Author Spotlight: Advancing Structural and Biochemical Studies of Proteins Through Thermal Shift Assays
Summary
Here, we present the thermal shift assay, a high-throughput, fluorescence-based technique used to investigate the binding of small molecules to proteins of interest.
Abstract
Defining the biological importance of proteins with unknown functions poses a significant obstacle in understanding cellular processes. Although bioinformatic and structural predictions have contributed to the study of unknown proteins, in vitro experimental validations are often hampered by the optimal conditions and cofactors required for biochemical activity. Cofactor binding is not only essential for the activity of some enzymes but may also enhance the thermal stability of the protein. One practical application of this phenomenon lies in utilizing the change in thermal stability, as measured by alterations in the protein’s melting temperature, to probe ligand binding.
Thermal shift assay (TSA) can be used to analyze the binding of different ligands to the protein of interest or find a stabilizing condition to perform experiments such as X-ray crystallography. Here we will describe a protocol for TSA utilizing the pseudokinase, Selenoprotein O (SelO), for a simple and high-throughput method for testing metal and nucleotide binding. In contrast to canonical kinases, SelO binds ATP in an inverted orientation to catalyze the transfer of AMP to the hydroxyl side chains of proteins in a posttranslational modification known as protein AMPylation. By leveraging the shift in the melting temperatures, we provide crucial insights into the molecular interactions underlying SelO function.
Introduction
Much of the human proteome remains poorly characterized. The "streetlight effect" described by Dunham and Kustatscher et al. refers to the phenomenon where extensively researched proteins receive more attention, leaving understudied proteins overlooked1,2. Factors contributing to this effect include the biological importance and disease relevance of certain proteins. Moreover, prior research that offers foundational knowledge, as well as the availability of tools for functional analysis, further stimulates research on highly studied proteins1,2. Projects such as the Understudied Proteins Initiative, Structural Genomics Consortium, and Enzyme Function Initiative aim to characterize understudied proteins using structural and functional proteomics2,3,4. A complementary approach to identifying the molecular functions of understudied proteins is to examine the interactions of these proteins with small molecules to gain valuable insights into the regulation and substrate specificity.
The binding of small molecules can increase the thermal stability of a protein5. This phenomenon, known as ligand-induced conformation stabilization, often results in an increase in the melting temperature of a protein, providing a measurable indication of ligand binding6. The thermal stability of a protein can be measured using Differential Scanning Fluorimetry (DSF), also known as Thermofluor, or Thermal Shift Assay (TSA)7,8,9,10. In TSA, a protein sample is subjected to increasing temperatures in the presence of an environmentally sensitive dye6. As the protein unfolds and denatures, the dye binds to the exposed hydrophobic regions of the protein and emits fluorescence. Extrinsic fluorescent dyes, or environmentally sensitive dyes, lack intrinsic fluorescence and instead fluoresce upon interacting with their target molecule9,11,12. The most commonly used dye, SYPRO Orange, offers several advantages such as enhanced stability and minimal background fluorescence8,9,12. Notably, SYPRO Orange is compatible with Real-Time Polymerase Chain Reaction (RT-PCR) systems given its excitation and emission wavelengths around 470 nm and 570 nm, respectively. This unique feature allows for high-throughput, accurate, and sensitive measurements that are compatible with fluorescence detection systems commonly used in RT-PCR systems8.
TSA is a versatile tool for investigating protein interactions with potential cofactors or drugs and for identifying stabilizing conditions for crystallography. Some of its advantages over other screening methods are its relative simplicity of setup and high throughput with 96- or 384-well plates13,14,15. The development of this technique has been transformative for drug discovery studies, offering convenience and enabling the study of diverse additives such as ions, ligands, and drugs6,16. Moreover, the adaptability of TSA has encouraged scientists to expand its utility, facilitating the measurement of binding affinities alongside the screening assays17,18. TSA is an effective tool in structural biology studies to identify conditions that promote the stabilization of the protein of interest for crystallization19. Thus, its flexibility and relative ease have positioned TSA as a cornerstone technique in characterizing proteins. Here, we will use TSA to analyze the binding of metals and nucleotide to the understudied pseudokinase, Selenoprotein O (SelO), as an example.
Kinases are one of the most targeted protein families in drug discovery20. Approximately 10% of human kinases are predicted to be inactive and named pseudokinases because they lack key catalytic residues required for catalysis21,22. Selenoprotein O (SelO) is an evolutionarily conserved pseudokinase that lacks the conserved aspartate in the catalytic HRD motif23. In eukaryotes, SelO localizes to the mitochondria and protects cells from oxidative stress24,25. Structural and biochemical analyses show that SelO transfers adenosine monophosphate (AMP) from ATP to protein substrates in a posttranslational modification known as AMPylation25. Recent studies indicate that enzymes that catalyze AMPylation may bind alternative nucleotides such as UTP in vitro26,27. Notably, studies have demonstrated that the SelO homolog from Salmonella typhimurium catalyzes protein UMPylation, or the transfer of UMP, in a manganese-dependent manner28. Given these intriguing observations, we test the binding of SelO to ATP and UTP in the presence of magnesium and manganese, serving as a representative example of TSA's applications. The following protocol can be readily adapted to optimize and examine the interactions of other proteins and additives of interest.
Protocol
1. Experimental setup
- Use a thermal cycler that can detect fluorescence, such as an RT-PCR instrument, to perform the described protocol. If using SYPRO Orange, as described in this protocol, use a scanning mode that allows for excitation around 470 nm and emission detection around 570 nm. Program the thermocycler to hold the sample at 20 °C for 2 min, followed by an increase in temperature of 0.5 °C/1 min up to 95 °C; measure fluorescence intensity every 1 °C.
NOTE: We recommend an optional step at the end of the cycle to return the sample block to 20 °C (Figure 1). - Each reaction contains four components: Buffer, protein of interest, additive, and extrinsic fluorescent dye. Design the experiment to include controls such as no protein, no dye, protein without additives, and additives alone to determine any background fluorescence that may influence the interpretation of the results. If available, include a positive control such as an additive known to bind and stabilize the protein of interest.
- Use purified protein for the assay. To follow this example, use E. coli SelO, expressed and purified as previously described25,29. Briefly, express His-sumo tagged SelO (E. coli SelO ppSumo) in Rosetta DE3 cells and purify it using Ni2+-NTA affinity. Cleave the His-sumo tag using Ulp protease and purify SelO further by gel filtration chromatography.
- The fluorescence of SYPRO Orange will depend on its interaction with the protein of interest. As these interactions will vary from protein to protein, optimize the protein and dye concentration for obtaining the maximum signal to noise of fluorescence signal.
NOTE: SYPRO Orange is not compatible with some proteins and additives such as detergents11,30. Additional extrinsic dyes listed in 12 may be screened as necessary.
2. Determination of the optimal dye concentration (Figure 2A)
- Prepare 20 µL dilutions of 50x, 40x, 30x, 20x, and 10x SYPRO Orange from the stock solution, which is provided at 5,000x in DMSO.
- Dispense 8 µL of 6.25 µM protein diluted in TSA buffer into six separate wells of a 384-well PCR plate.
NOTE: In our example, we used the following TSA buffer to perform the dilutions: 10 mM Tris pH 8, 150 mM NaCl, and 1 mM DTT. Samples may be run in triplicate to improve the reliability of outcomes. - Add 1 µL of TSA buffer to each well.
NOTE: This volume of buffer will be replaced with the addition of small molecules after optimization of dye and protein concentration. - Add 1 µL of each serial dilution of SYPRO Orange dye solution to each well. Add 1 µL of buffer without dye to the final well for the no dye control.
- Cover the plate with optically clear adhesive film.
- Briefly spin down the plate at 1,000 × g for 1 min in a centrifuge equipped with a PCR plate adapter.
- Place the plate in an RT-PCR machine and start the thermocycling protocol described in step 1.1.
3. Determination of the optimal protein concentration (Figure 2B)
- Prepare 20 µL of serial dilutions of 25 µM, 12.5 µM, 6.25 µM, 3.125 µM, and 1.56 µM of protein using TSA buffer (10 mM Tris pH 8, 150 mM NaCl, and 1 mM DTT).
- Dispense 8 µL of serial dilutions of the protein into five separate wells of a 384-well plate.
- Dispense buffer only in the 6th well as a no protein control.
- Add 1 µL of TSA buffer to each well.
NOTE: This volume of buffer will be replaced with the addition of small molecules after optimization of dye and protein concentration. - Add 1 µL of each 50x SYPRO Orange dye solution to each well.
- Cover the plate with optically clear adhesive film.
- Briefly spin down the plate at 1,000 × g for 1 min in a centrifuge equipped with a PCR plate adapter.
- Place the plate in an RT-PCR machine and start the thermocycling protocol described in step 1.1.
4. Setting up a TSA experiment (Figure 3A)
NOTE: After optimization of the protein and SYPRO Orange dye concentrations, the assay can be performed with the addition of the desired small molecules to analyze binding.
- Define the number of conditions by considering the replicates and adequate controls.
NOTE: For example, we will test the binding of SelO to eight conditions in triplicate. Including the controls (no additive, no protein, and no dye), we have 11 reactions x 3 (triplicate) = 33 total reactions. - Based on the optimal concentration of protein and dye observed for SelO, make sure that each reaction contains 8 µL of 6.25 µM protein, 1 µL of additive, and 1 µL of 50x SYPRO Orange dye for a total of 10 µL reaction volume. Thus, the final concentration used is 5 µM protein and 5x SYPRO Orange dye.
- Prepare 288 µL of 6.25 µM protein solution using TSA buffer. This volume of protein working solution accounts for 36 reactions (33 reactions with an additional 10% for variations in pipetting).
- Dispense 8 µL of 6.25 µM protein into separate wells of a 384-well plate. Dispense buffer only for the no protein control.
- Add 1 µL of small molecules at 10x concentration to achieve final 1x. To follow this example, add 1 µL of 20 mM MgCl2 to achieve a final concentration of 2 mM. Dispense buffer only for the no additive control.
- Add 1 µL of each 50x SYPRO Orange dye solution to each well.
- Cover the plate with optically clear adhesive film.
- Briefly spin down the plate at 1,000 × g for 1 min in a centrifuge equipped with a PCR plate adapter.
- Place the plate in an RT-PCR machine and start the thermocycling protocol described in step 1.1.
5. Data analysis
- Export data from the RT-PCR machine for relative fluorescence units (RFU) with respect to temperature (°C).
- Use the software of choice to extract and plot data points up to the highest intensity measured for each melting curve. Importantly, confirm that the no protein and no dye controls exhibit low background fluorescence without temperature-dependent increases in fluorescence (Figure 3A,B and Supplemental Table S1). Perform data analysis using freely accessible software such as MoltenProt31 or DSFWorld32.
- Determine the melting temperature by using Boltzmann Sigmoidal Fit for the data. The melting temperature (Tm) is defined as the temperature at which 50% denaturation or half maximal intensity is observed (Figure 3C).
- A thermal shift in the melting curve may suggest stabilizing or destabilizing additives. To calculate the change of melting temperature (Tm), use the formula: Tm= Tm additive– Tm buffer. A positive value describes a stabilizing condition or interacting additive; a negative value describes a destabilizing condition (Figure 3D).
Representative Results
Eukaryotic SelO consists of an N-terminal mitochondrial targeting sequence, a kinase-like domain, and a highly conserved selenocysteine at the C-terminus of the protein23. This mitochondrial-resident enzyme encodes a pseudokinase domain that is conserved from bacteria to humans23. Structural analysis of the SelO homolog from Pseudomonas syringae revealed amino acid alterations in the active site that facilitate the binding of ATP in an inverted orientation in comparison to canonical kinases25. Accordingly, SelO catalyzes AMPylation, rather than phosphorylation, of multiple proteins involved in antioxidant signaling to protect cells from oxidative damage and cell death25. In addition to AMPylation, some prokaryotic and eukaryotic AMPylases have been shown to catalyze UMPylation27,28.
We used purified recombinant E. coli SelO in TSA to examine the binding of ATP and UTP nucleotides to SelO in the presence of divalent cations. Similar to canonical kinases, SelO harbors the DFG motif in which the aspartate binds the metal ion to coordinate nucleotide in the active site. First, we optimized the concentration of protein and dye for robust fluorescence measurements in TSA, determining that 5 µM to 10 µM E. coli SelO was optimal with 5x SYPRO Orange (Figure 2). Furthermore, we showed that the no protein, as well as the no dye controls, had low fluorescence signal that was not responsive to the temperature increase. We detected approximately +4 °C and +12 °C increases in the thermal stability of E. coli SelO in the presence of ATP-Mg2+ and ATP-Mn2+, respectively (Figure 3). However, we did not observe a shift in thermal stability upon incubation with UTP, indicating that E. coli SelO may not exhibit detectable binding to UTP.
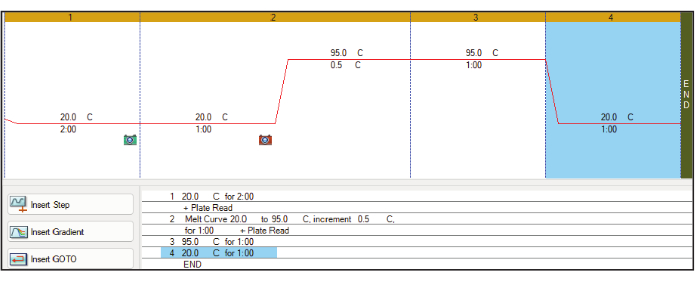
Figure 1: Thermocycler setup. An example of the thermocycler program used to increase sample temperature by +0.5 °C/min while measuring SYPRO Orange fluorescence using the FRET channel. The recommendation also includes an ambient temperature incubation at the start and end of the protocol. Abbreviation: FRET = Förster resonance energy transfer. Please click here to view a larger version of this figure.
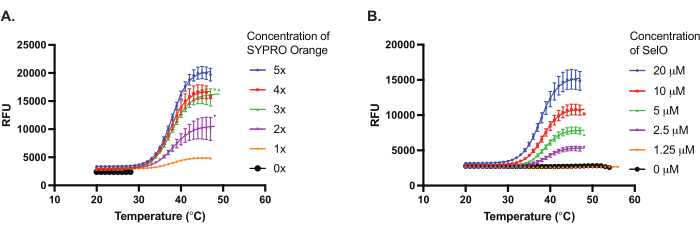
Figure 2: Optimization of protein and dye concentrations. (A) Representative results of optimizing the SYPRO Orange dye concentration using 5 µM Escherichia coli SelO. (B) Representative results of optimizing the E. coli SelO concentration using 5x SYPRO Orange dye. Thermal denaturation curve depicts relative fluorescence units (y-axis) with respect to temperature (x-axis). Results represent mean ± SD from triplicate wells (n = 3). Please click here to view a larger version of this figure.
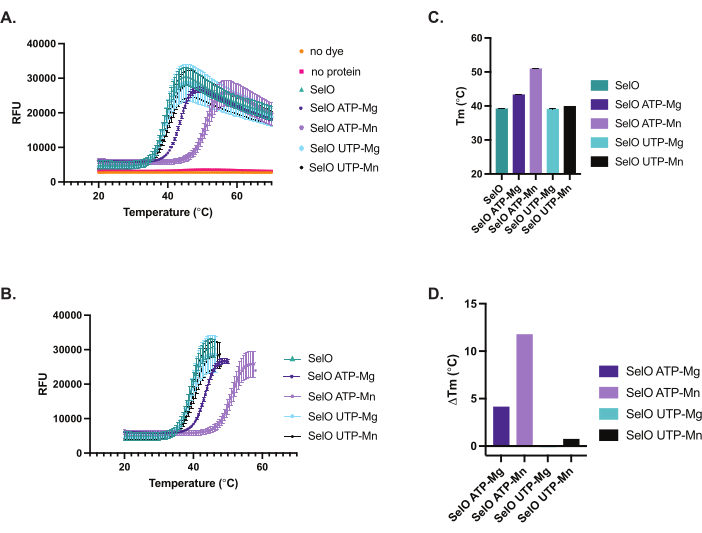
Figure 3:Thermal denaturation curves and ΔTm analyses of SelO in the presence of nucleotide and divalent cations. (A) Raw data of thermal denaturation curves of samples (5 µM Escherichia coli SelO in the presence of 200 µM ATP or UTP and 2 mM divalent cations, 5x SYPRO Orange) exported from RT-PCR machine and plotted. SelO ATP-Mg represents samples with SelO, ATP, and MgCl2, SelO ATP-Mn represents samples with SelO, ATP, and MnCl2, SelO UTP-Mg represents samples with SelO, UTP, and MgCl2, SelO UTP-Mn represents samples with SelO, UTP, and MnCl2. Note that the no dye and no protein controls exhibit low fluorescence. Results represent mean ± SD from triplicate wells (n = 3). (B) Thermal denaturation curves plotted to the highest intensity and fit with the Boltzmann sigmoidal equation. Although thermal denaturation of SelO was not affected by UTP, we observed a marked shift in thermal denaturation in the presence of ATP (purple curves). Results represent mean ± SD from triplicate wells (n = 3). (C) Histogram depicting Tm values as derived from Boltzmann sigmoidal fit of data represented in Figure 3B. Results represent mean ± SD from triplicate wells (n = 3). SelO 39.2 °C ± 0.085, SelO ATP-Mg 43.3 °C ± 0.133, SelO ATP-Mn 50.9 °C ± 0.085, SelO UTP-Mg 39.1 °C ± 0.056, SelO UTP-Mn 39.9 °C ± 0.087. (D) Histogram depicting the change in melting temperature ΔTm (Tm additive– Tm buffer). Please click here to view a larger version of this figure.
Supplemental Table S1: Example data for TSA of SelO in the presence of metals and nucleotides. Spreadsheet of raw data exported from the RT-PCR machine, and data processed to delete values after maximum RFU intensity as described in protocol steps 5.1 and 5.2. Please click here to download this File.
Discussion
The Thermal Shift Assay (TSA) serves as an efficient method for screening protein-ligand interactions, including those with cofactors and inhibitors. In this protocol, we used TSA to measure the binding of the pseudokinase SelO to nucleotides and divalent cations. Our findings show that SelO exhibits increased thermal stability in the presence of ATP and Mg2+/Mn2+. This observation aligns with previous reports indicating that SelO homologs from E. coli, S. cerevisiae, and H. sapiens catalyze the transfer of AMP from ATP to the hydroxyl side chain of protein substrates25,29. Although TSA provides valuable insights into nucleotide binding, it may not identify the in vivo substrate. Other molecules, not just the substrate, can also influence the thermal stability of a protein. To accurately identify a substrate, factors such as cellular concentration and binding affinity must be considered. Nevertheless, TSA is an excellent tool for screening a large array of conditions, such as the binding of small molecules, using thermal stabilization as a readout.
The utility of TSA extends beyond its application in analyzing small molecule binding to active or regulatory sites of proteins. TSA has significantly contributed to the biophysical characterization of proteins by providing stabilizing conditions that render the protein more conducive to crystallography experiments. Notably, TSA offers several advantages, including its simple setup and high-throughput capabilities using commonly available lab materials such as PCR plates and RT-PCR machines. These advantages allow for the testing of numerous conditions that may not be feasible or as straightforward with other techniques. Multiple studies have used TSA to measure the thermal stability of recombinant proteins with a library of compounds to determine the optimal conditions for protein crystallization19,33.
Including appropriate controls is necessary to avoid misinterpretation of melting curves, with autofluorescence from the plates or additives being a common concern. TSA may not be suitable for compounds that exhibit autofluorescence or quench the fluorescence of SYPRO orange. A potential solution is to screen alternative dyes described in 12. The TSA protocol outlined here can be easily adapted for other proteins and additives of interest. The additives are not solely limited to water-soluble compounds, as demonstrated in our protocol with nucleotides and divalent cations. Instead, additives can encompass a wide range of conditions such as varying pH, ligands, or drugs solubilized in DMSO8. Furthermore, preincubation of the ligand with the protein may provide more reproducible results by reducing interference and artifacts from the dye. Additives such as lipids and detergents may lead to high fluorescence due to their interactions with SYPRO Orange. An example of a denaturation curve is shown in Figure 3A which depicts low background fluorescence paired with a significant increase in fluorescence upon thermal denaturation of the protein. The optimal concentration of dye and protein will yield the maximum signal-to-noise ratio while maintaining a consistent fluorescence signal across replicates.
TSA offers the advantage of simplicity with its minimal components, thus reducing the number of variables for optimization. Critical steps include determining the optimal concentrations of both the dye and the protein. It is important to note that TSA requires purified, "well-behaved" protein. The protein and additive must be stable in the buffer at the concentrations used in TSA. Proteins or conditions that are prone to aggregation or oligomerization may result in erroneous fluorescence readings. Aggregated or unfolded proteins may exhibit high fluorescence initially with only a small increase upon denaturation. Oligomerization of proteins can also result in multiple transition states, complicating analysis10. The concentration of protein may be fine-tuned to prevent aggregation.
TSA is compatible with a wide range of buffers and salts, allowing for optimization of the stabilizing conditions for the protein and additive11. The volumes of the protein and additive solutions may be adjusted to overcome the limitations of protein and additive concentrations. For example, lower concentrations of additive such as 5x could be used instead of the 10x solution used in the representative results. Notably, the relative amount of protein and additive required to perform the assay is low since the 384-well PCR plates can accommodate volumes as low as 10 µL of total sample.
Given the strengths of TSA, it is ideal for investigating understudied proteins such as pseudokinases. Pseudoenzymes are catalytically inactive proteins that are structurally homologous to active enzymes34. Recent structural and biochemical studies demonstrated that a few pseudoenzymes are active enzymes while most have non-catalytic functions35,36. Although catalytically inactive, pseudoenzymes are implicated in various diseases and play important roles including scaffolding and allosteric modulation37. However, the mechanism of action and functional significance of many pseudoenzymes are unknown. The activities of some pseudoenzymes are regulated by ligand binding which results in increased thermal stability that is associated with conformational changes of the protein14. We propose that TSA is a valuable tool for examining the binding of ligands to pseudoenzymes to determine the optimal ligand for functional activity.
Disclosures
The authors have nothing to disclose.
Acknowledgements
A.S. is a W.W. Caruth, Jr. Scholar in Biomedical Research, Cancer Prevention and Research Institute of Texas (CPRIT) Scholar, and Charles and Jane Pak Center for Mineral Metabolism and Clinical Research Faculty Scholar. This work was supported by NIH Grant K01DK123194 (A.S.), CPRIT Grant RR190106 (A.S.), Welch (I-2046-20200401) and Welch (I-2046-20230405).
Materials
| Adenosine 5′-triphosphate disodium salt hydrate | Sigma Aldrich | A2383-10G | used for representative results but not required to perfom TSA |
| Avanti J-15R with microplate carrier assembly | Beckman Coulter | C19416 | |
| CFX Opus 384 Real-time PCR system | Bio-Rad | 12011452 | |
| Hard-Shell 384-Well PCR Plates, thin wall, skirted, clear/white | Bio-Rad | HSP3805 | |
| Magnesium Chloride | Sigma Aldrich | M8266-100G | used for representative results but not required to perfom TSA |
| Manganese (II) chloride tetrahydrate | Sigma Aldrich | M3634-500G | used for representative results but not required to perfom TSA |
| Microseal 'B' PCR Plate Sealing Film, adhesive, optical | Bio-Rad | MSB1001 | |
| SYPRO Orange Protein Gel stain | Sigma Aldrich | S5692-500UL | |
| Uridine 5′-triphosphate trisodium salt hydrate | Sigma Aldrich | U6625-100MG | used for representative results but not required to perfom TSA |
References
- Dunham, I. Human genes: Time to follow the roads less traveled. PLoS Biol. 16 (9), e3000034 (2018).
- Kustatscher, G., et al. An open invitation to the understudied proteins initiative. Nat Biotechnol. 40 (6), 815-817 (2022).
- Jones, M. M., et al. The structural genomics consortium: A knowledge platform for drug discovery: A summary. Rand Health Q. 4 (3), 19 (2014).
- Oberg, N., Zallot, R., Gerlt, J. A. Efi-est, efi-gnt, and efi-cgfp: Enzyme function initiative (efi) web resource for genomic enzymology tools. J Mol Biol. 435 (14), 168018 (2023).
- Pace, C. N., Mcgrath, T. Substrate stabilization of lysozyme to thermal and guanidine hydrochloride denaturation. J Biol Chem. 255 (9), 3862-3865 (1980).
- Pantoliano, M. W., et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J Biomol Screen. 6 (6), 429-440 (2001).
- Huynh, K., Partch, C. L. Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc Protein Sci. 79, 21-29 (2015).
- Niesen, F. H., Berglund, H., Vedadi, M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2 (9), 2212-2221 (2007).
- Wu, T., et al. Conformationally responsive dyes enable protein-adaptive differential scanning fluorimetry. bioRxiv. , (2023).
- Wu, T., et al. Protocol for performing and optimizing differential scanning fluorimetry experiments. STAR Protoc. 4 (4), 102688 (2023).
- Gao, K., Oerlemans, R., Groves, M. R. Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophys Rev. 12 (1), 85-104 (2020).
- Hawe, A., Sutter, M., Jiskoot, W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 25 (7), 1487-1499 (2008).
- Lucet, I. S., Murphy, J. M. Characterization of ligand binding to pseudokinases using a thermal shift assay. Methods Mol Biol. 1636, 91-104 (2017).
- Murphy, J. M., et al. A robust methodology to subclassify pseudokinases based on their nucleotide-binding properties. Biochem J. 457 (2), 323-334 (2014).
- Senisterra, G., Chau, I., Vedadi, M. Thermal denaturation assays in chemical biology. Assay Drug Dev Technol. 10 (2), 128-136 (2012).
- Lo, M. C., et al. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal Biochem. 332 (1), 153-159 (2004).
- Bai, N., Roder, H., Dickson, A., Karanicolas, J. Isothermal analysis of thermofluor data can readily provide quantitative binding affinities. Sci Rep. 9 (1), 2650 (2019).
- Vivoli, M., Novak, H. R., Littlechild, J. A., Harmer, N. J. Determination of protein-ligand interactions using differential scanning fluorimetry. J Vis Exp. (91), 51809 (2014).
- Vedadi, M., et al. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc Natl Acad Sci U S A. 103 (43), 15835-15840 (2006).
- Taujale, R., et al. Informatic challenges and advances in illuminating the druggable proteome. Drug Discov Today. 29 (3), 103894 (2024).
- Bailey, F. P., Byrne, D. P., Mcskimming, D., Kannan, N., Eyers, P. A. Going for broke: Targeting the human cancer pseudokinome. Biochem J. 465 (2), 195-211 (2015).
- Kung, J. E., Jura, N. Prospects for pharmacological targeting of pseudokinases. Nat Rev Drug Discov. 18 (7), 501-526 (2019).
- Dudkiewicz, M., Szczepinska, T., Grynberg, M., Pawlowski, K. A novel protein kinase-like domain in a selenoprotein, widespread in the tree of life. PLoS One. 7 (2), e32138 (2012).
- Han, S. J., Lee, B. C., Yim, S. H., Gladyshev, V. N., Lee, S. R. Characterization of mammalian selenoprotein o: A redox-active mitochondrial protein. PLoS One. 9 (4), e95518 (2014).
- Sreelatha, A., et al. Protein ampylation by an evolutionarily conserved pseudokinase. Cell. 175 (3), 809-821.e19 (2018).
- Frese, M., et al. The alarmone diadenosine tetraphosphate as a cosubstrate for protein ampylation. Angew Chem Int Ed Engl. 62 (8), e202213279 (2023).
- Mostert, D., et al. Pronucleotide probes reveal a diverging specificity for ampylation vs umpylation of human and bacterial nucleotide transferases. Biochemistry. 63 (5), 651-659 (2024).
- Yang, Y., et al. The ydiu domain modulates bacterial stress signaling through Mn2+-dependent umpylation. Cell Rep. 32 (12), 108161 (2020).
- Mukherjee, M., Sreelatha, A. Identification of selenoprotein o substrates using a biotinylated atp analog. Methods Enzymol. 662, 275-296 (2022).
- Kroeger, T., et al. Edta aggregates induce sypro orange-based fluorescence in thermal shift assay. PLoS One. 12 (5), e0177024 (2017).
- Kotov, V., et al. In-depth interrogation of protein thermal unfolding data with moltenprot. Protein Sci. 30 (1), 201-217 (2021).
- Wu, T., Gale-Day, Z. J., Gestwicki, J. E. Dsfworld: A flexible and precise tool to analyze differential scanning fluorimetry data. Protein Sci. 33 (6), e5022 (2024).
- Reinhard, L., Mayerhofer, H., Geerlof, A., Mueller-Dieckmann, J., Weiss, M. S. Optimization of protein buffer cocktails using thermofluor. Acta Crystallogr Sect F Struct Biol Cryst Commun. 69 (Pt 2), 209-214 (2013).
- Ribeiro, A. J. M., et al. Emerging concepts in pseudoenzyme classification, evolution, and signaling. Sci Signal. 12 (594), eaat9797 (2019).
- Goldberg, T., Sreelatha, A. Emerging functions of pseudoenzymes. Biochem J. 480 (10), 715-728 (2023).
- Pon, A., Osinski, A., Sreelatha, A. Redefining pseudokinases: A look at the untapped enzymatic potential of pseudokinases. IUBMB Life. 75 (4), 370-376 (2023).
- Murphy, J. M., Mace, P. D., Eyers, P. A. Live and let die: Insights into pseudoenzyme mechanisms from structure. Curr Opin Struct Biol. 47, 95-104 (2017).

.
