In Vitro Generation of Somite Derivatives from Human Induced Pluripotent Stem Cells
Summary
We present here a protocol for the differentiation of human induced pluripotent stem cells into each somite derivative (myotome, sclerotome, dermatome, and syndetome) in chemically defined conditions, which has applications in future disease modeling and cell-based therapies in orthopedic surgery.
Abstract
In response to signals such as WNTs, bone morphogenetic proteins (BMPs), and sonic hedgehog (SHH) secreted from surrounding tissues, somites (SMs) give rise to multiple cell types, including the myotome (MYO), sclerotome (SCL), dermatome (D), and syndetome (SYN), which in turn develop into skeletal muscle, axial skeleton, dorsal dermis, and axial tendon/ligament, respectively. Therefore, the generation of SMs and their derivatives from human induced pluripotent stem cells (iPSCs) is critical to obtain pluripotent stem cells (PSCs) for application in regenerative medicine and for disease research in the field of orthopedic surgery. Although the induction protocols for MYO and SCL from PSCs have been previously reported by several researchers, no study has yet demonstrated the induction of SYN and D from iPSCs. Therefore, efficient induction of fully competent SMs remains a major challenge. Here, we recapitulate human SM patterning with human iPSCs in vitro by mimicking the signaling environment during chick/mouse SM development, and report on methods of systematic induction of SM derivatives (MYO, SCL, D, and SYN) from human iPSCs under chemically defined conditions through the presomitic mesoderm (PSM) and SM states. Knowledge regarding chick/mouse SM development was successfully applied to the induction of SMs with human iPSCs. This method could be a novel tool for studying human somitogenesis and patterning without the use of embryos and for cell-based therapy and disease modeling.
Introduction
Developing a directed differentiation method for a desired cell type from PSCs is a necessary step for translating the study of PSC-derived cells into clinical applications. Forced expression of key genes is a promising strategy for organ-cell differentiation from PSCs and has improved our understanding of the genetic regulation of cell fate determination, organ morphogenesis, and organization during embryogenesis1. In addition, recapitulating the endogenous signaling environments, using the development of mouse and chick embryos as a roadmap, is considered essential for the directed differentiation of PSCs. However, given the application of PSC-derived cells in clinical studies such as cell-based therapies, the latter strategy is more suitable because it does not require gene manipulation.
Several studies have reported the induction of mesoderm from human and mouse PSCs in chemically defined conditions. Typically, these methods have relied on activin/nodal/transforming growth factor β (TGFβ) signaling and bone morphogenetic protein (BMP) signaling, believed to perform meso-endoderm and mesoderm differentiation, resulting in a low induction efficiency of the paraxial mesoderm (approximately 20%)2. In other words, the PSC-derived mesoderm induced by these signaling pathways was mainly lateral plate mesoderm, and not paraxial mesoderm. Recently, a few studies have demonstrated the efficient production of PSC-derived paraxial mesoderm based on different strategies3,4,5,6,7,8. In these studies, PSCs were cultured with relatively high concentrations of glycogen synthase kinase 3 (GSK3) inhibitors (WNT signaling activators), consequently the induction efficiency of paraxial mesoderm reached 70%–95%6,7.
In somitogenesis, the paraxial mesoderm first forms the presomitic mesoderm (PSM) posteriorly, and then forms somites (SMs) in the anterior part through mesenchyme-to-epithelial transition9,10. Notch ligand Delta-like 1 (DLL1) is known to have a pivotal role during somitogenesis, as oscillatory control of DLL1 expression, both in mRNA and protein level, regulates SM segmentation. SMs eventually subdivide into two parts, giving rise to the dermomyotome (DM) dorsally and sclerotome (SCL) ventrally11. Subsequently, the DM differentiates into the dermatome (D), a precursor of the dermis, and myotome (MYO), a precursor of skeletal muscle; additionally, a ventral portion of SCL forms the syndetome (SYN), a precursor of tendons and ligaments12 (Figure 1). Some researchers have reported the induction of PSC-derived SM derivatives such as MYO4,13 and SCL14; however, there are several limitations in these studies. Notably, since our knowledge of the signaling environments of D and SYN is fragmentary, induction protocols for D and SYN have not yet been systematically established. To demonstrate the full-competence of SMs induced from PSCs, it is essential to show the multi-differentiation capacity of induced SMs into all four derivatives (D, MYO, SCL, and SYN), while previous studies have only focused on specific SM derivatives. Here, we report on how to generate all four SM derivatives, including D and SYN, through PSM and SM fates from human iPSCs15. We believe that establishing an in vitro stepwise method that models the SM development process could contribute to the study of how human SM develops during embryogenesis, without using embryos.
Protocol
All experimental protocols involving human iPSCs were approved by the Ethics Committee of the Department of Medicine and Graduate School of Medicine, Kyoto University.
1. Human iPSCs Preparation Before Induction
NOTE: Culture human iPSCs (201B7-PAX3-GFP) on SNL feeder cells16 with primate ES cell medium supplemented with 4 ng/mL recombinant human basic fibroblast growth factor (FGF2) and 0.5% penicillin and streptomycin (hereinafter referred to as hESC medium, see Table 1). When the confluence ratio reaches 70%–80%, passage the cells as previously described17.
- Passaging of human iPSCs on SNL feeder cells17
- For passaging, add PBS into the cell culturing dish and rinse cells. Then, remove the PBS (hereafter, this process will be referred to as wash with PBS).
- Add 1 mL of CTK solution (see Table 1) at room temperature (RT) and wait until the SNL feeder cells start detaching from the bottom of the dish.
- Remove the CTK solution and then wash twice with PBS.
- Add 1 mL of hESC medium (see Table 1) into the dish, then scrape the cells using a scraper and collect into a 15 mL conical tube.
- Gently pipette the contents five times using a 1,000 µL tip, and then transfer to a new dish filled with hESC medium. Use a split ratio of 1:4 to 1:10, depending on the confluence ratio before passaging. Also, vary the volume of hESC medium depending on the scale of the dish (e.g., 3 mL for a 6 cm dish, 8 mL for a 10 cm dish).
- Incubate the human iPSCs at 37 °C and 5% CO2.
- Change the medium everyday (except for the day after passaging) and culture the cells until the next passaging procedure.
- Feeder-free culturing of human iPSCs before PSM induction
NOTE: To minimize the effect of growth factors secreted from SNL feeder cells, culture the human iPSCs under feeder-free conditions with a feeder-free cell culture medium (see Table 1) on extracellular matrix (ECM) solutions (see Table 1) coated dish for 3 days prior to PSM induction.- Day -4 (4 days prior to starting PSM induction)
- To prepare the ECM solution-coated dish, add 4 mL of ECM solution onto a 10 cm dish at 4 °C overnight.
NOTE: Place ECM solution on ice while preparing.
- To prepare the ECM solution-coated dish, add 4 mL of ECM solution onto a 10 cm dish at 4 °C overnight.
- Day -3
- First, remove the ECM solution from the dish and add 8 mL of feeder-free cell culture medium.
- To start feeder-free culturing, wash once with PBS to rinse the cultured cells.
- Add 1 mL of CTK solution at RT until the SNL feeder cells start detaching from the bottom of the dish.
NOTE: Use microscopy to confirm all feeder cells are detached from the bottom. - Remove the CTK solution and wash with PBS twice, so that all SNL feeder cells are fully removed.
- Add 1 mL of feeder-free cell culture medium into the dish, then scrape the cells using a scraper and collect them into a 15 mL conical tube.
- Gently pipette the contents three times using a 1,000 µL tip, then transfer to a new ECM solution-coated 10 cm dish (prepared during step 1.2.1). Use a split ratio of roughly 1:2 to 1:4, depending on the confluence ratio before feeder-free culturing.
- Incubate the human iPSCs at 37 °C and 5% CO2 for 3 days, changing the medium on day -1.
- Day -4 (4 days prior to starting PSM induction)
2. PSM Differentiation and Isolation by Fluorescence-activated Cell Sorting (FACS)
- PSM differentiation (Day 0–Day 4)
- Aspirate the feeder-free cell culture medium and add 8 mL of PSM induction medium (CDM basal medium supplemented with 10 µM SB431542, 10 µM CHIR99021, 2 µM DMH1, and 20 ng/mL FGF2, see Table 1).
NOTE: Cell confluence at the initiation of PSM differentiation is critical for induction efficiency. Use microscopy to confirm the confluent ratio is approximately 30%. - Incubate cells at 37 °C, with 5% CO2, for 4 days, changing the medium on Day 3.
- Harvest cells for FACS on Day 4 (section 2.2, below).
- Aspirate the feeder-free cell culture medium and add 8 mL of PSM induction medium (CDM basal medium supplemented with 10 µM SB431542, 10 µM CHIR99021, 2 µM DMH1, and 20 ng/mL FGF2, see Table 1).
- Isolation of DLL1 positive PSM cells by fluorescence-activated cell sorting (FACS)
NOTE: Below is a procedure for cell preparation before FACS sorting of DLL1 positive cells. Perform FACS sorting using a flow cytometer, according to the manufacturer’s protocol.- Aspirate the medium, then wash with PBS. Subsequently, add 1 mL of cell dissociation reagent and leave for 3 min at RT.
- Add 4 mL of CDM basal medium, scrape the cells using a scraper, and collect them into a 15 mL conical tube.
- Count the number of cells using an automated cell counter, then centrifuge at 280 x g for 3 min.
- Carefully remove the supernatant by aspiration and resuspend the cells in FACS buffer (see Table 1) at a concentration of 1.0 x 107 cells/mL. For a negative control sample (isotype control, or in convention without antibody), transfer 50 µL into a 15-mL conical tube and then suspend with 450 µL of FACS buffer.
- Add DLL1 antibody (see Table of Materials) in a ratio of 1/200. Protect the tube from light and keep on ice for 30 min.
- Centrifuge at 280 x g for 3 min.
- Carefully aspirate the supernatant and resuspend in FACS buffer (1.0 x 107 cells/mL) supplemented with 1 mg/mL DAPI.
- Transfer into a collection tube, incorporated with a 35 µm nylon mesh in the cap for filtering, then place the tube on ice until sorting is complete. Perform the same procedure with the negative control sample (step 2.2.4).
- Perform sorting using a flow cytometer according to the manufacturer’s protocol.
- Collect the sorted DLL1 positive cells into a 15 mL conical tube, containing 4 mL of CDM basal medium supplemented with 10 µM of Y27632. For total RNA extraction, centrifuge at 280 x g for 3 min then resuspend in RNA lysis buffer and store at -30 °C. See the RNA extraction, reverse transcription, and RT-qPCR procedures (section 5.1) for more detailed information.
- Perform SM differentiation using the sorted cells according to the below protocol (section 3).
3. SM Differentiation from PSM
- SM differentiation from sorted DLL1 positive PSM cells (Day 4–Day 8)
NOTE: Prepare the ECM solution-coated 12-well plates the day before FACS sorting. To prepare an ECM solution-coated 12-well plate, add 1 mL of ECM solution into each well at 4 °C and leave overnight. Keep the ECM solution on ice while preparing.- Following step 2.2.10, centrifuge at 280 x g for 3 min.
- Carefully aspirate the supernatant and resuspend in 1 mL of SM induction medium (CDM basal medium supplemented with 10 µM SB431542 and 5 µM CHIR99021, see Table 1).
- Count the number of cells using an automated cell counter.
- Seed 1.0 x 105 cells onto each well of the ECM solution-coated 12-well plates containing 1 mL of SM induction medium supplemented with 10 µM of Y27632.
- Incubate at 37 °C with 5% CO2 for 4 days until Day 8. Change the medium not containing Y27632 on Day 5 (the day after FACS sorting) and Day 7.
- Perform SM derivatives differentiation using induced SM cells according to the protocols below. For total RNA extraction from induced SM cells, collect the cells into a 15-mL conical tube and centrifuge at 280 x g for 3 min, then resuspend in RNA lysis buffer and store at -30 °C.
4. SM Derivatives (DM, MYO, D, SCL, SYN) Differentiation from SM
NOTE: To demonstrate the full-competence of SM cells, first perform DM (dermomyotome) and SCL (sclerotome) induction accordingly using iPSC-derived SM cells. Subsequently, perform MYO (myotome) and D (dermatome) induction using the DM cells, and conduct SYN (syndetome) induction using the SCL cells. Below are the protocols for the induction of each derivative (DM, MYO, D, SCL, and SYN) from induced SM cells in vitro.
- DM differentiation from SM cells (Day 8–Day 11)
- Aspirate the medium, then add 1 mL of DM induction medium (CDM basal medium supplemented with 5 µM CHIR99021 and 10 ng/mL BMP4, see Table 1).
- Incubate the cells at 37 °C, with 5% CO2, for 3 days until Day 11. Change the medium on Day 10 (Day 2 of DM induction).
- Perform MYO and D differentiation using induced DM cells according to the protocols below.
- MYO differentiation from DM cells (Day 11–Day 41)
- Aspirate the medium, then add 1 mL of MYO induction medium (CDM basal medium supplemented with 5 µM CHIR99021, see Table 1).
- Incubate the cells at 37 °C, with 5% CO2, for 30 days until Day 41. Change the medium every 3 days.
- D differentiation from DM cells (Day 11–Day 20)
- Aspirate the medium, then add 1 mL of D induction medium (CDM basal medium supplemented with 5 µM CHIR99021 and 10 ng/mL BMP4, see Table 1).
- Incubate the cells at 37 °C, with 5% CO2, for 9 days until Day 20. Change the medium every 3 days.
- SCL differentiation from SM cells (Day 8–Day 11)
- Aspirate the medium, then add 1 mL of SCL induction medium (CDM basal medium supplemented with 100 nM SAG and 0.6 µM LDN193189, see Table 1)14.
- Incubate the cells at 37 °C, with 5% CO2, for 3 days. Change the medium on Day 10 (Day 2 of SCL induction).
- Perform SYN differentiation using induced SCL cells according to the protocol below.
- SYN differentiation from SCL cells (Day 11–Day 32)
NOTE: Prepare the ECM solution-coated 24-well plates the day before initiating SYN induction. To prepare an ECM solution-coated 24-well plate, add 0.5 mL of ECM solution into each well at 4 °C and leave overnight. Keep the ECM solution on ice while preparing.- Aspirate the medium then wash with PBS, then add 0.2 mL of cell dissociation reagent to each well and leave for 3 min at RT.
- Add 0.8 mL of CDM basal medium to each well then scrape and collect all cells into a 15 mL conical tube.
- Centrifuge at 280 x g for 3 min.
- Carefully aspirate the supernatant and resuspend in 1 mL of SYN induction medium-1 (CDM basal medium supplemented with 20 ng/mL FGF8, see Table 1), then count the number of cells using an automated cell counter.
- Seed 5.0 x 104 cells into each well of the ECM solution-coated 24-well plates containing 1 mL of SYN induction medium-1.
- Incubate at 37 °C, with 5% CO2, for 3 days.
- On Day 14 (Day 3 of SYN induction), replace the medium with SYN induction medium-2 (CDM basal medium supplemented with 10 ng/mL BMP7 and 10 ng/mL TGFβ3, see Table 1).
- Incubate at 37 °C, with 5% CO2, for 18 days until Day 32. Change the medium every 3 days.
5. Characterization of iPSC-derived Products
NOTE: Upon differentiation, characterize human iPSCs derivatives using quantitative real-time PCR (RT-qPCR), immunocytochemistry (ICC), enzyme-linked immunosorbent assays (ELISA), and mechanical stretch stimulation assays, accordingly.
- Cell harvest, total RNA extraction, reverse transcription, and quantitative real-time PCR (RT-qPCR) analysis
- Collect the cell samples (procedures 2.2.10, 3.1.6, 5.4.3) into a 1.5 mL tube, then centrifuge at 280 x g for 3 min.
- Remove the supernatant, then resuspend in 350 µL of RNA lysis buffer, provided by an appropriate total RNA extraction kit.
- Extract total RNA using the kit according to the manufacturer’s protocol.
- Reverse transcribe the isolated 1 µg of total RNA to cDNA, according to the manufacturer’s protocol.
- Perform RT-qPCR using suitable enzymes, reagents, and primers according to the manufacturer’s protocol. The primer sequences used in this study are listed in Table 2.
- Immunocytochemistry (ICC)
- Prior to performing immunocytochemistry with antibodies, fix the cells with 2% paraformaldehyde at 4 °C for 10 min, and wash twice with PBS.
- For permeabilization, incubate with 0.2% methanol or 0.2% polysorbate 20/PBS (hereinafter referred to as PBS-T) at 4 °C for 15 min.
- Remove the permeabilization reagents and treat the cells with an appropriate blocking buffer or 1% bovine serum albumin/PBS at 4 °C for 60 min.
- Add the first antibody diluted with 10% blocking buffer in PBS-T and place on a shaking machine at 4 °C overnight.
- Wash three times with PBS-T (add PBS-T and place on the shaking machine at RT for 10 min).
- Add the second antibody, diluted with 10% blocking buffer in PBS-T and place on the shaking machine at RT for 60 min. The first and second antibodies for ICC used in this study are listed in Table 3.
NOTE: From this step onwards, protect the plate/dish from light by wrapping it in foil. - Wash twice with PBS-T.
- For counter staining, add 1/5000 DAPI diluted with PBS and place on the shaking machine at RT for 5 min.
- Remove the DAPI solution and add PBS into each well.
- Observe the cell staining using a fluorescence microscope. Alternatively, store the plate/dish at 4 °C for up to 1 month.
- Enzyme-linked Immunosorbent assay (ELISA) for functional analysis of iPSC-derived D
NOTE: Human dermal fibroblast cells (HDF) are commercially available. Culture HDF in DMEM supplemented with 10% fetal bovine serum (see Table 1).- Seed 1.0 x 105 cells of iPSC-derived D and HDF onto 24-well plates containing 1 mL of each culture medium (D: D induction medium, HDF: DMEM supplemented with 10% fetal bovine serum).
- After 3 days of cell culturing, collect 100 µL of each medium, place into a 1.5 mL tube, and store at 4 °C.
- Perform the series of procedures, such as the addition of detection antibodies and secondary antibodies, according to the manufacturer’s instruction, and quantify the number of targets by generating a standard curve against the concentration of the control analyte samples.
- Mechanical stretch stimulation assay for functional analysis of iPSC-derived SYN
NOTE: Adult human tenocytes are commercially available (see Table of Materials). Culture human iPSC-derived SYN and adult human tenocytes on a cell stretching device for the mechanical stretch stimulation assay as described below18,19. Use SYN induction medium-2 and tenocytes growth medium (see Table of Materials) as a culturing medium for each iPSC-derived SYN and adult human tenocytes, respectively.- At 24 h before stretching, plate 1.0 x 105 cells of iPSC-derived SYN and human tenocytes onto ECM solution-coated multi well-type silicon rubber chambers, each with a culture surface of 1.5 cm x 1.5 cm (see Table of Materials).
- Set the chambers on the device for cell stretching, and force monoaxial cyclic strain (0.5 Hz, 5%) for 12 h.
- For total RNA extraction, add 350 µL of RNA lysis buffer, then scrape and collect the cells into a 1.5 mL tube for total RNA extraction and subsequent RT-qPCR analysis (see procedure 5.1).
Representative Results
All figures in this report were obtained with 201B7-PAX3-GFP iPSCs, in which EGFP replaces one allele of the PAX3 coding sequence in exon 1. Establishment of 201B7-PAX3-GFP iPSCs will be described elsewhere (H. Sakurai, personal communication). The statistical significance was evaluated using statistical software. P-values lower than 0.05 were considered significant.
Characterization of human iPSC-derived PSM and SM cells
To assess the differentiation of human iPSCs toward SM through the PSM state (Figure 2A), FACS analysis, ICC analysis, and RT-qPCR analysis were performed. As shown in Figure 2B, over 85% of the cells were positive for DLL1, a marker of PSM, but negative for PAX3, a marker of SM, after 4 days of PSM induction with human iPSCs. Subsequently, this population became PAX3 positive SM cells after 4 days of SM induction. The PSM-SM transition was also confirmed by ICC (Figure 2C) and RT-qPCR (Figure 2D). TBX6, MSGN1, and WNT3A, PSM markers were expressed at the PSM state (day 4), but not expressed at the SM state (day 8). PARAXIS, MEOX1, and PAX3, SM markers, were expressed at SM, but not expressed at PSM. Furthermore, staining of CDH11, a marker of epithelialized SM, only accumulated at the cell-cell junction, following the addition of SB431542 with CHIR99021 (Figure 2E).
Characterization of SM derivatives induced from human iPSC-derived SM cells
To evaluate the differentiation potency of human iPSC-derived SM, differentiation toward DM, MYO, D, SCL, and SYN (Figure 3A) was assessed by ICC analysis and PAX3 (GFP)-fluorescence. As shown in Figure 3B, DM differentiation was confirmed by ALX4 and EN1 staining, and PAX3 (GFP)-fluorescence; MYO differentiation was confirmed by MYOD, MYOG, and Myosin heavy chain (MHC) staining; D differentiation was confirmed by EN1 and PDGFRa staining; SCL differentiation was confirmed by PAX1, PAX9, and NKX3.2 staining; and SYN differentiation was confirmed by SCX, MKX, COL1A1, and COL1A2 staining.
Characterization of induced D and SYN
1. Enzyme-linked immunosorbent assay (ELISA) for functional analysis of iPSC-derived D
In the human body, one of the primary functions of dermal fibroblasts is to secrete extracellular matrix (ECM) proteins, such as collagen and hyaluronic acid that hydrate the skin and help sustain the skin structure. To demonstrate that a comparable amount of collagen-type 1 and hyaluronic acid proteins were secreted in the culture medium of iPSC-derived D and HDF, ELISA was performed, as shown in Figure 4A.
2. Mechanical stretch stimulation assay for functional analysis of iPSC-derived SYN
As several studies have already reported, mechanical stimulation affects tendon development before and after birth, and promotes the differentiation of tenocytes from precursor cells18,19. Therefore, it is well known that reactivity to mechanical stress is one of the characteristics of tenocytes. To demonstrate the comparable reactivity of human iPSC-derived SYN and human adult tenocytes, a mechanical stretch stimulation assay was performed as shown in Figure 4B.
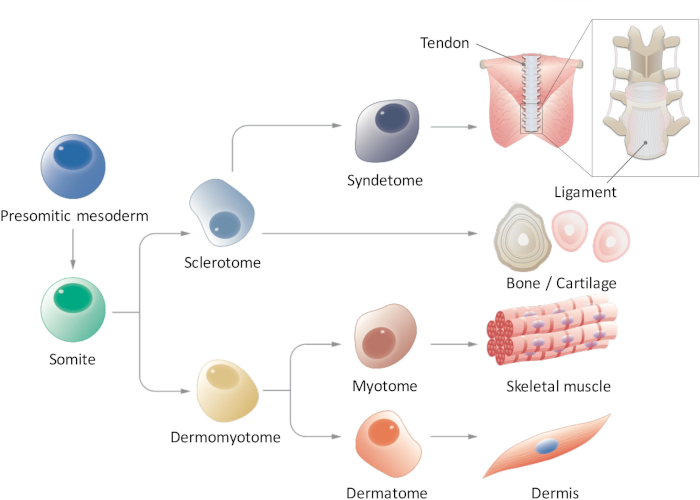
Figure 1: Schematic view of hierarchical differentiation of paraxial mesoderm. Presomitic mesoderm is a cell population that transiently emerges during early embryogenesis and undergoes segmentation to form somites. Somites are a transient stem cell population that gives rise to multiple cell types, such as sclerotome, dermomyotome, syndetome, dermatome, and myotome cells, which eventually differentiate into tendon/Ligament, bone/cartilage, skeletal muscle, and dermis cells. Please click here to view a larger version of this figure.
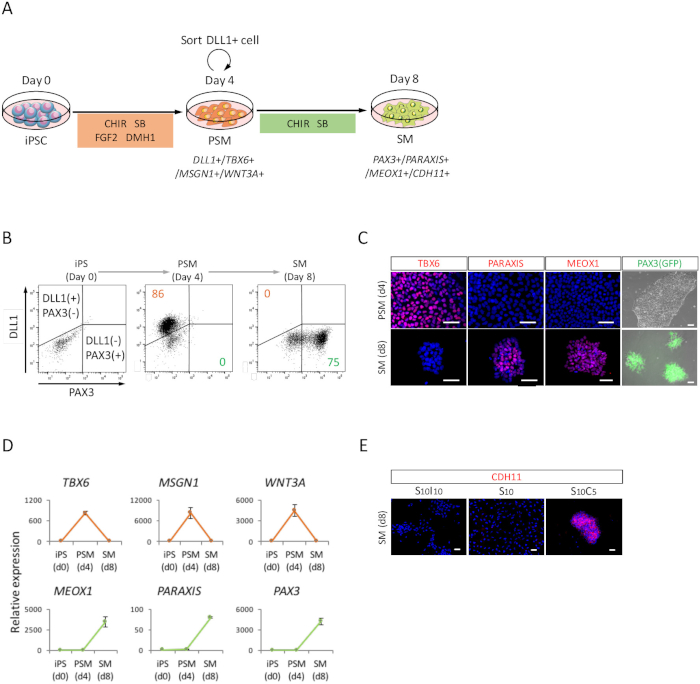
Figure 2: FACS, RT-qPCR, and ICC analysis of human iPSC-derived PSM and SM. (A) Schematic view of a protocol for SM differentiation through PSM. (B) Representative dot plot of DLL1 staining and PAX3 (GFP)-fluorescence on day 4 of PSM induction and day 4 (day 8 from iPSC) of SM induction. (C) Representative immunocytochemical images and PAX3 (GFP)-fluorescence on day 4 of PSM induction and day 4 (day 8 from iPSC) of SM induction. Cells were stained with anti-TBX6, PARAXIS, and MEOX1 antibodies (red) and co-stained with DAPI (blue) or detected with PAX3 (GFP)-fluorescence (green). (D) RT-qPCR analysis of markers for PSM and SM at iPSC, PSM and SM. The means ± standard error (S.E.) from three sets of experiments are shown. (E) Representative immunocytochemical images on day 4 (day 8 from iPSC) of SM, cultured in S10I10 (combination of SB431542 and IWR1, an inhibitor of WNT signaling), S10 (SB431542), and S10C5 (combination of SB431542 and CHIR99021) conditions. Cells were stained with anti-CDH11 antibody (red) and co-stained with DAPI (blue). iPS, induced pluripotent stem cell; PSM, presomitic mesoderm; SM, somite; S10, SB431542 10 μM; C5, CHIR99021 5 μM; I10, IWR1 10 μM; Scale bars = 50 μm. This figure has been modified from Nakajima et al. (2018)15. Please click here to view a larger version of this figure.
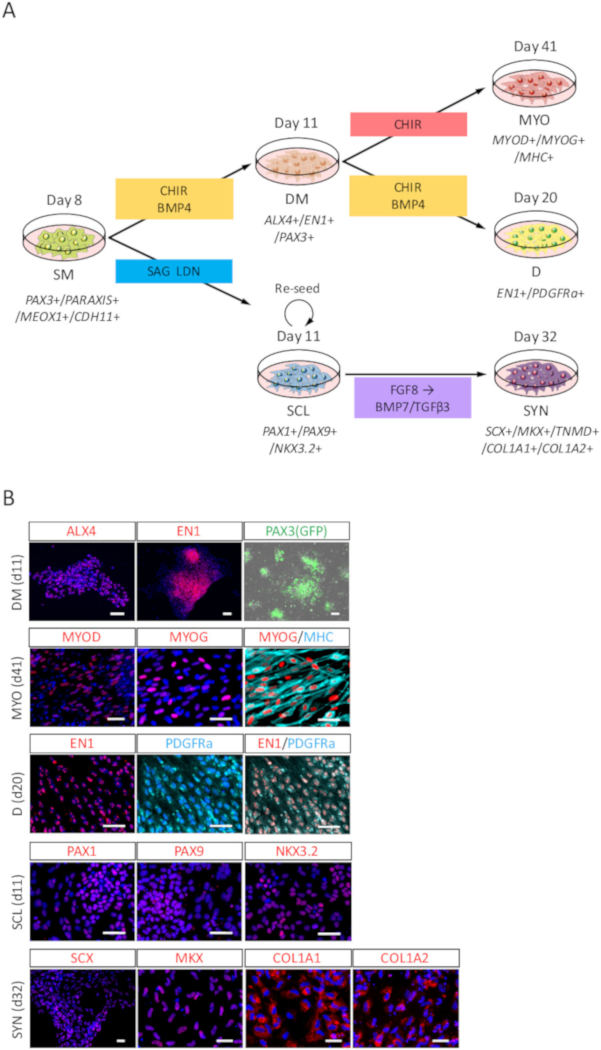
Figure 3: ICC analysis of DM, MYO, D, SCL, and SYN differentiated from human iPSC-derived SM. (A) Schematic view of protocols for SM derivatives differentiation. (B) Representative immunocytochemical images and PAX3 (GFP)-fluorescence on day 3 (day 11 from iPSC) of DM induction, day 30 (day 41 from iPSC) of MYO induction, day 9 (day 20 from iPSC) of D induction, day 3 (day 11 from iPSC) of SCL induction, and day 21 (day 32 from iPSC) of SYN induction. DM, cells were stained with anti-ALX4 and EN1 antibodies (red) and co-stained with DAPI (blue) or detected with PAX3 (GFP)-fluorescence (green); MYO, cells were stained with anti-MYOD, MYOG (red), and MHC (cyan) antibodies, also co-stained with DAPI (blue); D, cells were stained with anti-EN1 (red) and PDGFRa (cyan) antibodies and co-stained with DAPI (blue); SCL, cells were stained with anti-PAX1, PAX9, and NKX3.2 (red) antibodies, and co-stained with DAPI (blue); SYN, cells were stained with anti-SCX, MKX, COL1A1, and COL1A2 (red) antibodies, and co-stained with DAPI (blue). DM, dermomyotome; MYO, myotome; D, dermatome; SCL, sclerotome; SYN, syndetome; Scale bars = 50 μm. This figure has been modified from Nakajima et al. (2018)15. Please click here to view a larger version of this figure.
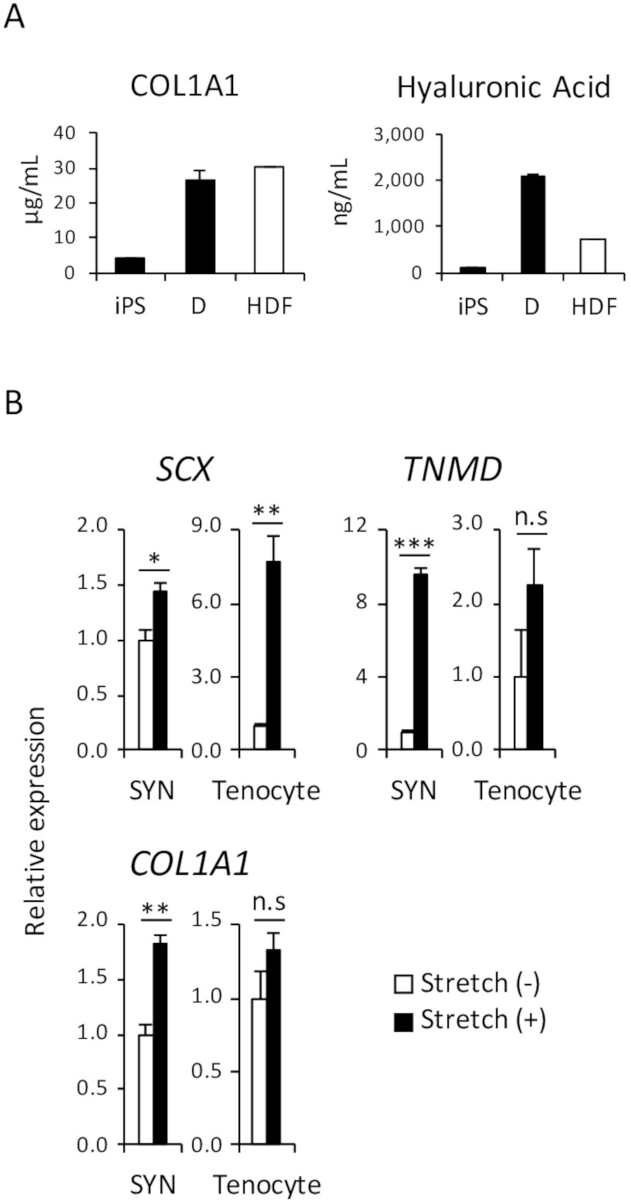
Figure 4: Functional assay of induced D and SYN. (A) The amount of collagen-type 1 and hyaluronic acid proteins in the culture medium were analyzed by ELISA. (B) The effect of mechanical stretch stimulation on induced SYN and human adult tenocytes was assessed by RT-qPCR. The means ± standard error (S.E.) from three sets of experiments are shown. *p < 0.05; **p < 0.01; *** p < 0.001 by Dunnett’s multiple comparisons t-test compared to Stretch (-); n.s, not significant, HDF, human adult dermal fibroblast. This figure has been modified from Nakajima et al. (2018)15. Please click here to view a larger version of this figure.
| Medium/solution | Reagant | Concentration |
| CDM basal medium | Iscove’s modified Dulbecco’s medium/Ham’s F12 | 1:1 |
| Penicillin/Streptomycin | 0.5 % | |
| Chemically defined lipid concentrate | 1 % | |
| Apo-transferrin | 15 mg/mL | |
| Monothioglycerol | 450 mM | |
| Bovine serum albumin | 5 mg/mL | |
| Insulin | 7 mg/mL | |
| CTK solution | Water | – |
| Trypsin | 0.25 % | |
| Collagenase IV | 0.1 mg/mL | |
| Calcium chloride | 1 mM | |
| Knockout SR | 20 % | |
| D induction medium | CDM basal medium | – |
| CHIR99021 | 5 µM | |
| BMP4 | 10 ng/mL | |
| DM induction medium | CDM basal medium | – |
| CHIR99021 | 5 µM | |
| BMP4 | 10 ng/mL | |
| ECM solution | Artificial extracellular matrix | 0.3 mg/mL |
| DMEM/F12 | – | |
| FACS buffer | PBS | – |
| Bovine serum albumin | 0.1 % | |
| Feeder-free cell culture medium | mTeSR1 | – |
| Penicillin/Streptomycin | 0.5 % | |
| HDF culture medium | DMEM | – |
| Fetal bovine serum | 10 % | |
| hESC medium | Primate ES cell medium | – |
| Penicillin/Streptomycin | 0.5 % | |
| FGF2 | 4 ng/mL | |
| MYO induction medium | CDM basal medium | – |
| CHIR99021 | 5 µM | |
| PSM induction medium | CDM basal medium | – |
| SB431542 | 10 µM | |
| CHIR99021 | 10 µM | |
| DMH1 | 2 µM | |
| FGF2 | 20 ng/mL | |
| SCL induction medium | CDM basal medium | – |
| SAG | 100 nM | |
| LDN193189 | 0.6 µM | |
| SM induction medium | CDM basal medium | – |
| SB431542 | 10 µM | |
| CHIR99021 | 5 µM | |
| SYN induction medium-1 | CDM basal medium | – |
| FGF8 | 20 ng/mL | |
| SYN induction medium-2 | CDM basal medium | – |
| BMP7 | 10 ng/mL | |
| TGFβ3 | 10 ng/mL |
Table 1: Media and solution recipes.
| NAME | Forward | Reverse |
| ACTB | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
| COL1A1 | GGACACAGAGGTTTCAGTGGT | GCACCATCATTTCCACGAGC |
| MEOX1 | GAGATTGCGGTAAACCTGGA | GAACTTGGAGAGGCTGTGGA |
| MSGN1 | GGAGAAGCTCAGGATGAGGA | GTCTGTGAGTTCCCCGATGT |
| PARAXIS | TCCTGGAGAGCTGTGAGGAT | CACACCCTGTCACCAACAGT |
| PAX3 | AGGAAGGAGGCAGAGGAAAG | CAGCTGTTCTGCTGTGAAGG |
| SCX | CCCAAACAGATCTGCACCTTC | GCGAATCGCTGTCTTTCTGTC |
| TBX6 | AGCCTGTGTCTTTCCATCGT | AGGCTGTCACGGAGATGAAT |
| TNMD | CCCTTCATGCTGAAGCCACTT | CTCACTTTCAGCAGAATTGGGG |
| WNT3A | CAAGATTGGCATCCAGGAGT | ATGAGCGTGTCACTGCAAAG |
Table 2: Primer sequences for RT-qPCR analysis.
| Concentration | ||
| 1st Antibody |
ALX4_Goat | 1/50 |
| CDH11_Mouse | 1/1000 | |
| COL1A1_Rabbit | 1/100 | |
| COL2A1_Mouse | 1-2 μg/mL | |
| EN1_Rabbit | 1/50 | |
| MEOX1_Rabbit | 1/50 | |
| MHC_Rabbit | 1/200 | |
| MKX_Rabbit | 1/50 | |
| MYOD_Rabbit | 1/500 | |
| MYOG_Mouse | 1/400 | |
| NKX3.2_Rabbit | 1/50 | |
| PARAXIS_Rabbit | 1/50 | |
| PAX1_Rabbit | 1/50 | |
| PAX9_Rabbit | 1/50 | |
| PDGFRa_Goat | 1/100 | |
| SCX_Rabbit | 1/50 | |
| TBX6_Goat | 1/50 | |
| 2nd Antibody |
Donkey anti Goat IgG(H+L) secondary antibody555 | 1/500 |
| Donkey anti Goat IgG(H+L) secondary antibody647 | 1/500 | |
| Goat anti Mouse IgG(H+L) secondary antibody555 | 1/500 | |
| Goat anti Rabbit IgG(H+L) secondary antibody555 | 1/500 | |
| Goat anti Rabbit IgG(H+L) secondary antibody647 | 1/500 |
Table 3: First and second antibodies for ICC.
Discussion
A well-known method for the induction of PSC-derived SM through PSM is the combination of CHIR99021 + A83-01 (TGFβ inhibitor) during PSM induction from PSC, but not during the PSM maturation process6. In the present study, WNT/beta-catenin signaling was inhibited using C59 to induce SM from PSM. However, we introduced the use of CHIR99021 to activate the WNT pathway during SM differentiation. This decision was made based on the finding that several WNTs are expressed in the surrounding tissues of SM and given the fact that WNT reporters are active in SM20. As a result, we observed epithelialization, a characteristic of SM in vivo, only under the condition with CHIR99021, based on the accumulation of CDH11 in cell-cell junctions (Figure 2E). This observation indicates the critical involvement of WNT signaling during PSM differentiation and SM epithelialization, therefore our protocol may better recapitulate the endogenous signaling environment. However, it also implies a further possibility of fine-tuning the WNT/beta-catenin signaling pathway during differentiation, because robustness and efficiency of differentiation might vary significantly depending on the cell types, cell lines, and various chemical compounds of WNT-inducers used by each researcher.
This method also allows us to generate all four SM derivatives, MYO, D, SCL, and SYN, from human iPSCs. Our stepwise protocols using CDM can be used to identify the signaling requirements during human somitogenesis/somite patterning, and provide important insights into human SM development. For example, our methods could be useful for studying segmentation clock mechanisms, a molecular oscillation system that regulates the formation of SM. It has been thoroughly investigated in mice, chicks, and zebrafish, but not in humans due to the lack of appropriate experimental tools.
Moreover, our method can be applicable to future clinical cell-based therapies. For example, human iPSC-derived D or SYN can be transplanted into severely injured skin or ruptured tendons for regeneration and treatment. However, several limitations need to be resolved before this method can be practically applied. Although in the present study, we used SNL feeder cells for iPSC maintenance and ECM solution, which is extracted from the Engelbreth-Holm-Swarm mouse sarcoma, as a surface coat on the dish during induction, these non-human animal-derived reagents should be removed to improve clinical quality. In addition, cell quantity and quality, which includes the purity and maturation of the desired cells, must also be improved. Furthermore, not only the cell number but also the cell strength is an important characteristic for tendon/ligament regeneration. Additionally, the development of surface markers for purification and a novel method for 3D reconstitution are indispensable in order to advance our protocols to clinical cell-based therapies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Junya Toguchida (CiRA) for his help with project administration and funding acquisition, Mr. Mitsuaki Shibata (CiRA) and Ms. Mei Terashima (CiRA) for their technical assistance, Dr. Yayoi Toyooka (CiRA) and Dr. Daisuke Kamiya (CiRA) for their proofreading of the manuscript, and Mr. Masaya Todani (CiRA) for providing an illustration (Figure 1). We also thank all the members of the Ikeya and Toguchida laboratories (CiRA) for their support during this study. This work was supported by Grants-in-aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (26670661), the Program for Intractable Diseases Research Utilizing Disease-Specific iPS Cells from the Japan Science and Technology Agency (JST) and the Japan Agency for Medical Research and Development (AMED), the Core Center for iPS Cell Research of the Research Center Network for the Realization of Regenerative Medicine (JST/AMED), and the iPS Cell Research Fund (in part to Makoto Ikeya and Junya Toguchida). Makoto Ikeya was also supported by Grants-in-aid for Scientific Research (JSPS) (16H05447) and the Acceleration Program for Intractable Diseases Research utilizing Disease-specific iPS cells (AMED).
Materials
| ALX4_Goat antibody | Santacruz | sc-22066 | |
| Apo-transferrin | Sigma | T1147 | |
| BMP4 | R&D | 314-BP-010 | |
| BMP7 | R&D | 354-BP-010 | |
| Bovine serum albumin | Sigma | A8806 | |
| Calcium chloride | Nacalai tesque | 067730-15 | |
| CDH11_Mouse antibody | Cell signaling | 13577 | |
| Cell streching device | Strex | STB-140 | |
| Chemically defined lipid concentrate | Gibco | 11905-031 | |
| CHIR99021 | Axon | 1386 | |
| COL1A1_Rabbit antibody | Abcam | ab34710 | |
| COL2A1_Mouse antibody | Thermo scientific | MS-235 | |
| Collagenase IV | Thermofisher | 17104019 | |
| DLL1 APC-conjugated_Mouse antibody | R&D | FAB1818A | For FACS |
| DMEM | Sigma | D6046 | |
| DMEM/F12 | Gibco | 11320-082 | |
| DMH1 | Tocris | 4126 | |
| EN1_Rabbit antibody | Abcam | ab70993 | |
| Fetal bovine serum | Nichirei | 171012 | |
| FGF2 | Wako | 060-04543 | |
| FGF8 | Peprotech | 100-25 | |
| Human dermal fibroblast | Cell applications | 160-05a | |
| Human tenocyte | Angio proteomie | cAP-0041 | |
| Insulin | Wako | 090-06474 | |
| Iscove’s modified Dulbecco’s medium/Ham’s F12 | Gibco | 21056023 | |
| Knockout SR | Gibco | 10828028 | |
| LDN193189 | Axon | 1509 | |
| Matrigel | BD bioscience | 354230 | Artificial extracellular matrix |
| MEOX1_Rabbit antibody | Abcam | ab75895 | |
| MHC_Rabbit antibody | Santacruz | sc-20641 | |
| MKX_Rabbit antibody | Atlas antibodies | A83377 | |
| Monothioglycerol | Sigma | M6145 | |
| mTeSR1 | Stemcell tech | 85850 | |
| Multi well-type silicon rubber chamber | Strex | STB-CH-4W | |
| MYOD_Rabbit antibody | Abcam | ab133627 | |
| MYOG_Mouse antibody | Santacruz | sc-12732 | |
| NKX3.2_Rabbit antibody | Sigma | HPA027564 | |
| Novex Donkey anti Goat IgG(H+L) secondary antibody555 | Invitrogen | A21432 | |
| Novex Donkey anti Goat IgG(H+L) secondary antibody647 | Invitrogen | A21447 | |
| Novex Goat anti Mouse IgG(H+L) secondary antibody555 | Invitrogen | A21422 | |
| Novex Goat anti Rabbit IgG(H+L) secondary antibody555 | Invitrogen | A21428 | |
| Novex Goat anti Rabbit IgG(H+L) secondary antibody647 | Invitrogen | A21245 | |
| PARAXIS_Rabbit antibody | Santacruz | sc-98796 | |
| PAX1_Rabbit antibody | Abcam | ab95227 | |
| PAX9_Rabbit antibody | Gene tex | GTX104454 | |
| PBS | – | – | |
| PDGFRa_Goat | R&D | AF307 | |
| Penicillin/Streptomycin | Invitrogen | 15140-122 | |
| Primate ES cell medium | Reprocell | RCHEMD001 | |
| SAG | Calbiochem | 566661 | |
| SB431542 | Selleckchem | SEL-S1067-10 | |
| SCX_Rabbit antibody | Abcam | ab58655 | |
| TBX6_Goat antibody | R&D | AF4744 | |
| Tendon cell growth medium | Angio-proteomie | cAP-40 | Tenocytes growth medium |
| TGFβ3 | R&D | 243-B3-200 | |
| Trypsin | Gibco | 15090046 |
References
- Tanaka, A., et al. Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PLoS One. 8 (4), e61540 (2013).
- Sakurai, H., et al. In vitro modeling of paraxial mesodermal progenitors derived from induced pluripotent stem cells. PLoS One. 7 (10), e47078 (2012).
- Chal, J., et al. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nature Protocl. 11 (10), 1833-1850 (2016).
- Chal, J., et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nature Biotechnology. 33 (9), 962-969 (2015).
- Umeda, K., et al. Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Scientific Reports. 2, 455 (2012).
- Loh, K. M., et al. Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell. 166 (2), 451-467 (2016).
- Xi, H., et al. In Vivo Human Somitogenesis Guides Somite Development from hPSCs. Cell Reports. 18 (6), 1573-1585 (2017).
- Chal, J., Pourquie, O. Making muscle: skeletal myogenesis in vivo and in vitro. Development. 144 (12), 2104-2122 (2017).
- Tam, P. P., Beddington, R. S. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 99 (1), 109-126 (1987).
- Aulehla, A., Pourquie, O. Signaling gradients during paraxial mesoderm development. Cold Spring Harbor Perspectives in Biology. 2 (2), a000869 (2010).
- Christ, B., Scaal, M. Formation and differentiation of avian somite derivatives. Advances in Experimental Medicine and Biology. 638, 1-41 (2008).
- Brent, A. E., Schweitzer, R., Tabin, C. J. A somitic compartment of tendon progenitors. Cell. 113 (2), 235-248 (2003).
- Sakurai, H., et al. Bidirectional induction toward paraxial mesodermal derivatives from mouse ES cells in chemically defined medium. Stem Cell Research. 3 (2-3), 157-169 (2009).
- Zhao, J., et al. Small molecule-directed specification of sclerotome-like chondroprogenitors and induction of a somitic chondrogenesis program from embryonic stem cells. Development. 141 (20), 3848-3858 (2014).
- Nakajima, T., et al. Modeling human somite development and fibrodysplasia ossificans progressiva with induced pluripotent stem cells. Development. 145 (16), (2018).
- McMahon, A. P., Bradley, A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 62 (6), 1073-1085 (1990).
- Takahashi, K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131 (5), 861-872 (2007).
- Suzuki, H., et al. targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proceedings of the National Academy of Sciences of the United States of America. 113 (28), 7840-7845 (2016).
- Marturano, J. E., Arena, J. D., Schiller, Z. A., Georgakoudi, I., Kuo, C. K. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proceedings of the National Academy of Sciences of the United States of America. 110 (16), 6370-6375 (2013).
- Maretto, S., et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proceedings of the National Academy of Sciences of the United States of America. 100 (6), 3299-3304 (2003).

