Flow Cytometry Assay to Quantify Oxidative Stress: An Assay to Quantify Reactive Oxygen Species in Intestinal Organoids Using Fluorogenic Probes
Abstract
Source: Stedman, A., et al., Analyzing Oxidative Stress in Murine Intestinal Organoids using Reactive Oxygen Species-Sensitive Fluorogenic Probe. J. Vis. Exp. (2021).
This video demonstrates a flow cytometry assay to quantify the ROS levels in 3D intestinal organoids obtained from GFP-expressing transgenic mice. This method provides an in vitro experimental model to investigate the efficacy of test compounds on ROS production.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of reagents and materials for culturing intestinal organoids
- To prepare growth culture medium, mix advanced DMEM/F-12 supplemented with 1x glutamine, 1x penicillin/streptomycin (P/S) solution, 10 mM of HEPES, 50 ng/mL of murine EGF, 20 µg/mL of murine Noggin 500 ng/mL of mouse R-Spondin1 (see Table of Materials). Leave the medium at room temperature (RT) during the crypt's extraction.
NOTE: Freeze the unused medium in aliquots at -20 °C. Avoid freeze and thaw. - Fill a 50 mL tube with 40 mL of Advanced DMEM/F-12 and keep it on ice.
NOTE: Keep the unused medium at 4 °C. It will be used for organoid passaging. - Pre-warm the cell culture plates (96-well round bottom) in the incubator at 37 °C.
- Thaw basement membrane matrix (BMM) (see Table of Materials) aliquots on ice (before starting the protocol or at least 1 h before plating crypts).
NOTE: The BMM will quickly solidify if not kept cold. - Prepare washing/flushing solution by adding 1% penicillin-streptomycin solution to DPBS (DPBS-P/S).
- Fill a 100 mm petri-dish with 10 mL of cold DPBS-P/S. Fill six 15 mL tubes with 10 mL of DPBS and label them from F1 to F6.
- Prepare 30 mL of 10 mM EDTA solution by dilution from 0.5 M EDTA in DPBS. Fill two 15 mL tubes with 10 mL of 10 mM EDTA, and label them E1 and E2.
- Keep all solutions pre-cooled at 4°C and keep them on the ice during the procedure.
2. Intestinal organoids culture
- Sacrifice an 8-10 weeks-old Lgr5-EGFP-IRES-creERT2 (Lgr5-GFP) mouse according to the national rules and regulations.
- Collect 5-8 cm of the jejunum encompassing the region between the duodenum (5 cm from the stomach) and the ileum (10 cm from the cecum) and keep in cold DPBS-P/S on ice.
- Clean the intestinal content by flushing with 5-10 mL of cold DPBS-P/S.
NOTE: Home-made flushing syringes can be obtained by plugging a 200 µL tip onto a 10 mL syringe nozzle. - Open the intestine longitudinally using ball-tip scissors (see Table of Materials) (to prevent damaging the tissue).
- Using forceps, transfer the tissue into a petri dish containing cold DPBS-P/S at room temperature and shake it to rinse.
- With a plastic Pasteur pipette, grab the intestine by aspiration and transfer it into a 15 mL tube labeled E1 containing 10 mL cold 10 mM EDTA.
- Invert the tube 3 times and incubate on ice for 10 min.
- Using a plastic Pasteur pipette, transfer the tissue in tube F1 containing 10 mL DPBS. Vortex for 2 min (on normal vortex, holding the tube by hand and ensuring that the intestine swirls nicely).
- Put 10 µL of the fraction in a petri dish and assess the quality of the fraction under a microscope.
NOTE: All vortex steps are performed at maximum speed, and the quality of each fraction should be assessed under the microscope (Figure 1). - With a plastic Pasteur pipette, grab the intestine by aspiration and transfer it in tube F2 containing 10 mL DPBS and vortex for 2 min.
- Repeat step 2.10, transferring the tissue in tube F3 containing 10 mL DPBS and vortex for 2 min.
- Repeat EDTA incubation as in step 2.6, transferring the tissue in tube E2 containing 10 mM EDTA.
- Invert the tube 3 times and incubate on ice for 5 min.
- Repeat step 2.10, transferring the tissue in tube F4 containing 10 mL DPBS and vortex for 3 min.
- Repeat step 2.14, transferring the tissue in tube F5 containing 10 mL DPBS and vortex for 3 min.
- Repeat step 2.15, transferring the tissue in tube F6 containing 10 mL DPBS and vortex for 3 min.
- Combine the best fractions filtering by gravity through a 70 µm cell strainer into a 50 mL tube (on ice) to eliminate villi and significant debris.
NOTE: Usually, F5 and F6 are the fractions containing numerous crypts and less debris. - Spin the crypts at 150 x g at 4 °C for 3 min.
- Empty the tube, disrupt the pellet mechanically, and add 5 mL of cold DMEM/F12.
- Put 10 µL of the suspension in a petri dish and count the number of crypts present in the aliquot manually under a microscope.
NOTE: Do not count single cells or small debris. - Calculate the volume (V) of crypts’ suspension required in µL, considering that 300 crypts are plated per well, W is the number of wells, and N is the number of crypts counted out of 10 µL of the suspension. Transfer the crypts to a new 15 mL tube.
NOTE: V = 300 x W x 10/N. If a small volume is used in the planned experiment, a 1.5 mL centrifuge tube can be used. - Spin the crypts at 200 x g at 4 °C for 3 min.
- Carefully remove the supernatant using a pipette.
- Mechanically disrupt the pellet and gently add growth culture medium to obtain a concentration of 90 crypts/µL.
- Add 2 volumes of undiluted BMM to have a final concentration of 30 crypts/µL. Carefully pipette up and down without introducing air bubbles into the mix.
NOTE: Always keep the tube on ice to avoid BMM solidification. - Plate 10 µL of the crypts/BMM mix into each well. For Flow cytometry analysis, use round-bottom 96-well plates. Distribute 10 µL at the center of each well as a dome.
NOTE: Plate the organoids as a thin layer for the imaging assay to enable their in-depth imaging. - Leave the plate for 5 min at RT to allow the BMM to solidify. Place the plate in the incubator at 37 °C and 5% CO2 for 15 min.
- Add 250 µL of growth medium into each well, taking care not to detach the BMM.
- Place the plates in the incubator at 37 °C and 5% CO2.
- Perform the ROS analysis between days 4 and 6 of culture. Otherwise, change the medium and split the organoids after the appearance of several long budding structures and when dead cells accumulate into the organoids' lumens.
3. Organoids passaging
- Start passaging small intestinal organoids from the 6th day of culture, when significant budding structures have formed, and the organoids’ lumens have become dark.
NOTE: The organoid's lumen becomes dark due to the accumulation of dead cells, debris, and mucus. Avoid letting the organoids overgrow before splitting. The splitting ratio depends on the organoids' growth. Passaging the organoids with a ratio of 1:2 at day 6 and 1:3 at day 10 is recommended. - Fill a 15 mL tube with 4 mL of cold Advanced DMEM/F-12 and keep it on ice.
NOTE: Here, volumes for a 96-well culture plate are provided. If a different format is used, adjust the volume accordingly. - Carefully aspirate the medium with a pipette or a vacuum pump from the wells without touching the BMM domes, and discard it.
- Add 100 µL of cold Advanced DMEM/F-12 per well. Pipette up and down to break the BMM and transfer the content of the well into the 15 mL tube.
- Wash the well with 200 µL cold Advanced DMEM/F-12 and collect it in the same tube.
NOTE: If passaging multiple wells from the same experimental condition, the contents of the wells can be pooled in the same 15 mL collecting tube. - Spin the 15 mL collecting tube at 100 x g for 5 min at 4°C.
- Discard the supernatant and add 1mL of cold Advanced DMEM/F-12 to the pellet. Using a P1000 tip, take up a P10 tip (without filter) and pipette up and down at least 20 times.
- Add 4 mL of cold Advanced DMEM/F-12 to the tube. Spin at 300 x g for 5 min at 4°C.
- Aspirate the supernatant with a pipette or a vacuum pump without disturbing the pellet. Then, disrupt the pellet mechanically.
- Add BMM diluted in growth culture medium (2:1 ratio). Carefully pipette up and down without introducing air bubbles into the mix.
- Plate 10 µL of the crypts/BMM mix into each well.
- Keep the plate for 5 min at RT to allow the BMM to solidify. Place the plate in the incubator at 37 °C and 5% CO2 for 15 min.
- Add 250 µL of growth medium into each well.
NOTE: Be careful not to detach the BMM. - Place the plates in the incubator at 37 °C and 5% CO2.
4. Preparation of reagents and materials to assess oxidative stress in intestinal organoids
- Prepare a 250 mM stock solution of inhibitor N-acetylcysteine (NAC) (see Table of Materials), and resuspend 10 mg with 245 µL of DPBS. Use at 1 mM final concentration.
- Prepare a 50 mM stock solution of inducer Tert-butyl hydroperoxide (tBHP), 70% in water, and dilute 3.22 µL with 496.8 µL of DPBS. Use at 200 µM final concentration.
- For the Flow cytometry study, prepare a 250 µM working solution of a fluorogenic probe (see Table of Materials) by diluting the stock solution 1/10 in DMSO. Use at 1 µM final concentration.
NOTE: As indicated in the manufacturer's instructions, the fluorogenic probe is sensitive to light and oxygen. Stocks and aliquots should not be opened and closed too many times. - Prepare a final solution of 0.1 µg/mL DAPI in DPBS, to be used for dead cell discrimination in the Flow cytometry assay.
NOTE: These steps describe using negative and positive controls that must be included in any assays, using the conditions indicated in Figure 2A. The assay can be used to test anti- or pro-oxidant compounds. The steps are the same, and the only difference is when the compounds are added before using the fluorogenic dye.
5. Quantification of the oxidative stress on the dissociated organoids using flow cytometer
- Add 1 µL NAC stock solution in the wells for negative controls to obtain a final concentration of 1 mM.
NOTE: Use the organoids plated in the 96-well round-bottom plates. - Incubate for 1 h at 37 °C and 5% CO2.
- Add 1 µL tBHP stock solution in the corresponding wells to obtain a final concentration of 200 µM.
- Incubate for 30 min at 37 °C and 5% CO2.
- With a multichannel pipette, remove the medium without disturbing the attached BMM and transfer it to another 96-well round bottom plate. Keep this plate aside.
- Add 100 µL of trypsin, and with a multichannel pipette, pipette up and down at least five times to destroy the BMM.
- Incubate for not more than 5 min at 37 °C and 5% CO2.
- With a multichannel pipette, pipette up and down at least five times to dissociate the organoids.
- Spin at 300 x g for 5 min at RT.
- Discard the supernatant by inverting the plate. Add back the medium collected in step 6.3 to the corresponding wells and resuspend the cells by pipetting up and down 5 times.
- Add the fluorogenic probe at the final concentration of 1 µM. Add 1 µL per well from the 250 µM dilution and incubate for 30 min at 37 °C and 5% CO2.
NOTE: Do not add the fluorogenic probe to the wells needed for the instrument's settings (Figure 2B). - Spin at 300 x g for 5 min at RT.
- Resuspend the cells with 250 µL of 0.1 µg/mL DAPI solution. Transfer the samples in the proper Flow cytometry tubes, keep the tubes on ice and proceed with the analysis.
NOTE: Add PBS instead of DAPI to the wells needed for the instrument's settings (Figure 2B). - Optimize the forward and side scatter voltage settings on unstained control and laser voltages for each fluorophore using mono-stained samples.
- Using an appropriate gating strategy (Figure 3A), collect a minimum of 20,000 events.
NOTE: 50,000 events are preferred. Detailed acquisition settings vary according to the instrument used.
Representative Results
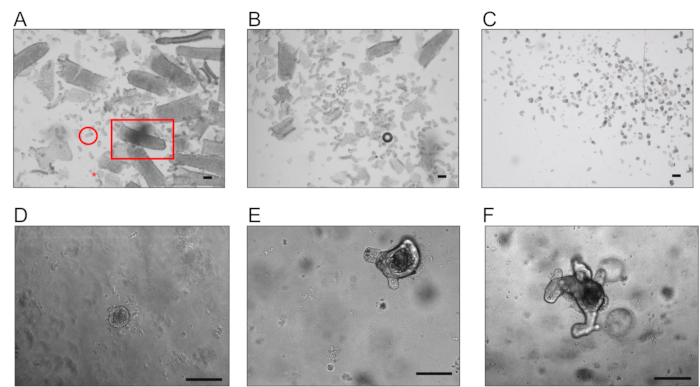
Figure 1: Representative images of crypts and organoids. (A) Example of fraction F1 obtained after the first incubation with EDTA, enriched in villi (square), with some debris (star) and crypts (circle). (B) Example of fraction F4 enriched in crypts. (C) Suspension presenting only isolated crypts obtained after the filtration with a 70 µm cell strainer (scale bar, 200 µm). (D, E, and F). Typical organoids were obtained after 1, 3, and 5 days respectively, after embedding the crypts in BMM (scale bar, 100 µm).
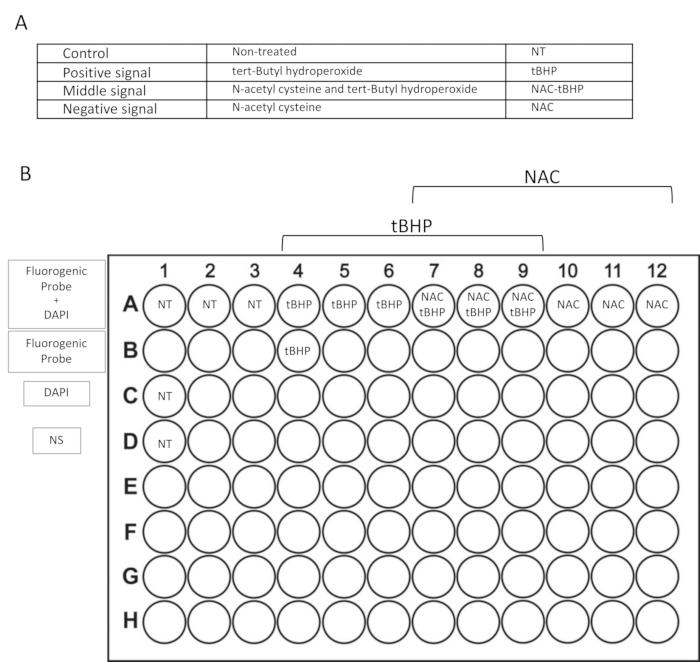
Figure 2: Outline of the experimental plan. (A) Conditions used in this protocol included in each experiment: non-treated wells (NT), inducer-treated wells (tert-Butyl hydroperoxide – tBHP), inhibitor-treated wells (N-acetyl cysteine – NAC), and inhibitor- and inducer-treated wells (NAC-tBHP). (B) Plate format for the flow cytometry assay. Each condition is plated in triplicate (line A). Lines B, C, and D include wells for flow cytometer setting with only the fluorogenic probe, only DAPI, or non-stained (NS) samples.
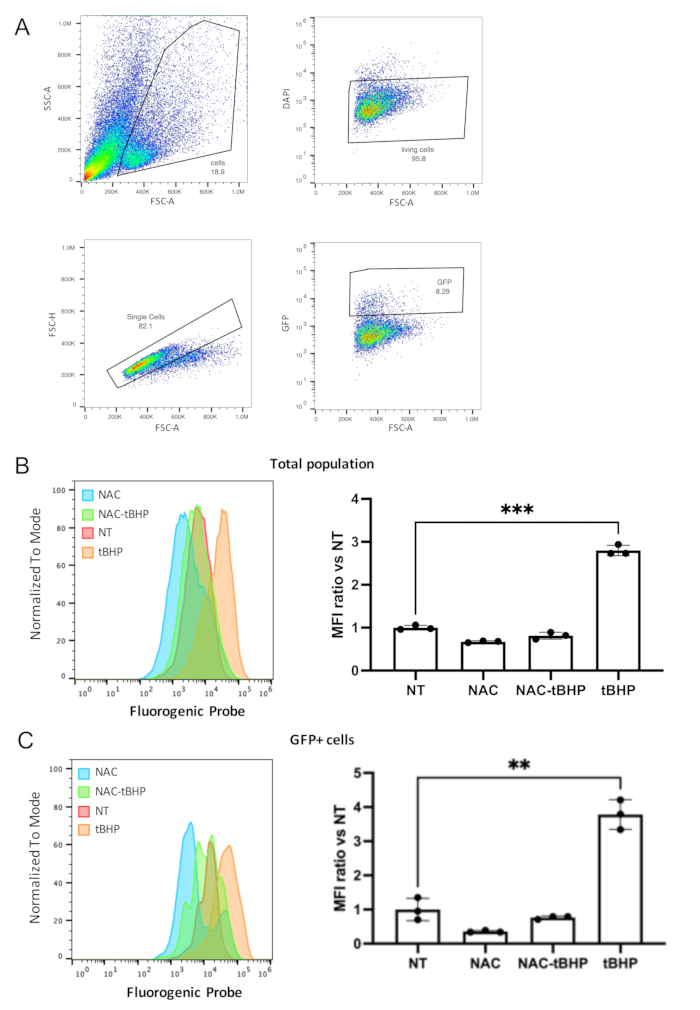
Figure 3: Representative flow cytometry analysis of ROS in cells derived from organoids. (A) Schematic representation of the gating strategy used in flow cytometry analysis: gating for cell shape (exclusion of dead cells and debris accumulated in the organoids lumen), gating for living cells (cells not incorporating DAPI-laser 405), gating for single cells (doublet discrimination), and stem cells (GFP positive cells-laser 488) (FSC: forward scatter, SSC: side scatter). The ROS signal has been acquired using the 630 laser. (B) On the left, histograms were obtained with an appropriate software showing the intensity ROS signals for the total living population (after gating around 10,000 events per condition) in the different samples NT: non-treated; NAC: inhibitor-treated; tBHP inducer-treated; NAC-tBHP: inhibitor- and inducer-treated. On the right, a typical example of the calculated ratio for MFI values over the NT samples obtained during an experiment starting from 3 samples per condition (mean ± SD) (*** P = 0.0003). (C) Same as in B for the GFP-positive population (1,000 events per condition) (* P = 0.02).
Offenlegungen
The authors have nothing to disclose.
Materials
| Mice | |||
| Lgr5-EGFP-IRES-creERT2 (Lgr5-GFP) | The Jackson Laboratory | ||
| Growth culture medium | |||
| Advanced DMEM F12 (DMEM/F12) | ThermoFisher | 12634010 | |
| B-27 Supplement, minus vitamin A | ThermoFisher | 12587010 | Stock Concentration: 50x |
| GlutaMAX (glutamine) | ThermoFisher | 35050038 | Stock Concentration: 100x |
| Hepes | ThermoFisher | 15630056 | Stock Concentration: 1 M |
| Murine EGF | R&D | 2028-EG-200 | Stock Concentration: 500 µg/mL in PBS |
| murine Noggin | R&D | 1967-NG/CF | Stock Concentration: 100 µg/mL in PBS |
| Murine R-spondin1 | R&D | 3474-RS-050 | Stock Concentration: 50 µg/mL in PBS |
| N-2 Supplement | ThermoFisher | 17502048 | Stock Concentration: 100x |
| Penicillin-Streptomycin (P/S) | ThermoFisher | 15140122 | Stock Concentration: 100x (10,000 units/mL of penicillin and 10,000 µg/mL of streptomycin) |
| Material | |||
| 70 µm cell strainer | Corning | 352350 | |
| 96-well round bottom | Corning | 3799 | |
| ball tip scissor | Fine Science Tools GMBH | 14086-09 | |
| CellROX® Deep Red Reagent | ThermoFisher | C10422 | |
| DAPI (4’,6-diamidino-2-phénylindole, dichlorhydrate) (fluorgenic probe) | ThermoFisher | D1306 | stock at 10 mg/mL |
| DPBS 1x no calcium no magnesium (DPBS) | ThermoFisher | 14190144 | |
| Matrigel Growth Factor Reduced, Phenol Red Free (Basement Membrane Matrix) | Corning | 356231 | once received thaw o/n in the fridge, keep for 1h on ice and, make 500 mL aliquots and store at -20 °C |
| N-acetylcysteine (NAC) | Sigma | A9165 | |
| tert-Butyl hydroperoxide (tBCHP)solution (70%wt. In H2O2) | Sigma | 458139 | |
| TrypLE Express Enzyme (1X), no phenol red (trypsin) | ThermoFisher | 12604013 | |
| UltraPure 0.5 M EDTA, pH8.0 | ThermoFisher | 15575020 | |
| Y-27632 | Sigma | Y0503 | Rock-inhibitor to be used to minimize cell death upon tissue dissociation |
| Programs and Equipment | |||
| Attune NxT (Flow Cytometer) | ThermoFischer | Flow cytometer analyzer | |
| FlowJo | BD Bioscience | FACS analysis | |
| Prism | GraphPad Software | statistical analysis |

