Intermittent Photic Stimulation in Rabbit Model: A Technique to Induce Epileptic Seizures and Simultaneously Record Multisystem Parameters
Abstract
Source: Bosinski, C., et al. Multi-system Monitoring for Identification of Seizures, Arrhythmias and Apnea in Conscious Restrained Rabbits. J. Vis. Exp. (2021).
In this video, we show a procedure for simultaneous recording of video-EEG-ECG-capnography-oximetry data generated by inducing seizures in a rabbit model.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Photic stimulation experiment
- Place a light source with a circular reflector 30 cm in front of the rabbit at eye level, with the flash intensity set to the maximum (16 candela). The light source is indicated by a white dot in Figure 1E.
NOTE: A dimly lit room should be used to elicit the photosensitive response. - As the rabbit's eyes are on the side of the head instead of the front of its head (as in humans), place 2 mirrors on either side of the rabbit, and 1 behind the rabbit so that the light enters the rabbit's eyes.
NOTE: A flat mirror that is ≥ 20 cm tall, by ≥ 120 cm long creates a triangular enclosure around the rabbit to ensure that the flashing light enters the rabbit's eyes, as seen in Figure 1E. - Connect the light source to a controller that has an adjustable rate, intensity, and duration.
- Record video using a camera with a red light and infrared recording capabilities.
- Expose the rabbits to each frequency for 30 s with their eyes open and then another 30 s with a surgical mask covering their face to simulate or cause eye closure at each frequency.
NOTE: Previous studies have shown that eye closure is the most provocative maneuver for eliciting photosensitivity to seizure. In addition, 10% of photosensitive patients only exhibit electroencephalographic signs while their eyes are closed. A seizure can be identified clinically by observing the presence of head and whole-body myoclonic jerks, clonus, or a tonic state. The EEG recording is more thoroughly analyzed for electroencephalographic correlation (e.g., spikes, poly-spikes, and rhythmic discharges) with motor manifestations for a definitive diagnosis of seizure activity. Movements in which the EEG is obscured by muscle artifact or waves of indeterminant epileptogenicity should be reviewed by an epileptologist for confirmation. - Increase the photic stimulator frequency from 1 Hz to 25 Hz in 2 Hz increments. Then perform the same photo-stimulation protocol, but this time decrease the frequency from 60 Hz to 25 Hz in 5 Hz increments.
NOTE: If a rabbit has a seizure, the experiment should be stopped. Continue to monitor the rabbit for 30 min. Then return the rabbit to the housing room and monitor every 1 h for 3 h for full recovery. However, if the photic stimulation induces a photoparoxysmal response, then the remainder of ascending frequencies are skipped, and the series is started again by descending from 60 Hz until another photoparoxysmal response occurs. This will allow for the determination of the upper and lower photic stimulation thresholds. No delay is necessary as the photoparoxysmal response will cease after the photic stimulation is discontinued. If it is unclear whether a photoparoxysmal response has occurred, the frequency is repeated after a 10 s delay. - After the experiment is completed, remove EEG and ECG leads from the rabbit and return it to its home cage for routine care by husbandry staff.
Representative Results
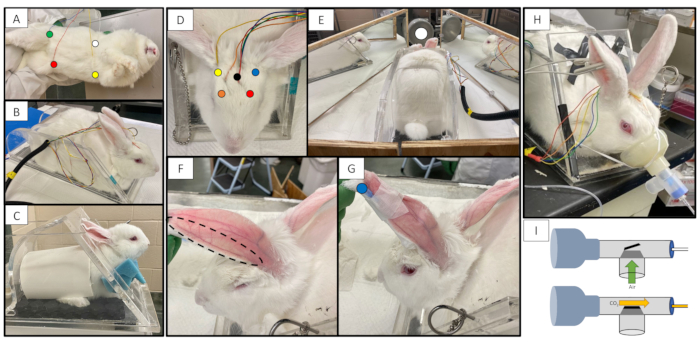
Figure 1: Rabbit connected to equipment. (A) Location of ECG electrodes, Left Arm is indicated by a yellow dot. Right Arm is indicated by a white dot. Left Leg is indicated by a red dot. Ground anterior to the right leg is indicated by a green dot. (B) Rabbit in restrainer with ECG and EEG electrodes attached. (C) Juvenile rabbit in a restrainer with appropriate modifications to accommodate a smaller rabbit, including a booster beneath the rabbit, neck foam and cut PVC pipe. (D) Rabbit in restrainer with location of EEG electrodes. Right Frontal is indicated by an orange dot. Left Frontal is indicated by a red dot. Right Occipital is indicated by a yellow dot. Left Occipital is indicated by a blue dot. The reference is indicated by a black dot. (E) Rabbit in restrainer with photic stimulator and mirror booth setup. Light source is indicated by a white dot. (F) Marginal ear vein after rabbit's ear has been shaven and wiped with alcohol. (G) Rabbit with angiocatheter securely taped in the left marginal ear vein. Site of injection plug is indicated with a blue dot. (H) Rabbit with facemask attached to the capnography tubing by a T-piece that contains a one-way valve. (I) Diagram of the facemask and T-piece connected to the capnography tubing. During inspiration, room air can enter the T-piece through a one-way valve (green arrow). During expiration, CO2 leaves the T-piece by entering the capnography tubing (yellow arrow.) Because of the small amount of dead space, very little CO2 is retained in the T-piece and is generally less than 5 mmHg.
Offenlegungen
The authors have nothing to disclose.
Materials
| Computer | Dell | Optiplex 5040 | Acquisition computer |
| ECG Electrode | RhythmLink | RLSND116-2.5 | 13mm 35-degree bent (0.4 mm diameter) subdermal pin electrodes |
| EEG Electrode | RhythmLink | RLSP513 | 5-twist 13mm straight (0.4mm diameter) subdermal pin electrodes |
| EEGLAB (2020) | Swartz Center for Computational Neuroscience | Open Access | Can perform spectral analysis of EEG |
| Ethernet-to-ethernet adapter | Linksys | USB3G16 | Adapter for connecting the camera to the computer |
| Foam padding | Generic | N/A | Reduces pressure applied to the neck of small rabbits by the restrainer in order to prevent the adverse cardiorespiratory effects of neck compression |
| IR Light | Bosch | EX12LED-3BD-8W | Facilitates recordings in the dark |
| LabChart Pro (2019, Version 8.1.16) | ADInstruments | N/A | ECG Analysis |
| MATLAB (R2019b, Update 5) | MathWorks | N/A | Required to run EEGLAB |
| Microphone | Sony Stereo | ECM-D570P | Recording of audible manifestions of seizures |
| Micropore Medical Tape, Paper, White | 3M | 1530-1 | Used to secure wires and create ear splint |
| Natus NeuroWorks | Natus | LC101-8 | Acquisition and review software |
| Photic Stimulator | Grass | PS22 | Stimulator to control frequency, delay, duration, intensity of the light pulses |
| Plastic wire organizer / bundler | 12Vwire.com | LM-12-100-BLK | Bundle wires to cut down on noise |
| PS 22 Photic Stimulator | Grass Instruments | BZA641035 | Strobe light with adjustable flash frequency, delay, and intensity |
| PVC pipe | Generic | N/A | Prevents small rabbits from kicking their hind legs and causing spinal injury |
| Quantum Amplifier | Natus | 13926 | Amplifier / digitizer |
| Quantum HeadBox Amplifier | Natus | 22134 | 64-pin breakout box |
| Rabbit Restrainer | Plas-Labs | 501-TC | Various size rabbit restrainers are available. 6" x 18" x 6" in this study. |
| Rubber pad (booster) | Generic | N/A | Raises small rabbits up in the restrainer to prevent neck compression |
| SpO2 ear clip | NONIN | 61000 | PureSAT/SpO2 |
| SpO2 sensor adapter | NONIN | 13931 | XPOD PureSAT/SpO2 |
| SRG-X120 1080p PTZ Camera with HDMI, IP & 3G-SDI Output | Sony | SRG-X120 | Impela Camera |

