A Three-Dimensional Digital Model for Early Diagnosis of Hepatic Fibrosis Based on Magnetic Resonance Elastography
Summary
The objective of this study was to develop a novel three-dimensional digital model for the early diagnosis of hepatic fibrosis, which includes the stiffness of each voxel in the patient’s liver and can thus, be used to calculate the distribution ratio of the patient’s liver at different fibrosis stages.
Abstract
Hepatic fibrosis is an early stage of liver cirrhosis, and there are no better non-invasive and convenient methods for the detection and evaluation of the disease. Despite the good progress made with the liver stiffness map (LSM) based on magnetic resonance elastography (MRE), there are still some limitations that need to be overcome, including manual focus determination, manual selection of regions of interest (ROIs), and discontinuous LSM data without structural information, which makes it impossible to evaluate the liver as a whole. In this study, we propose a novel three-dimensional (3D) digital model for the early diagnosis of hepatic fibrosis based on MRE.
MRE is a non-invasive imaging technique that employs magnetic resonance imaging (MRI) to measure the liver stiffness at the scanning site through human-computer interaction. Studies have indicated a significant positive correlation between the LSM obtained through MRE and the degree of hepatic fibrosis. However, for clinical purposes, a comprehensive and precise quantification of the degree of hepatic fibrosis is necessary. To address this, the concept of Liver Stiffness Distribution (LSD) was proposed in this study, which refers to the 3D stiffness volume of each liver voxel obtained by the alignment of 3D liver tissue images and MRE indicators. This provides a more effective clinical tool for the diagnosis and treatment of hepatic fibrosis.
Introduction
Hepatic fibrosis refers to the formation of excessive scar tissue in the liver, usually as a result of liver damage or disease1,2,3,4. It frequently arises as a consequence of chronic liver injury and is commonly associated with liver diseases, such as chronic viral hepatitis, non-alcoholic fatty liver disease, and alcoholic liver disease. If left untreated, hepatic fibrosis can progress to cirrhosis, a potentially life-threatening condition associated with significant morbidity and mortality.
Active research in this area aims to elucidate the cellular and molecular mechanisms underlying the pathogenesis of hepatic fibrosis, as well as to develop novel diagnostic and therapeutic strategies to improve patient outcomes. Another objective is the noninvasive detection of the hepatic fibrosis stage, which is a critical aspect that directly correlates with disease diagnosis, treatment selection, and prognosis evaluation. Despite the importance of accurate diagnosis and the monitoring of hepatic fibrosis, traditional diagnostic methods, such as liver biopsy, are invasive and associated with significant risks. In contrast, magnetic resonance elastography5,6 (MRE) is a promising non-invasive imaging technique that has demonstrated potential in the diagnosis and monitoring of hepatic fibrosis by quantifying liver stiffness.
In recent years, there has been significant research focused on evaluating the accuracy and reliability of MRE in the diagnosis of hepatic fibrosis, as well as its potential advantages over traditional diagnostic methods. The liver stiffness metric of MRE has been granted approval by the United States Food and Drug Administration (FDA) for clinical diagnosis, and extensive comparative analysis with pathological results has been conducted in clinical practice. The results have shown that the stiffness maps generated by MRE exhibit a strong positive correlation with various stages of liver fibrosis7,8,9,10,11,12. Yet so far, the work of accurately evaluating and tracking the progression of liver fibrosis in patients through quantitative analysis of liver stiffness distribution (LSD) by matching liver structure images with MRE has not made much progress.
In this study, the medical imaging group analysis technique13,14,15 is introduced to achieve accurate alignment of the liver structure images with the stiffness map generated by MRE in 3D space, enabling the calculation of liver stiffness values for each voxel of the entire liver. Based on the 3D-digital model of LSD, the exact distribution of patient-specific liver fibrosis staging can be calculated and evaluated. This lays a solid foundation for the precise quantitative diagnosis of early-stage liver fibrosis.
Protocol
This study utilized 3D-digital LSD modeling to reconstruct the liver of a typical patient with clinically confirmed hepatic fibrosis. The patient was recruited from a well-known liver disease treatment institution, "You An Hospital" in Beijing, China, and underwent routine upper abdominal magnetic resonance imaging (MRI) and MRE imaging after providing consent. The patient was chosen as the case study for this research method due to the confirmation of hepatic fibrosis staging through pathological examination and the absence of obvious clinical symptoms, which emphasizes the applicability and clinical value of this research in diagnosing early-stage hepatic fibrosis patients. This paper also provides a quantitative comparison between the liver of this patient and a healthy liver. The software tools used in this study are listed in the Table of Materials.
1. Data collection and preparation
NOTE: The parameter difference is not sensitive to the research method.
- MRI scanning strategies
NOTE: This study utilized actual DICOM data obtained from clinical imaging using a magnetic resonance imaging (MRI) device manufactured by GE. The content of the data includes IDEAL (Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation) water-fat separation imaging and magnetic resonance elastography (MRE) imaging.- Ensure that the IDEAL data have a horizontal resolution of 256 pixels by 256 pixels, with a pixel spacing of 1.5625 mm and a slice thickness of 10 mm.
NOTE: The scanning strategy could be further optimized, but the methodology employed in this study is applicable to higher-precision medical imaging.
- Ensure that the IDEAL data have a horizontal resolution of 256 pixels by 256 pixels, with a pixel spacing of 1.5625 mm and a slice thickness of 10 mm.
- Rename the folder of every sequence.
NOTE: As the DICOM data exported from the equipment does not explicitly provide sequence names, during the preprocessing stage, it is necessary to add explicit names for each sequence to facilitate subsequent analysis and processing.- Copy all DICOM data to a customized working directory.
- Navigate to the directory containing the data in MATLAB's working directory.
- Execute the Description_Name function to add descriptive names to the folders for each sequence.
- See Figure 1 for a comparison before and after renaming. Add a Description Name to each image sequence folder to facilitate the identification of the necessary image sequences for various analytical purposes.
- Quickly check images of IDEAL.
- Change the directory of different phases' folders, including the in-phase, out-phase, water, and fat phases, which were stored in separate folders for imaging using IDEAL.
- Execute the Slice_View function to view the impact sequences for each phase.
- See Figure 2 for an image of the interactive graphic user interface (GUI) for the MRI-IDEAL sequence. Use the scroll bar at the bottom of the GUI to quickly browse through the different sequences.
- Use the MRI-IDEAL out-phase sequence as the type of MRI sequence for providing clearer descriptions of liver tissue boundaries.
NOTE: In the following operations, the focus will be on using IDEAL's out-phase sequence to delineate the 3D region of the liver.
2. Extract the 3D region of the liver
NOTE: The individual voxels in the 3D region of the liver serve as spatial carriers for LSD, with the stiffness value of each voxel being derived from MRE. Extracting the 3D region of the liver tissue is a necessary step before fusion. While deep learning can be used to accomplish this task more efficiently, it is not the focus of this study. Therefore, mature software tools (e.g., MIMICS) are still used here to extract the 3D region of the liver tissue.
- To initiate the MIMICS software, select New Project and in the ensuing dialog box, navigate to the folder containing the IDEAL out-phase images. Proceed by clicking on NEXT | the Convert button, thereby gaining entry into the sequence-editing state.
- To create an empty Mask, click on the New button in the MASK dialog box located on the right-hand side and select the maximum threshold.
- To delimit the area of the liver in all horizontal views, utilize the Edit Masks tool located beneath the Segment label.
- To generate the 3D spatial part of the liver, select the liver mask that has been delineated and click on the Calculate Part from Mask button. The extracted 3D region of the liver is shown in Figure 3.
- Click on File | Export | select the Dicom command. In the popup dialog box, choose the liver mask, set the file path and files' names, and click the OK button to complete the export of the 3D region of the liver to the specified DICOM files.
3. The Liver stiffness map sequence
NOTE: The MRE stiffness range in patients with early fibrosis is typically below 8 kpa. To view this, the sequence image labeled 'SE27_ST8K_(Pa)' should be selected.
- Change the directory to the folder of 'SE27_ST8K_(Pa)', which contains the liver stiffness map sequence.
- To browse through each stiffness map, execute the MRE_show function in Matlab's workspace, with the function's argument being the filename located in the specified path.
- The liver stiffness map shown in Figure 4 is an RGB true-color image, with a data structure of 512 pixels by 512 pixels by 3 matrix, where each pixel point has three values representing the three primary colors, RBG. Observe the color bar on the left that displays the corresponding stiffness values of different colored pixels. Calculate the exact stiffness of each pixel by using their respective correlations.
- The supplementary information in Figure 4 includes data such as sequence description, scan position, time, patient information, and image parameters. Use these data, particularly the image parameters, to establish the spatial relationship between MRE and IDEAL sequences.
4. 3D-Volume of liver stiffness distribution
NOTE: Each voxel in the 3D liver stiffness volume represents the stiffness value of a corresponding voxel in the 3D liver region, which is derived from the stiffness value of each pixel in Figure 4. By aligning the 3D liver region in Figure 3 with the stiffness map in Figure 4, the stiffness value of each voxel can be extracted, resulting in the generation of the 3D liver stiffness volume.
- Invoke the LSD_Slice function with the 3D liver region shown in Figure 3 and the Liver stiffness map in Figure 4 as input parameters to obtain the 3D-Volume of liver stiffness distribution, as shown in Figure 5.
- View the stiffness map of each layer of the liver by dragging the scroll bar below the GUI shown in Figure 5.
NOTE: However, unlike Figure 4, only liver tissue is accurately retained here. - Observe the icons in the upper right corner of the GUI (Figure 5) such as zooming in, zooming out, returning to the global view, and marking the coordinates of the pixel selected.
NOTE: The default color bar is the colormap of "jet" which means that the corresponding values (Unit kpa) from blue to red are low to high. - Execute the LSD_Volume function with the same input as LSD_Slice to obtain the spatial distribution of the 3D liver LSD, as shown in Figure 6. View the 3D-volume of LSD from any perspective by holding down the left mouse button and dragging the screen (Figure 6).
5. LSD quantitative analysis
NOTE: An important quantitative analysis focus of this study is to provide the proportion of different stages of LSD voxels in the patient's liver. Figure 6 shows that the distribution of liver fibrosis in patients is uneven in different spatial locations. The reason why clinical symptoms are not yet obvious is mainly due to a considerable proportion of liver tissue being in a normal stage. Therefore, it is necessary to quantify precisely the difference between patients and healthy individuals. This is an important quantitative concept of this study.
- Determine the numerical ranges of stiffness values for different stages of hepatic fibrosis, as shown in Figure 7.
- Calculate the distribution of the patient's entire liver voxels in different fibrosis stages (Figure 8) by invoking the Hepatic_Fibrosis function with the input parameter of the 3D-volume of LSD shown in Figure 6.
- Use the same steps to calculate and compare the results of a completely healthy liver with the typical liver fibrosis patient described above (Figure 9).
Representative Results
By utilizing the information in the Description_Name field of DICOM files, the original MRI folder can be renamed to facilitate the rapid localization of the required imaging sequence during the analysis process in the imaging group. The MRI-IDEAL out-phase sequence is the type of MRI sequence used for providing clearer descriptions of liver tissue boundaries. This is because the MRI-IDEAL out-of-phase sequence can better differentiate the magnetization strength and angle of different tissues through specific image processing techniques.
The MRI-IDEAL out-phase sequence works by using gradient echo sequences (GREs) to generate images and utilizing out-phase control during image acquisition. This reduces the magnetic field inhomogeneity between tissues during imaging, thus improving the resolution and contrast of images for tissue structures. Additionally, the MRI-IDEAL out-phase sequence can also suppress the fat signal, thus reducing the interference of fat in imaging and providing a better display of adjacent tissue structures. In summary, the MRI-IDEAL out-phase sequence can improve imaging resolution and contrast by using techniques such as magnetic field control and fat signal suppression, resulting in clearer tissue boundaries.
Although deep learning tools can be used to extract the 3D anatomical structure of the liver, this method has a certain degree of machine learning error. The focus of this study is the precise quantification of hepatic fibrosis; therefore, the MIMICS tool was used for the extraction of the 3D liver tissue region, combined with expert experience to extract a relatively accurate 3D region of liver tissue.
The MRE stiffness map can display the shear stiffness of various spatial positions within each horizontal scan in the upper abdomen. This study focused on the early stages of hepatic fibrosis; therefore, the numerical range was 0-8 kPa. Figure 4 is the standard version of the MRE-Liver Stiffness Map on the GE device, which includes the stiffness map, but it is difficult to distinguish the liver's anatomical structure. The core innovation of this study is the precise quantification achieved by aligning the MRE-Liver Stiffness Map with the liver's anatomical structure shown in Figure 3.
Figure 5 provides an accurate stiffness map for the liver, which allows doctors and patients to have an exact understanding of the location and size of early hepatic fibrosis lesions, instead of a vague sense. This clears the way for further numerical quantification analysis.
Figure 6 is obtained by reconstructing the stiffness map of each liver scanned layer along the horizontal axis in 3D space; Figure 6 is the 3D version of Figure 5. In 3D space, the degree and location of a patient's hepatic fibrosis can be more clearly identified.
The results of comparing, analyzing, and studying pathological examination results with MRE stiffness can be found elsewhere9. To further quantify the numerical distribution of liver fibrosis stage in patients, Figure 7 lists the range of stiffness intervals for different stages used in this study based on previous research results.
According to the stiffness numerical range for different stages of liver fibrosis depicted in Figure 7, it is possible to calculate the specific proportion of liver 3D voxels in different stages for the patient. This calculation is based on the data from the 3D LSD as shown in Figure 6. Consequently, Figure 8 presents the quantitative results of the patient's hepatic fibrosis, indicating the proportion of the patient's liver that falls under different stages of hepatic fibrosis.
Based on the results shown in Figure 8, the data of a healthy liver were calculated as a comparison to illustrate the quantitative effect of the method researched in this study, as shown in Figure 9. The precise quantification difference between the two can be visualized. Based on this research paradigm, in subsequent studies, this group will conduct further investigation into the LSD of a healthy liver and the quantitative classification of early-stage hepatic fibrosis.
Figure 1: Description name of every MRI sequence. Shown here are the folder names of the MRI scan sequences. Please click here to view a larger version of this figure.
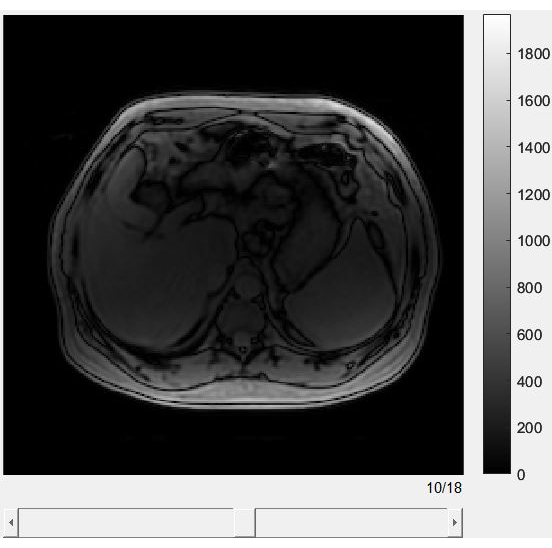
Figure 2: The graphic user interface of the slices of each IDEAL phase sequence. An example of browsing through MRI-IDEAL. MRI-IDEAL is a powerful tool that enhances the quality and interpretability of MRI images, particularly in cases where fat and water separation is critical. Please click here to view a larger version of this figure.
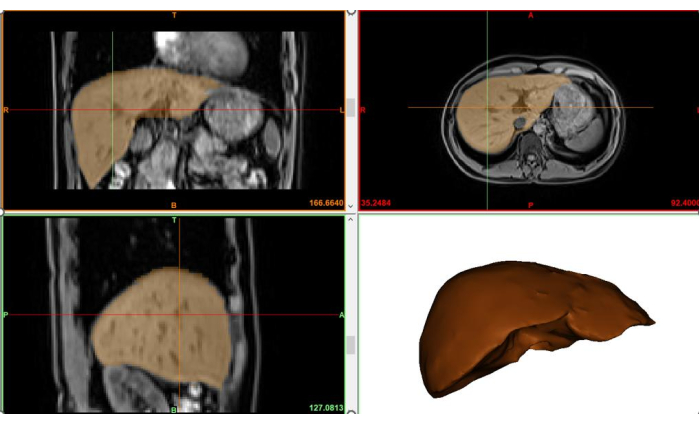
Figure 3: The extracted 3D region of the liver. Shows the 3D spatial extent of the liver based on structural images of the liver. Please click here to view a larger version of this figure.
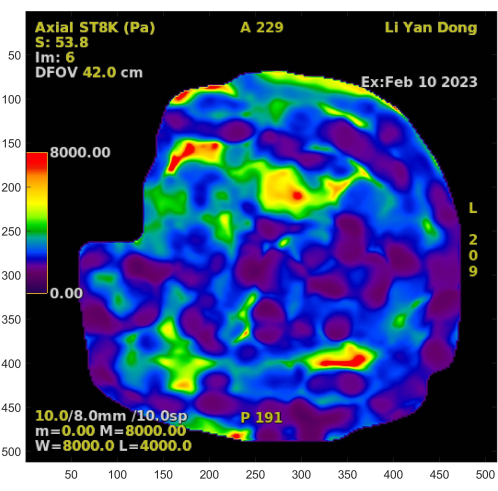
Figure 4: The Liver stiffness map. The standard version of the MRE-Liver Stiffness Map. Please click here to view a larger version of this figure.
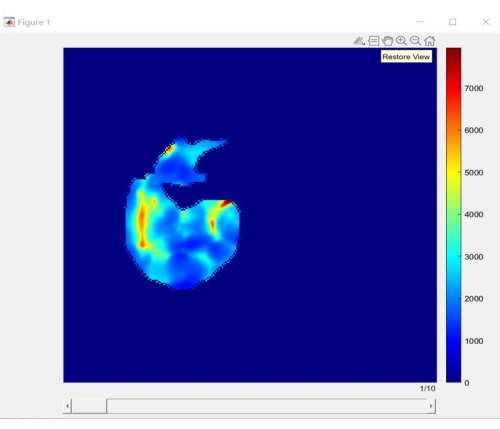
Figure 5: Slices of liver stiffness distribution. An accurate stiffness map belonging to the liver. Please click here to view a larger version of this figure.
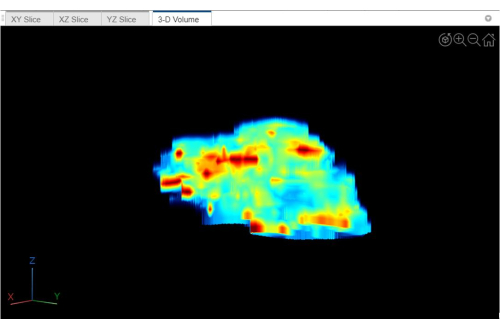
Figure 6: 3D-volume of liver stiffness distribution. This is the 3D version of Figure 5. Please click here to view a larger version of this figure.
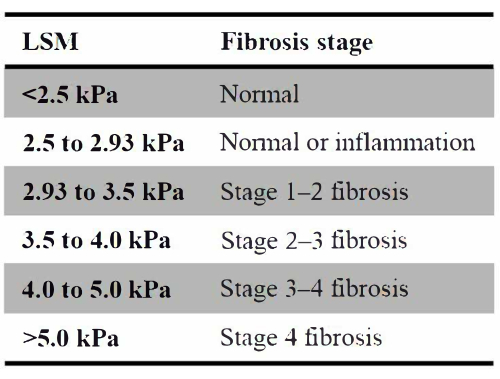
Figure 7: Different stages of hepatic fibrosis. List of the range of stiffness intervals for different stages used in this study based on previous research results. Please click here to view a larger version of this figure.
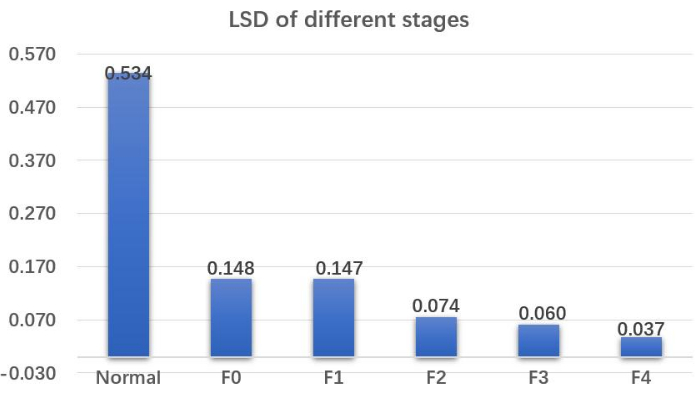
Figure 8: Liver stiffness distribution of the different stages. Quantitative results of the patient's hepatic fibrosis indicate the proportion of the patient's liver that falls under different stages of hepatic fibrosis. Abbreviation: LSD = liver stiffness distribution. Please click here to view a larger version of this figure.
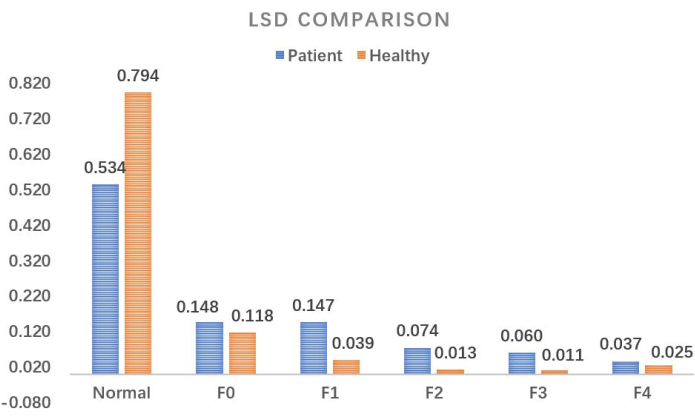
Figure 9: Comparison of liver stiffness distribution. A detailed quantitative comparison between a healthy liver and a patient with early-stage liver fibrosis. Abbreviation: LSD = liver stiffness distribution. Please click here to view a larger version of this figure.
Discussion
In clinical practice, it is challenging to accurately quantify and track the condition of early-stage hepatic fibrosis patients. The comparison shown in Figure 9 fully reflects the degree of hepatic fibrosis in the patient compared to a healthy liver; of course, this figure can also be a comparison between two different periods for the patient, used for evaluation of treatment efficacy. This precise quantification method is the core critical step of this study. Furthermore, the calculation method of the 3D volume of LSD shown in Figure 5 and Figure 6 can accurately locate the spatial location and size of fibrotic lesions in the patient’s liver, providing a solid quantitative basis for the accurate diagnosis of early-stage hepatic fibrosis. It can also provide scientific support for precisely guided liver puncture using the 3D-digital model.
This study proposes the concept of 3D LSD and its precise quantification in different stages of hepatic fibrosis. The results show that this method can effectively quantitatively assess the disease progression of early hepatic fibrosis patients. Possible further improvements and evolution include improving the scanning accuracy of MRE scans, especially the horizontal scanning interval; improving the imaging accuracy of liver magnetic resonance structural images; introducing deep learning technology to assist in the rapid extraction of the liver’s 3D region; accumulating more LSD data for healthy livers to establish a baseline for diagnostic comparisons; and accumulating more patient data for each stage of hepatic fibrosis to develop more accurate classification standards.
Although the method proposed in this study can quantitatively stage early hepatic fibrosis based on 3D LSD, it does not address the underlying mechanisms of the disease development2. Different equipment and scanning strategies may lead to inconsistent results. Developing a more standardized and universal computational protocol remains challenging.
Compared to traditional invasive diagnostic methods for hepatic fibrosis, the work presented in this paper has the following prominent advantages. First, both routine upper abdominal MRI and MRE are non-invasive. Second, 3D LSD can accurately characterize the size and location of hepatic fibrosis lesions in 3D space. Third, the quantitative results can provide clinicians with a clear understanding of the proportion of liver voxels at different stages of hepatic fibrosis. Finally, this study achieved accurate alignment of liver tissue structure with the MRE stiffness map, allowing clinicians to index stiffness values from structural images or vice versa, to index the spatial location of liver tissue from lesions in the stiffness map. This approach is very valuable for the precise quantification of early hepatic fibrosis.
The MRI-IDEAL technique works by acquiring MRI images at multiple echo times, which allows for the separation of water and fat signals by exploiting their different resonance frequencies. This separation is achieved through an iterative decomposition process that calculates the relative proportions of water and fat in each pixel of the image. The resulting images can provide clinicians with valuable information about the distribution and amount of body fat, which can be useful in diagnosing and monitoring conditions such as obesity, diabetes, and liver disease.
The quantitative method proposed in this study is not only applicable for the quantitative diagnosis of early hepatic fibrosis but also for the diagnosis of middle- and late-stage liver cirrhosis. It can also be used as a grouping and screening technique to exclude patients with hepatic fibrosis or liver cirrhosis, as well as a prognostic tool for various types of liver cirrhosis. The 3D LSD can also be used as a navigation tool for precise liver puncture or surgery.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This publication was supported by the fifth national traditional Chinese medicine clinical excellent talents research program organized by the National Administration of Traditional Chinese Medicine. The official network link is 'http://www.natcm.gov.cn/renjiaosi/zhengcewenjian/2021-11-04/23082.html. '
Materials
| MATLAB | MathWorks | 2022B | Computing and visualization |
| Mimics | Materialise | Mimics Research V20 | Model format transformation |
| Tools for 3D_LSD | Intelligent Entropy | HepaticFibrosis V1.0 | Beijing Intelligent Entropy Science & Technology Co Ltd. Modeling for CT/MRI fusion |
References
- Henderson, N. C., Rieder, F., Wynn, T. A. Fibrosis: from mechanisms to medicines. Nature. 587 (7835), 555-566 (2020).
- Parola, M., Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Molecular Aspects of Medicine. 65, 37-55 (2019).
- Ramachandran, P., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 575 (7783), 512-518 (2019).
- Stefan, N., Häring, H. -. U., Cusi, K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. The Lancet. Diabetes & Endocrinology. 7 (4), 313-324 (2019).
- Castera, L., Friedrich-Rust, M., Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 156 (5), 1264.e4-1281.e4 (2019).
- Godoy-Matos, A. F., Silva Júnior, W. S., Valerio, C. M. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetology & Metabolic Syndrome. 12 (1), 1-20 (2020).
- Venkatesh, S. K., Xu, S., Tai, D., Yu, H., Wee, A. Correlation of MR elastography with morphometric quantification of liver fibrosis (Fibro-C-Index) in chronic hepatitis B. Magnetic Resonance in Medicine. 72 (4), 1123-1129 (2014).
- Yin, M., et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clinical Gastroenterology and Hepatology. 5 (10), 1207-1213 (2007).
- Venkatesh, S. K., Wang, G., Lim, S. G., Wee, A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. European Radiology. 24, 70-78 (2014).
- Ichikawa, S., et al. Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magnetic Resonance in Medical Sciences. 11 (4), 291-297 (2012).
- Chen, J., et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 259 (3), 749-756 (2011).
- Singh, S., et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clinical Gastroenterology and Hepatology. 13 (3), 440.e6-451.e6 (2015).
- Ferro, M., et al. Radiomics in prostate cancer: an up-to-date review. Therapeutic Advances in Urology. 14, 17562872221109020 (2022).
- Nam, D., Chapiro, J., Paradis, V., Seraphin, T. P., Kather, J. N. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction. JHEP Reports. 4 (4), 100443 (2022).
- Wu, Y. -. J., Wu, F. -. Z., Yang, S. -. C., Tang, E. -. K., Liang, C. -. H. Radiomics in early lung cancer diagnosis: from diagnosis to clinical decision support and education. Diagnostics. 12 (5), 1064 (2022).

