mice to immunize. Glassware should be preferred to plasticware to minimize the waste of part of the emulsion.
- With a glass syringe holding a 19 G needle blend for about 30 min, always keeping the emulsion on ice.
- To verify if the emulsion is ready, drop a small amount of emulsion on water. The emulsion is ready to be injected only if the drop remains as it is and does not disperse.
- Immunize the control group of mice with CFA only. Treat experimental mice (MOG immunized) with MOG in CFA. The expected variance of disease onset in MOG immunized mice is 11.3 (±1.8) dpi.
- Transfer the 19 G needle to a 1 ml plastic syringe and aspirate the emulsion. Avoid bubbles, change the needle with a 25 G and leave the syringe on ice. Syringes filled with the emulsion are ready to be used and should be kept on ice for a couple of hr.
- On a glass beaker prepare an emulsion composed of Incomplete Freund’s adjuvant containing 4 mg/ml of Mycobacterium tuberculosis [otherwise defined as Complete Freund’s Adjuvant (CFA)] and 200 μg of MOG (peptide 35-55), considering that the total amount of emulsion per mouse will be 300 μl.
- Immunization
- Place the mice (6-8 weeks old, female) into a warming box and wait for about 10 min to allow the tail vein to dilate.
- Transfer one mouse from the warming box to a mouse restrainer. Close the restrainer to avoid any movement of the mouse.
- Fill a 1 ml insulin syringe with 100 µl of pertussis toxin (5 μg/ml) avoiding making bubbles. Slowly inject the solution in the tail vein. Remove the needle and press for a few seconds with a piece of paper to plug the bleeding.
- Place the mouse back in the cage and repeat the same procedure for all the mice.
- Anesthetize a mouse with continuous isoflurane inhalation (1-2%, 2 L/min) and inject 100 µl of the emulsion subcutaneously at the level of the two flanks and the base of the tail (300 μl emulsion/mouse). Slowly remove the syringe, avoiding the leakage of the emulsion.
- Mark the mouse with an ear puncher, place the mice in the cage and check until full recovery. Repeat for all the mice.
- After 48 hr (2 days post immunization, dpi) repeat section 2.2.3.
- Behavioral analysis of EAE mice
- Starting from 5 dpi, weigh the mice daily and monitor their locomotor performances by using the scale scoring system described in Table 1.
3. Injection of Neural Stem/Progenitor Cells into the Tail Vein (i.v.)
- Cell preparation
- Harvest neurospheres by centrifugation at 300 x g for 10 min.
- Remove the supernatant and add 200 μl of cell aggregate dissociation solution. Incubate 10 min at 37 °C, 5% CO2.
- Gently resuspend 10-12x (avoiding bubbles) with a P200 until obtaining a single cell suspension and take to 800 μl with basal medium without Ca2+ and Mg2+.
- Count the cells by using a vital stain to assay cell viability and dilute the suspension with basal medium without Ca2+ and Mg2+ to obtain a final density of 106 cells/150 μl. Keep the cell suspension on ice until use.
- NPC i.v. injection
NB: The ideal dissociation into single cell suspension and the absence of air bubbles are two key aspects to consider to reduce the risk of either clumps/aggregates vs emboli formation which might result in vein occlusion and consequent death.
- At the peak of the disease (16-18 dpi), weigh and score the mice. Distribute the mice according to their score in order to have homogeneous groups.
- Place the mice into a warming box and wait for about 10 min to allow the tail vein to dilate.
- Transfer one mouse from the warming box to a mouse restrainer. Close the restrainer to avoid any movement of the mouse.
- Fill a 1 ml insulin syringe with 150 μl of cell suspension and make sure to remove all the bubbles. Slowly inject the solution into the tail vein. Remove the needle and press for a few seconds with a piece of paper to plug the bleeding (Figures 6A and 6B).
- Place the mouse in the cage and check until recovery. Repeat the same procedure for all the mice.
4. Injection of Neural Stem/Progenitor Cells into the Cisterna Magna (i.c.v)
- Cell preparation
- Harvest NPCs by centrifuging at 300 x g for 10 min.
- Remove the supernatant and add 200 μl of cell aggregate dissociation solution. Incubate 10 min at 37 °C, 5% CO2.
- Gently resuspend 10-12x with a P200 until obtaining a single cell suspension and take to 800 μl with basal medium without Ca2+ and Mg2+.
- Count the cells and dilute the suspension with basal medium without Ca2+ and Mg2+ to obtain the desired cell density. Keep the cell suspension on ice until use.
- 4.2) NPC i.c.v. injection
- At the peak of the disease (16-18 dpi), weigh and score the mice. Distribute the mice according to their score in order to have homogeneous groups.
- Place the mouse on a stereotaxic apparatus under continuous isoflurane inhalation (1-2%, 2 L/min). Secure the head of the mouse with the ear bars. Then adjust both the mouth and nose pieces. Make sure the head of the mouse is flat and fixed.
- Swab the head of the mouse with Povidone-iodine (PVP-I) and make an incision to cut the skin in the posterior portion of the head to expose the skull.
- Using a micromanipulator, insert the tip of a microliter syringe into the cleft between the occiput and the atlas vertebra through the intact muscles and ligaments in the midline at the back of the neck. The bent part of the needle is kept in close contact with the internal surface of the occiput for the entire length, as described22.
- Slowly insert the needle and wait a few sec. The operator is meant to learn to recognize the feeling of the needle being “hooked” to the mouse skull, prior to start injecting the cell preparation. Slowly inject the volume of cells (within 3-5 min). Leave the needle in place for additional few seconds after the injection and remove slowly the syringe.
- Stitch up the skin and place the mouse into a recovery cage until full recovery.
5. Tissue Processing
- Deeply anaesthetize the mice with a mixture of 0.5 ml ketamine (100 mg/ml), 0.25 ml xylazine (23.32 mg/ml) and 4.25 ml sterile water and transcardically perfuse, washing with saline-EDTA and fixing with 4% PFA. Remove both the vertebral columns and brains and postfix in 4% PFA for 12 hr at 4 °C. Wash the tissue in PBS, remove the spinal cords from the bones and leave the tissue at least 48 hr in 30% sucrose (in PBS) at 4 °C. Embed the tissues in optimal cutting temperature (OCT) compound and snap freeze with liquid nitrogen, as described2.
- Use a microtome to cut the tissues in 10-12 μm thick slices.
- Alternatively, embed fresh tissue in agarose and cut tissue using a vibratome 50-80 μm thick slices.
- In both the cases, incubate O/N at 37 °C in 5-bromo-4-chloro-3-indolyl-β D-galactopyranoside (X-gal) solution to detect nuclear β-galactosidase activity.
- Recut sections containing β-gal+ cells into 5 µm slices and double stain using antiglial fibrillary acidic protein (GFAP) for astrocytes (50 μg/ml), antineuronal nuclear antigen (NeuN) for neurons (1μg/ml) and antiNestin for undifferentiated neural cells (10 μg/ml).
- Proceed as described in sections 1.7.11 and 1.7.12. For multiple stainings make sure to use secondary antibodies conjugated with different fluorophores.
- For tissues containing GFP labeled cells proceed as explained in steps 5.1-5.3 and proceed with the multiple stainings.
- Add some drops of mounting medium to the slides and cover with a tissue coverslip.
- For sections stained with biotin conjugated antibodies prepare a solution of avidin biotin complex (ABC solution, see Table 2) and leave in agitation for 45 min.
- Incubate the slices 5 min with 1% H2O2 to block the activity of endogenous peroxidase.
- Wash twice with PBS.
- Incubate with ABC solution for 1hr at RT.
- Wash twice with PBS.
- Incubate with 3,3′-diaminobenzidine solution and 0.3% H2O2. Monitor the reaction under a microscope and wash with PBS as soon as the slice start getting brown (usually around 5-10 min).
- Wash twice with PBS and proceed as described in step 5.7.
Comprehensive list of materials and reagents is shown in Table 2.
Representative Results
NPC derivation and characterization
SVZ dissections are performed on pools (n= 5-7 mice/pool) of 6-8 week old C57Bl/6 mice by means of mechanical and enzymatic dissociation (Figure 1A). After a few days of culturing in CGM, free-floating neurospheres begin forming (Figures 1A and 1B). Primary spheres are collected and mechanically passaged every 4-5 DIV. Upon passaging, numbers of live and dead cells are ascertained and cumulative cell numbers plotted to generate a growth curve (Figure 1C). This gives an indication of the propagation rate, thus providing an indirect parameter to evaluate the global stability of the NPC preparation. When proliferating as neurospheres, NPCs express markers of mitotic activity (phospho-histone H3, PHH3; 5 μg/ml) and of undifferentiated neural cells (e.g. Nestin) (Figure 2A). Undifferentiated NPCs that are to be therapeutically efficacious in EAE after transplantation must express cell surface adhesion molecules that include CD44 (5 μg/ml), the α4 subunit of the VLA-4 (10 μg/ml) (Figure 2F). When plated under appropriate differentiation conditions, NPCs express markers typical of the three neural lineages, (Figure 2B), such as the astroglial marker GFAP (Figure 2C), the neuronal marker microtubule associated protein-2 (MAP-2; 5 μg/ml) (Figure 2D) and the oligodendroglial markers O4 (5 μg/ml) and myelin basic protein (MBP; 10 μg/ml) (Figure 2E), thus confirming that NPCs are multipotent towards the three main neural lineages. To facilitate the identification of transplanted cells in vivo, NPCs are transduced with lentiviruses engineered to stably express reporter genes such as GFP. Transduced NPCs express GFP both as proliferating neurospheres (Figures 3A and 3C) as well as when differentiating in vitro upon growth factor withdrawal (Figure 3B). To verify the efficiency of transduction, the expression of GFP is analyzed by flow cytometry, starting as early as 3 passages after cell transduction (that is allowing enough time to have robust expression of the introduced transgene at the protein level). When applying third generation lentiviruses to NPCs in vitro, we consistently observe >90% of cells showing high levels of GFP over multiple independent experiments (Figure 3C), with neither overt toxicity nor changes in proliferation, when comparing the growth curves of wild type and lenti-GFP transduced NPCs (Figure 3D).
Chronic EAE induction
EAE is one of the best-characterized models of MS, since it recapitulates most of the pathological and clinical events of MS. Immunization of C57Bl/6 mice with MOG35-55 leads to the development of a chronic form of EAE. When approaching the disease onset (11.3±1.8 dpi) during the induction phase (preclinical), MOG immunized mice start losing weight (Figure 4A). At the beginning of the effector/invasion phase (peak clinical; 16.8±3.2 dpi) EAE mice reach the peak of the disease (score 3.5±0.7), and shortly after peaking they show a progressive and partial recovery that finally stabilizes during the chronic phase (stable clinical) from around 30 dpi onwards (score 2.8±0.9) (Figure 4B). Numerous studies, including some investigating EAE by either intravital microscopy23 or magnetic resonance imaging24, paired with in vivo tissue pathology, have identified in the peak of the effector/invasion phase (16-22 dpi) the ideal window of opportunity (WoO) in which to perform systemic experimental therapies with stem cells intended to enter the chronically inflamed EAE CNS and protect it from secondary damage.
NPC injection
Syngeneic NPCs injected systemically in EAE mice (Figure 6) are found almost exclusively in perivascular areas of CNS damage, both in the brain (Figure 5A) and spinal cord (Figures 5B-D), up to 45 days post transplantation (dpt), as described5. i.v. injected NPCs preferentially retain an immature, Nestin+ (Figure 5C) phenotype, while few NPCs moving out of the perivascular area express NeuN (Figure 5D). Of note, NPCs injected i.v. into healthy controls fail to enter the CNS via the vascular route (Figure 5E). After i.v. NPC injection, significant numbers of injected NPCs are found at the level of non-CNS organs such as the liver, gut, spleen, lung and kidney, but not the heart, as early as 10 dpt (Figure 5F). Non-CNS accumulating NPCs are completely cleared out of these peripheral organs by 30 dpt (Figure 5G).
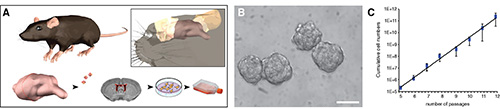
Figure 1. Isolation of NPCs from the SVZ of adult mice and generation of stably expandable NPC lines. A, Schematic representation of the main critical steps of the generation of stably expandable, and preparation of injectable mouse NPCs. B, Neurospheres in vitro. C, NPCs cultured as neurospheres are passaged every 4-5 days. Numbers of live and dead cells are collected and cumulative cell numbers plotted to generate a growth curve. The scale bar in B is 200 μm. Data in C are mean cumulative number of cells ± SD (n= 3).
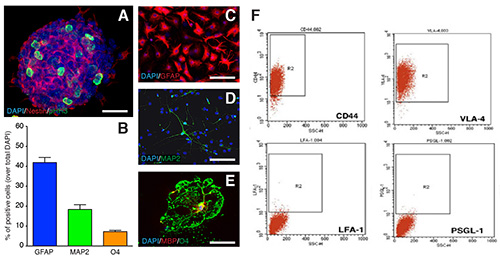
Figure 2. NPCs characterization. A, proliferating mouse neurospheres express the PHH3 core histone protein (green), and the type VI intermediate filament protein Nestin (red). B-E, After 6 DIV in differentiation conditions, NPCs are multipotent towards astroglial (GFAP, red in C), neuronal (MAP-2, green in D) and oligodendroglial (O4, green in E; and MBP, red in E) lineages. Quantification of GFAP-, MAP-2-, and O4-expressing cells at 6 DIV after NPC differentiation in vitro are showed in B. Data in B are as mean % ± SD (n= 3). The scale bars in C-E are 50 μm. F, Flow cytometry analysis of the expression of the main cell surface adhesion molecules regulating leukocyte extravasation in mouse neurospheres. Figure 2F is reproduced from Pluchino et al.2
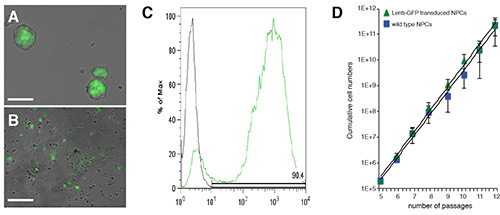
Figure 3. Transduction of NPCs with lentiviruses expressing reporter genes (e.g. GFP. A and B, Phase contrast images of neurospheres (A) and differentiating NPCs (B) that have been transduced with a 3rd generation lenti-GFP vector. Flow cytometry analysis of the NPCs in A. C, Wild type (not transduced) and lenti-GFP transduced NPCs cultured as neurospheres are passaged every 4-5 days. Numbers of live and dead cells are collected and cumulative cell numbers plotted to generate a growth curve. The scale bar in A and B is 200 μm. Data in C are mean cumulative number of cells ± SD (n=3).
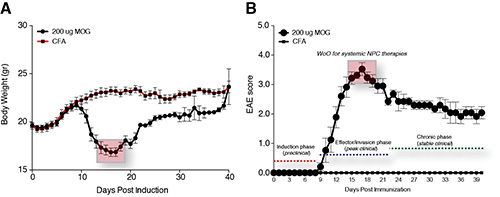
Figure 4. Functional characterization of chronic EAE. A, Daily weight monitoring in MOG- and CFA-immunized C57Bl/6 mice over a total follow up of 40 dpi. B, Daily EAE score monitoring in MOG- and CFA-immunized C57Bl/6 mice over a total follow up of 40 dpi. Data are expressed as mean numbers ± SD (n=10 mice/group).
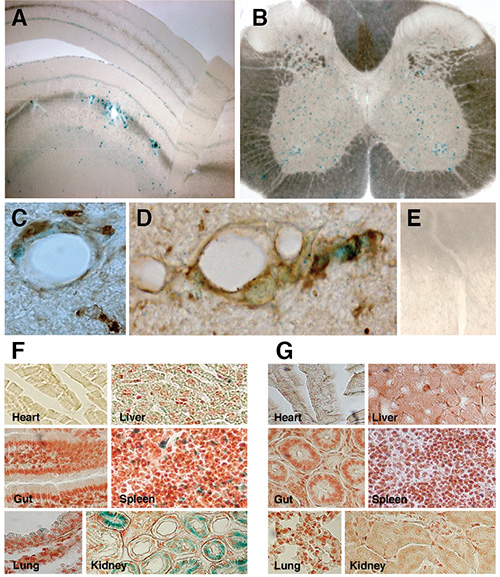
Figure 5. Systemically injected NPCs migrate in the parenchyma of EAE mice. A-E, X-gal staining of vibratome-cut (70 µm) brain (A) and spinal cord (B) tissue sections from EAE mice injected i.v. with syngenic NPCs, showing transplanted β-gal+ cells (blue cells) persisting within perivascular CNS areas up to the end of the clinical follow up (106 dpi). C, β-gal+ i.v.-injected NPCs (blue) persisting within perivascular CNS areas, retain a Nestin+ (brown) phenotype. D, Few migrating NPCs express NeuN+ (brown) as long as they move out of the perivascular area. E, X-gal staining in a representative spinal cord section from a sham-treated EAE mouse. Scale bars, 20 μm. F-G, Analysis of tissues other than the CNS shows that i.c.- or i.v.-injected NPCs (blue cells) reach virtually all the organs (e.g. lung, liver, spleen, heart, gut, kidney) within 10 dpt (F), but are cleared from the same organs by 30 dpt (G). Figures 5A-E is reproduced from reference 5, while Figures 5F-G is reproduced from Pluchino et al.2
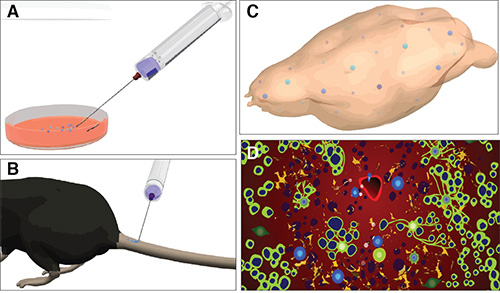
Figure 6. Schematic representation of the protocol for systemic injection of NPCs in mice with EAE. Main critical steps of the systemic injection of NPCs in mice with EAE: from the preparation of injectable CAM expressing single cell dissociated NPCs the cell culture room (A), to the injection into the tail vein of EAE mice at the peak of the effector/invasion disease phase (B), towards the in vivo detection of the injected NPCs that have targeted the CNS (C), to the in vivo tissue pathological studies allowing the study of the mechanisms of therapeutic plasticity of injected NPCs (D). In D, green cells in are oligodendrocytes, dark blue cells are axons, light blue cells are transplanted NPCs, orange cells are astrocytes, and red cells are endothelial cells.
| Score | Locomotor Responses |
| 0 | Normal mouse. No overt signs of disease |
| 1 | Limp tail: complete flaccidity of the tail, and absence of curling at the tip of the tail when a mouse is picked up |
| 1.5 | Hind limb weakness: the mouse shows occasional and brief trippings when walking on a waddling gait |
| 2 | Limp tail and hind limb weakness: when placed on its back, the mouse cannot turn to its normal position |
| 2.5 | Partial hind limb paralysis: the mouse can no longer use hind limbs to maintain rump posture or walk but can still move one or both hindlimbs to some extent, forelimbs remain unaffected |
| 3 | Complete hind limb paralysis: total loss of movement in hindlimbs. The mouse drags itself only on its forelimbs, which remain unaffected. Mice at this stage are given food on the cage floor, long sipper tubes, and daily subcutaneous injection of hydrating solutions to prevent dehydration. If mice develop sores or skin lesions that do not recover with treatment, they will be killed humanely |
| 3.5 | Forelimb weakness: when place on a vertical grid, the mouse is not able to climb and slowly falls |
| 4 | Moribund state |
| 5 | Mouse found dead or culled according to humane endpoints |
Table 1. Five stage scale of clinical signs and ascending paralysis in EAE mice.
Discussion
Somatic stem cell based therapies are emerging as one of the most promising strategies for treating chronic inflammatory CNS disorders such as MS211. While the mechanisms sustaining their therapeutic effects still need to be completely elucidated, the significant impact of NPC transplantation in different experimental models of neurodegenerative diseases has given rise to the somewhat provocative belief that stem cells may soon be applied into human studies. However, before envisaging any potential human applications of such innovative therapies we need to face some key issues and address some unanswered questions, such as the identification of the ideal stem cell source for transplantation (autologous vs allogeneic, pluripotent vs multipotent) and the best route of administration.
Neurodegenerative diseases differ in their pathophysiologies, with some being spatially confined (e.g. spinal cord injury, stroke) while other being characterized by a spatial temporal dissemination (e.g. MS). It derives that the focal injection of (stem) cells may be a suitable treatment for the former, while it may be inadequate or simply unfeasible for the latter, where the presence of multiple sites of brain damage represents an additional challenge to be overcome.
Consistent evidence has shown that intravenous delivery may represent a valid (and low invasive) protocol for cell administration, supported by the intrinsic ability of stem cells to sense the environment and specifically home towards the site(s) of damage. However, translation of systemic NPC therapies into clinics is still hindered by a few limitations, such as the possible accumulation of transplanted cells in peripheral non-CNS organs (e.g. lungs, spleen, lymph nodes, and kidneys) that may or may not be sites of local inflammatory responses in vivo. While this nonspecific accumulation of injected NPCs out of the target CNS is quickly cleared in mice with chronic EAE2, there is evidence of long term NPC persistence (or in some cases exclusive targeting) at the level of the secondary lymphoid organs (e.g. lymph nodes and spleen) after systemic injection in mice with relapsing EAE519,20. The fact that significant numbers of transplanted NPCs recirculate toward non-CNS organs creates a dilution effect, inevitably reducing the absolute number of NPCs in the CNS. Interestingly, systemically injected NPCs are therapeutically efficacious (e.g. via immune regulatory actions) even when accumulating exclusively out of the CNS19,20. And the overall therapeutic effects of injected NPCs targeting the CNS only5 or the lymph nodes only19 are comparable. This may in part due to an intrinsic plasticity of the cells, which respond differently to the molecular components of the surrounding microenvironment. Notably, if on one hand transplanted NPCs may feel the presence of inflammatory cytokines and exert their therapeutic effect through immune modulatory mechanisms16, on the other hand they may come in contact with hostile microenvironments ultimately leading to the formation of tumors25,26. For these reasons, the preferred option is at present to concentrate injected NPCs in the CNS. As such, the intrathecal administration (via a lumbar or cisternal route) is still the most accepted prospective route of administration in clinical trials. However, the multifocality and pathological heterogeneity of MS lesions may limit the efficacy of such an approach.
Here we describe an efficient protocol to isolate, maintain and characterize mouse neural progenitors from the adult SVZ and successively, how to systemically (both i.v and i.c.v) inject NPCs in mice affected by chronic EAE, one of the most studied models of MS. Even though relatively simple and straightforward, these protocols present some critical steps that need to be taken into consideration. At first, it is mandatory to obtain a stably expandable and healthy cell preparation. As described throughout the protocol, the stability of the cells may be indirectly inferred by looking at their growth rate. Ideally, neurospheres should reach a diameter of 150-200 μm every 4-5 DIV and the percentage of cell death upon passages must be very low (less than 10%). Also, neurospheres must present a compact and regular round shape at the microscope. The infection with lentiviruses may interfere with the survival and stability of the cells. To obtain an optimal infection, it is mandatory to obtain a titration of the virus of interest sufficient to obtain 3 x 106 T.U./ml. Indeed, while on one hand a low amount of virus would lead to a small percentage of infected cells, on the other hand the use of a high amount would likely lead to a toxicity effect (strongly dependent on the gene to be infected). NPCs can be infected multiple times with integrating viruses, should the outcome of the first transduction lead to low percentage of transgene positive viable cells. It is always a good practice to have intra experimental comparison of stability and viability parameters with wild type, nontransduced, controls NPCs. As for the induction of chronic EAE, the most problematic part is represented by the preparation of the emulsion. As described, glassware and ice must be use at all times. The preparation of the emulsion takes 30-45 min, and it can be considered ready to be injected only when it does not disperse when dropped on water. If the emulsion dissipates in water, this means it is not yet ready and requires further blending.
Good accuracy during the i.v. injection procedure at the time of cell administration is critical. First, it is extremely important to have a single cell suspension, as the injection of aggregated cells may obstruct the veins of the injected mouse and lead to its immediate death. To assure the correct positioning of the needle into the vein, it is good practice to aspirate with the syringe few microliters of blood. In the case of no blood coming into the syringe, the operator should slowly move the needle backward or forward until the correct location is found. Only at this point can the cell suspension be slowly injected. Importantly, the operator should typically not be required to apply any pressure on the syringe since the liquid should easily flow in the vein. An improper injection would inevitably result in a subcutaneous swelling corresponding to the injection site.
It is worth noting that in the very near future systemically injected NPCs2,27, genetically modified or not, may themselves represent a therapeutic option, as well as providing an important tool for delivering immune modulatory and/or neuroprotective drugs and pro remyelinating agents directly into the CNS28. Therefore, while some limitations related to the systemic delivery of NPCs do exist, both i.v. and i.c.v. routes may ultimately represent valid alternative therapeutic approach in those diseases where the focal injection of cells may not constitute the gold standard protocol of treatment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Jayden Smith for critically reviewing and proof editing the manuscript. This work has received support from the National Multiple Sclerosis Society (NMSS, partial grants RG-4001-A1), the Italian Multiple Sclerosis Association (AISM, grant 2010/R/31), the Italian Ministry of Health (GR08-7), Wings for Life, Banca Agricola Popolare di Ragusa (BAPR), the European Research Council (ERC) under the ERC- 2010-StG Grant agreement no 260511-SEM_SEM and the European Community (EC) 7th Framework Program (FP7/2007-2013) under Grant Agreement n*deg; 280772- iONE.
Materials
| Cell culture | |||
| EBSS | Sigma | E2888 | |
| L-Cystein | SIGMA-ALDRICH CO LTD | C7352 | |
| Papain | WORTHINGTON | 30H11965 | |
| EDTA | Fisher scientific | D/0700/50 | |
| Mouse NeuroCult basal medium | Stem Cell technologies | 05700 | |
| NeuroCult proliferation supplements | Stem Cell technologies | 05701 | |
| Heparin | Sigma | H3393 | |
| Basic fibroblast growth factor | Peprotech | 100-18B-1000 | |
| Epidermal growth factor | Peprotech | AF-100-15-1000 | |
| Pen/Strep | Invitrogen | 1514012 | |
| Matrigel (coating solution) | BD biosciences | 354230 | |
| NeuroCult® Differentiation Kit (Mouse) | Stem cell technologies | 05704 | |
| Accumax | eBioscience | 00-4666-56 | |
| Dulbecco's PBS (DPBS) (10x) without Ca& Mg | PAA LABORATORIES LTD | H15-011 | |
| Myco trace | PAA LABORATORIES LTD | Q052-020 | |
| Dimethyl sulfoxide (DMSO) | SIGMA | D2650 | |
| immunofluorescence | |||
| Normal goat serum | PAA LABORATORIES LTD | B11-035 | |
| Polyethylene glycol p-(1,1,3,3-tetramethylbutyl)-phenyl ether | SIGMA-ALDRICH CO LTD | T8787 | |
| Mouse anti Nestin | Abcam | ab11306 | |
| Rabbit anti GFAP | DAKO | 203344 | |
| Mouse anti Histone H3 (phospho S10) | Abcam | ab14955 | |
| Rabbit anti MAP-2 | Abcam | ab32454 | |
| Rat anti MBP | AbD SEROTEC | MCA409S | |
| Anti-O4 Antibody, clone 81 | MAB345 | Millipore | MAB345 | |
| DAPI | Invitrogen | D1306 | |
| Mounting solution | DAKO | S3023 | |
| EAE | |||
| Freund's Adjuvant Incomplete | SIGMA-ALDRICH CO LTD | F5506 | |
| Mycobacterium tuberculosis | DIFCO | H37Ra | |
| MOG(35–55) | Espikem | ||
| Pertussis toxin | List Biological Laboratories | 181 | |
| Tissue processing | |||
| Iris scissor straight | Fine Sciences Tolls | 14060-09 | |
| Blunt/bended forceps | Fine Sciences Tolls | 11080-02 | |
| Brain slicer | Zivic instruments | BSMAS005-1 | |
| Surgical blades | Swann-Morton | 324 | |
| P200, P1000 pipettes | |||
| Ketamine (Vetalar) | Boehringer Ingelheim | 01LC0030 | |
| Xylazine (Rompun) | Bayer | 32371 | |
| Stereotaxic frame | KOPF | Model 900 | |
| Hamilton syringe | Hamilton | 7762-04 | |
| Paraformaldehyde (PFA) | SIGMA | 158127 | |
| VECTASTAIN Elite ABC Kit | vector laboratories | PK-6100 |
References
- Ben-Hur, T., et al. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 41, 73-80 (2003).
- Pluchino, S., et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 422, 688-694 (2003).
- Chu, K., Kim, M., Jeong, S. W., Kim, S. U., Yoon, B. W. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci. Lett. 343, 129-133 (2003).
- Bottai, D., Madaschi, L., Di Giulio, A. M., Gorio, A. Viability-dependent promoting action of adult neural precursors in spinal cord injury. Mol. Med. 14, 634-644 (2008).
- Pluchino, S., et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 436, 266-271 (2005).
- Einstein, O., et al. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol. Cell Neurosci. 24, 1074-1082 (2003).
- Chu, K., et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 1023, 213-221 (2004).
- Jeong, S. W., et al. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 34, 2258-2263 (2003).
- Aboody, K. S., et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. U.S.A. 97, 12846-12851 (2000).
- Muller, F. J., Snyder, E. Y., Loring, J. F. Gene therapy: can neural stem cells deliver. Nat. Rev. Neurosci. 7, 75-84 (2006).
- Martino, G., Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 7, 395-406 (2006).
- Deckert-Schluter, M., Schluter, D., Hof, H., Wiestler, O. D., Lassmann, H. Differential expression of ICAM-1, VCAM-1 and their ligands LFA-1, Mac-1, CD43, VLA-4, and MHC class II antigens in murine Toxoplasma encephalitis: a light microscopic and ultrastructural immunohistochemical study. J. Neuropathol. Exp. Neurol. 53, 457-468 (1994).
- Hemmer, B., Archelos, J. J., Hartung, H. P. New concepts in the immunopathogenesis of multiple sclerosis. Nat. Rev. Neurosci. 3, 291-301 (2002).
- Butcher, E. C., Picker, L. J. Lymphocyte homing and homeostasis. Science. 272, 60-66 (1996).
- Rampon, C., et al. Molecular mechanism of systemic delivery of neural precursor cells to the brain: assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 26, 1673-1682 (2008).
- Pluchino, S., et al. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann. Neurol. 66, 343-354 (2009).
- Martino, G., Pluchino, S., Bonfanti, L., Schwartz, M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 91, 1281-1304 (2011).
- Aharonowiz, M., et al. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS ONE. 3, e3145 (2008).
- Pluchino, S., et al. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PLoS One. 4, (2009).
- Einstein, O., et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann. Neurol. 61, 209-218 (2007).
- Gritti, A., et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 16, 1091-1100 (1996).
- Furlan, R., Pluchino, S., Marconi, P. C., Martino, G. Cytokine gene delivery into the central nervous system using intrathecally injected nonreplicative viral vectors. Methods Mol. Biol. 215, 279-289 (2003).
- Constantin, G. Visualization and analysis of adhesive events in brain microvessels by using intravital microscopy. Methods Mol. Biol. 239, 189-198 (2004).
- Politi, L. S., et al. Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem Cells. 25, 2583-2592 (2007).
- Melzi, R., et al. Co-graft of allogeneic immune regulatory neural stem cells (NPC) and pancreatic islets mediates tolerance, while inducing NPC-derived tumors in mice. PLoS One. 5, (2010).
- Amariglio, N., et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 6, (2009).
- Ben-Hur, T., et al. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol. Cell Neurosci. 24, 623-631 (2003).
- Giusto, E., Donega, M., Cossetti, C., Pluchino, S. Neuro-immune interactions of neural stem cell transplants: From animal disease models to human trials. Exp. Neurol. , (2013).

