Bioscaffold Fabrication by Electrospraying: A Technique to Generate Microcarrier Scaffolds Derived from Extracellular Matrix of Decellularized Tissue
Abstract
Source: Kornmuller, A. et al. Fabrication of Extracellular Matrix-derived Foams and Microcarriers as Tissue-specific Cell Culture and Delivery Platforms. J. Vis. Exp. (2017)
This video demonstrates the procedure of extracellular matrix-derived microcarrier fabrication by electrospraying. These microcarriers simulate the native tissue-specific microenvironment and are stable in long-term 3D in vitro cell culture models.
Protocol
1. ECM-derived Microcarrier Fabrication via Electrospraying
NOTE: An overview of the electrospraying set-up is shown in Figure 1.
- Incubate a previously prepared cryomilled ECM suspension at 37 °C with continuous agitation at 100 rpm O/N.
NOTE: Cryomilled ECM is used to fabricate the ECM-derived microcarriers to ensure greater uniformity in the suspension and prevent clogging during electrospraying. - Load 3 mL of ECM suspension into a 3 mL Luer-Lock syringe and attach a winged infusion set onto the bore of the syringe.
NOTE: A range of needle gauges can be used, recognizing that the selection may influence the size and shape of the resultant microcarriers. To fabricate the DAT, DDT, and DLV microcarriers, use a 25G needle. - Secure the syringe within the syringe pump. Fasten the needle to a retort stand and position the needle tip vertically at a distance of 4–6 cm from the top of a low-form Dewar flask (250–500 mL size range recommended).
- Attach an alligator clip electrode to the tip of the needle and connect it to the positive terminal of the high-voltage power supply.
- Fold a strip of aluminum foil (12 x 5 cm) over the edge of the vacuum flask.
- Attach a second alligator clip electrode to the outer edge of the foil and connect it to the ground source terminal of the power supply.
- Fill the Dewar flask with liquid nitrogen to approximately 1 cm from the top so that ¾ of the foil is submerged.
NOTE: It is important to keep the Dewar flask filled with liquid nitrogen. The surface of the liquid nitrogen must not be less than 2 cm from the top of the flask during the electrospraying process. Continuous refilling of the liquid nitrogen is required to maintain a constant level during electrospraying. - Set the syringe pump to an infusion rate of 30 mL/h.
NOTE: Varying the infusion rate can alter the microcarrier shape and diameter, but the recommended range is 25–35 mL/h. While it is highly dependent on the viscosity of the suspension, flow rates below 25 mL/h may result in the generation of larger microcarriers. At very low flow rates, the size and shape of the microcarriers may become less homogeneous. At infusion rates above 35 mL/h, the uniformity of the droplets generated via electrospraying may be disrupted, resulting in non-spherical microcarriers with a broader size distribution. - Apply a voltage of 20 kV and turn on the syringe pump to initiate electrospraying.
NOTE: The voltage may be varied to tune the size of the microcarriers and tends to have a greater impact on the diameter than the infusion rate. The recommended voltage range is 15–25 kV. A decrease in microcarrier diameter is expected with an increase in voltage. - Once the infusion is complete, carefully pour off excess liquid nitrogen from the Dewar, leaving the microcarriers suspended in ~25 mL of liquid nitrogen to ensure they remain frozen.
- Immediately transfer the microcarriers with the liquid nitrogen into a 50 mL centrifuge tube by pouring in one smooth motion. Collect any frozen microcarriers remaining in the Dewar with a scoopula and add them to the centrifuge tube.
NOTE: Depending on the size of the Dewar, the microcarriers in liquid nitrogen can be initially poured into a mid-sized vessel and then transferred immediately into the 50 mL centrifuge tube. - Cover the centrifuge tube containing the microcarriers in liquid nitrogen with aluminum foil perforated with small holes in preparation for lyophilization.
- Place the covered centrifuge tubes into a lyophilizer flask. Immediately connect the flask to the lyophilizer and dry the samples O/N.
NOTE: It is very important to keep the microcarriers suspended in liquid nitrogen to ensure they do not thaw before this step, as thawing may result in collapse and/or aggregation. DAT, DDT, and DLV microcarriers fabricated at a concentration of 35 mg/mL using the electrospraying conditions described above will typically have a hydrated diameter ranging between 350–500 μm in size. The size distribution may vary depending on the ECM source. - Store the lyophilized microcarriers in a desiccator until required.
Representative Results
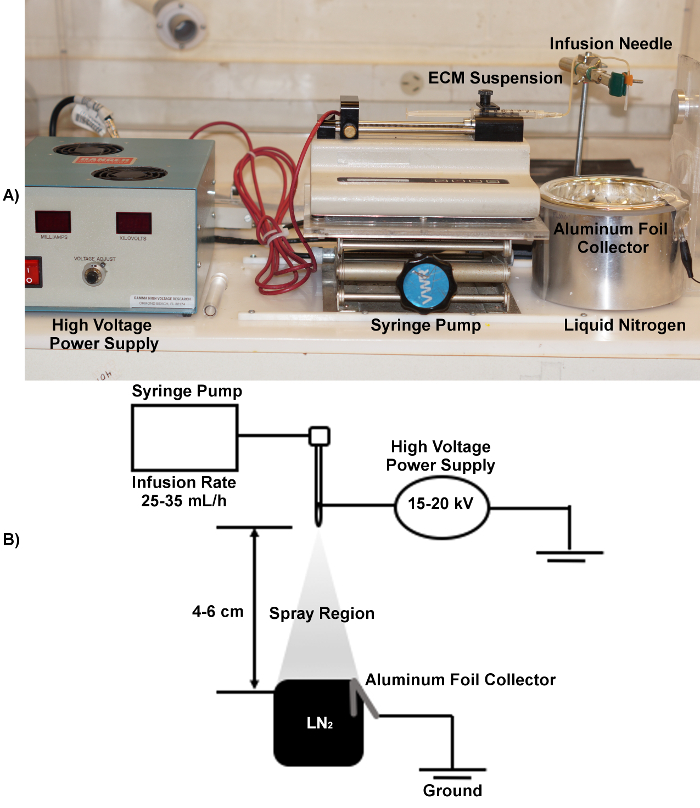
Figure 1. Overview of the electrospraying apparatus used in microcarrier fabrication. A: Image showing the arrangement of the key electrospraying equipment including the syringe pump and high voltage power supply, as well as the positioning of the needle relative to the Dewar of liquid nitrogen. B: Electrospraying schematic, including the recommended ranges for the voltage, infusion rate, and distance.
Offenlegungen
The authors have nothing to disclose.
Materials
| Alligator clip leads | VWR | 470149-728 | |
| Aluminium foil | Fisher Scientific | 01-213-101 | |
| Centrifuge tubes (15 mL) | Sarstedt | 62.554.205 | |
| Centrifuge tubes (50 mL) | Sarstedt | 62.547.205 | |
| Dessicator | Fisher Scientific | 8624426 | For lyophilized ECM and bioscaffold storage |
| Dewar flask | Fisher Scientific | 10-196-6 | Low form; volume range of 250 – 500 mL |
| ECM (Extracellular Matrix) | Isolated from human adipose tissue, porcine dermis or porcine myocardium, as described in Flynn et al. 2010, Reing et al. 2010, and Wainwright et al. 2010 (ref # 28, 8, 32) | ||
| Liquid nitrogen | For electrospraying | ||
| Lyophilizer | Labconco | 7750021 | FreeZone4.5 |
| Orbital incubator shaker | SciLogex | 8.3201E+11 | Temperature controlled (37 °C) |
| Retort stand | VWR | 470019-526 | |
| Retort stand clamp | VWR | 21573-606 | |
| Safety-Lok Syringe | BD | 309606 | 3 mL Luer lock syringe for microcarrier fabrication and dispensing ECM suspension |
| Scoopula | VWR | 89259-968 | For collecting microcarriers |
| Syringe pump | VWR | 10117-490 | Microprocessor controlled |
| High voltage power supply | Gamma High Voltage Research | ES30P-5W/DDPM | Capable of recommended 15 – 25 kV voltage range |
| Winged infusion set | Terumo | 22258092 | 30 cm tubing length, 25 G 3/4" recommended; Other needle gauges can be used and may influence microcarrier diameter |

