Protein Phase Separation Assay: An Optogenetic Method for Mutant RNA-Binding Protein Phase Separation in Spinal Motor Neurons of Zebrafish Larvae
Abstract
Source: Asakawa, K. et al. Optogenetic Phase Transition of TDP-43 in Spinal Motor Neurons of Zebrafish Larvae. J. Vis. Exp. (2022)
In this video, we describe a phase separation assay, wherein intracellular proteins comprising intrinsically disordered regions fused to a photosensitive oligomerization domain are induced via blue-light exposure to self-associate into membrane-less liquid-like condensates.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board
1. Preparation of LED for blue light illumination
- Turn on an LED panel by using the associated application installed on a tablet/phone. Put the probe of a spectrometer into an empty well of a 6-well dish and adjust the LED light to the wavelength peaking at ~456 nm through the application. Place the optical sensor of an optical power meter in the empty well and adjust the power of the LED light (~0.61 mW/cm2). The LED light setting can be saved and is retrievable in the application.
- Introduce the dish/LED panel setting to the incubator at 28 °C. Finish this step before imaging of fish starts at 48 hpf.
2. Imaging of zebrafish larvae expressing optogenetic TDP-43
- Select Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish at least before 47 hpf, based on RFP (opTDP-43h) or EGFP (EGFP-TDP-43z) fluorescence in the spinal motor column using the epifluorescence microscope equipped with a Plan-Neofluar 5x/0.15 objective lens.
- Dechorionate the Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish.
- Preheat 1% low melting temperature agarose containing 250 µg/mL of ethyl 3-aminobenzoate methanesulfonate salt at 42 °C.
- Briefly anesthetize Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43] double-transgenic fish at 48 hpf in E3 buffer containing the same concentration of Tricane.
- Put a drop of the preheated 1% low melting temperature agarose on the glass base dish at the room temperature. The diameter of the dome-shaped agarose drop on the glass dish is 8-10 mm.
- Using a Pasteur pipette, add the anesthetized fish to the low melting temperature agarose on the glass base dish, and then mix by pipetting a few times. Minimize the amount of the E3 buffer added to the agarose along with the fish.
- Maintain the fish on its side by using a syringe needle during the solidification of agarose (typically ~1 min) to ensure that the spinal cord is in an appropriate horizontal position. After the solidification, put a couple of drops of E3 buffer onto the dome-shaped agarose-mounted fish.
- Acquire serial confocal z-sections of the spinal cord by scanning with a confocal microscope equipped with a 20x water immersion objective lens with the numerical aperture 1.00, using a scan speed of 4.0 µs per pixel (12 bits per pixel), a step size of 1.0 µm per slice for the objective, and a combination of excitation/emission wavelengths: Channel 1) 473/510 nm for EGFP and Channel 2) 559/583 nm for mRFP1.
NOTE: The cloaca on the ventral side of the fish is included in the regions of interest (ROI) as a reference, which helps to identify and compare the spinal segments (levels 16-17) across the time points. - Remove the fish from the agarose by carefully cracking the agarose with a syringe needle as soon as the imaging is complete. Keep the amount of time the fish is embedded in the agarose as short as possible, although the agarose embedding for <30 min does not affect the viability of the fish.
3. Light stimulation of opTDP-43h-expressing fish by field illumination of a blue light-emitting diode (LED) light
- Add 7.5 mL of E3 buffer to the well and place the imaged Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish into the well. Place the six-well dish on the LED panel by keeping the dish and LED panel 5 mm apart with a spacer (for example, with five slide glasses stacked).
- Turn on the blue LED light. Keep some of the Tg[mnr2b-hs:opTDP-43h] Tg[mnr2b-hs:EGFP-TDP-43z] double-transgenic fish in a separate six-well dish covered with aluminum foil when unilluminated control fish are necessary (i.e., in dark conditions).
- After the illumination (e.g., for 24 h at 72 hpf in Figure 1), image the spinal cord of the illuminated fish by repeating the steps 2.3 – 2.9.
Representative Results
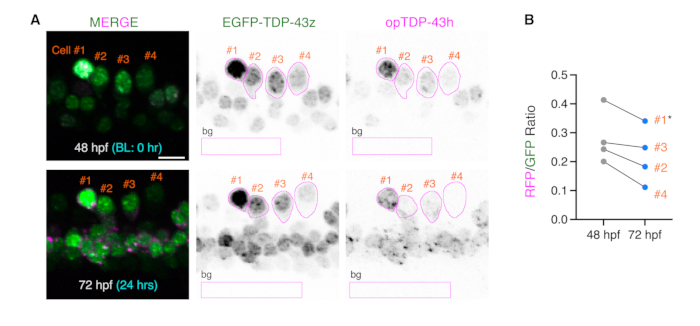
Figure 1: Ratiometric comparisons of opTDP-43h and EGFP-TDP-43z before and after light stimulation. (A) ROIs covering the somas of four single mnr2b-positive cells at 48 and 72 hpf were drawn based on the EGFP-TDP-43z signal and are shown in magenta. The rectangular ROIs (bg) were used to subtract the background signal (background ROI). Figures are adapted from Asakawa et al. (B) The relative intensities of opTDP-43h to EGFP-TDP-43z were plotted for each cell at each time point as the RFP/GFP ratio. Cell #1, indicated by the asterisk, was not suitable for the ratiometric comparison because its EGFP-TDP-43z signal was saturated and its RFP/GFP value was overestimated. The bar indicates 10 µm.
Offenlegungen
The authors have nothing to disclose.
Materials
| Confocal microscope | Olympus | FV1200 | |
| Epifluorescence microscope | ZEISS | Axioimager Z1 | |
| Fluorescence stereomicroscope | Leica | MZ16FA | |
| Glass base dish | IWAKI | 3910-035 | |
| Incubator | MEE | CN-25C | |
| LED panel | Nanoleaf Limited | Nanoleaf AURORA smarter kit | |
| Objective lens | Olympus | XLUMPlanFL N 20×/1.00 | |
| Objective lens | ZEISS | Plan-Neofluar 5x/0.15 | |
| Optical power meter | HIOKI | 3664 | |
| Optical sensor | HIOKI | 9742-10 | |
| Six-well dish | FALCON | 353046 | |
| Spectrometer probe BLUE-Wave | StellerNet Inc. | VIS-50 | |
| Syringe needle | TERUMO | NN-2725R | |
| Tricane | Sigma-Aldrich | A5040 | |
| NuSieve GTG Agarose | LONZA | 50181 |

