Establishing Villous and Decidual Organ Cultures as Ex Vivo Models of Human Maternal-Fetal Interface
Abstract
Source: Rizzuto, G. A., et al. Human Placental and Decidual Organ Cultures to Study Infections at the Maternal-fetal Interface. J. Vis. Exp. (2016).
This video demonstrates the establishment of primary human placental villous and decidual organ cultures in ex vivo conditions. These models are well-suited for studying pathogenesis at the human maternal-fetal interface.
Protocol
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board.
1. Preparation, Prior to Collection Day
- Autoclave strainers/forceps for collection, and scissors/forceps for micro-dissection.
- Prepare an adequate volume (500 ml per specimen) of Wash buffer (refer to Table 1 for the recipe).
- Prepare an adequate volume (100 ml per specimen) of 0.22 µM sterile-filtered Collection media (refer to Table 1 for the recipe).
- Aliquot Extracellular Matrix (ECM, refer to Table of Reagents for details) for long-term storage.
- Store ECM in solid form at -20 °C. For best performance, avoid repeat freeze-thaw. Thaw bottle to a viscous solution at 4 °C, and prepare 300 µl aliquots in the cold room with chilled pipette tips. Store aliquots at -20 °C.
2. Collection of Fresh Tissue
CAUTION: When working with fresh human tissue, researchers must follow Universal Precautions (OSHA) for preventing transmission of Bloodborne Pathogens, and must have received proper institutional training. Proper personal protective equipment, including gloves, eye protection, and lab coats are necessary.
- Transport autoclaved strainers (one per specimen), autoclaved forceps, Wash buffer (carboy), Collection media (25 ml per specimen in a 50 ml conical tube), spray bottle, 10% bleach, 70% ethanol, tissue collection tray, sharpie, and ice bucket/cooler to hospital/clinic.
NOTE: Specimens are received in a research-designated space located in a room close to the operating suite. A sterile tissue culture hood is not required, but the collection should occur expeditiously and the researcher should appropriately sanitize surfaces, as described below. - Place carboy of Wash buffer adjacent to the sink, and spray the spigot with 10% bleach and 70% ethanol. Place a glass tissue collection tray on the light source (light pad or lightbox), and sanitize with 10% bleach and 70% ethanol. Allow to air dry.
- Have the clinician carry specimens from the operating suite to the specimen preparation room. At the sink, pour the specimen atop a sterile hand-held strainer, rinse the tissue several times in Wash buffer, and transfer the entire specimen in the buffer to a sanitized glass tray atop the light source.
NOTE: Assessment of tissue by the clinician is of the highest priority, thus the following steps are only performed after the clinician has verbally indicated that he/she has finished the evaluation. - Use sterile forceps to select placental villi and decidua (Figures 2A and 3A) for collection, and transfer to separate 50 ml conical tubes. Label the tubes with gestational age.
NOTE: Preferred gestational ages are less than 9 weeks and less than 16 weeks for placenta and decidua, respectively. At the institution, only a fraction of each specimen is procured for research purposes, as adequate tissue must remain for clinical pathologic diagnosis. - Store and transport the specimen on ice to the research lab.
- To collect more than one specimen, sanitize the glass tray as described above with 10% bleach and 70% ethanol between the collection of each specimen.
3. Preparation of Organ Culture Plates
NOTE: The following steps should be performed using sterile technique in a tissue culture hood. It is ideal for the dissecting microscope to be located in a sterile field. However, this is not absolutely necessary as long as micro-dissection is performed expeditiously, and instruments are dipped frequently in 70% ethanol.
- Thaw ECM vial(s) on ice. Dilute the ECM 1:1 with ice-cold Collection media, and mix by pipetting. Pipette slowly to avoid introducing bubbles. Each well of a 6-well tissue culture plate requires 100 µl of this suspension and will accommodate 3-4 organ cultures.
- Place transwell inserts (pore size 0.4 µM) into each well of the 6-well tissue culture plate.
- Coat each transwell insert with a thin layer (100 µl) of the ECM/media suspension.
- Place the plate on ice and immediately proceed to the establishment of organ cultures. Alternatively, prepare the plate prior to tissue collection, in which case wrap it in para-film and store it at 4 °C for later use. Storage longer than 6 hr is not recommended as the ECM will dry out.
4. Establishment of Organ Cultures
NOTE: This step describes how to place decidual organ cultures and villous organ cultures in separate transwells. The tissues are not in contact.
- Rinse specimens twice in Collection media, and centrifuge at 1,000 x g between each rinse. Transfer tissue to a sterile petri dish and place it on the stage of a dissecting microscope. Keep a 6-well plate on ice adjacent to the microscope.
- Micro-dissect tissue with sterile springer dissecting scissors and forceps, to establish 2-3 mm villous organ cultures (Figure 1A).
NOTE: Villous organ cultures are small, well-vascularized "trees" with 2 or 3 branches, and with prominent extravillous trophoblast (EVT) cell columns11. It is important to utilize only villous tissue from < 9-week first trimester specimens, as the invasion of extravillous trophoblasts into ECM is most pronounced at these young ages. - Select well-vascularized villi with prominent EVT cell columns. EVT cell columns are visualized as bulbous, "fuzzy", "fluffy" ends (refer to arrowheads in Figure 1A).
- Use sterile forceps to transfer 3 or 4 villous trees into each transwell. Adjust branches so the tree is positioned flat atop ECM and separate clumped branches.
- Micro-dissect decidual tissue with sterile springer dissecting scissors and forceps, to establish 3 mm3 decidual organ cultures (Figure 2A).
NOTE: Specimens contain two distinct types of decidual tissues, decidua parietalis and decidua capsularis (left and right tissue fragments, respectively, Figure 2A). - Trim with scissors to create 3 mm3 pieces of decidua parietalis. Decidua capsularis is not used for these cultures. Use forceps to tease away any clotted maternal blood.
- Use sterile forceps to transfer 3-4 pieces of decidua into each transwell.
- Add 1 ml of Collection media to the bottom of the well. Incubate plate overnight at 37 °C in 5% CO2
- For experiments where it is important to mimic normal first-trimester placental oxygen tension, maintain villous organ cultures in relative hypoxia (3% O2).
NOTE: Laboratory options include small hypoxia incubator chambers that fit inside existing incubators or a tri-gas incubator that introduces external N2 gas to lower oxygen concentration. - Add 1 ml of Villous or Decidual medium (Table 1) to the top of the transwell after 12-16 hr of culture.
NOTE: The absence of media during the first overnight in culture allows the extravillous trophoblast cell columns to invade (villous culture), and for the tissue (both villous and decidual) to become securely embedded in ECM. In our experience, decidual organ cultures remain viable for 72 hr and villous organ cultures for 96 hr. We recommend experimental endpoints that fall within this timeframe. See Table 1 for the composition of Villous and Decidual medium. The villous medium has a high percentage of FBS, while the Decidual medium is supplemented with pregnancy hormones (progesterone, 17β-estradiol) that maintain the decidualized state (Table 1).
Table 1: Media recipes. Components and concentrations for preparation of Wash buffer, Collection medium, Villous medium, and Decidual medium.
| Wash buffer | Collection medium | Villous medium | Decidual medium |
| PBS | DMEM/F-12 with GlutaMAX | DMEM/F-12 with GlutaMAX | DMEM/F-12 with GlutaMAX |
| Penicillin 100 IU/ml | Fetal Bovine Serum 2.5% | Fetal Bovine Serum 20% | Fetal Bovine Serum 2.5% |
| Streptomycin 100 μg/ml | Penicillin 100 IU/ml | Penicillin 100 IU/ml | 17β-estradiol 300 pg/ml |
| Gentamicin 50 μg/ml | Streptomycin 100 μg/ml | Streptomycin 100 μg/ml | Progesterone 20 ng/ml |
| Amphotericin B 1.25 μg/ml | Gentamicin 50 μg/ml | ||
| Amphotericin B 1.25 μg/ml |
Representative Results
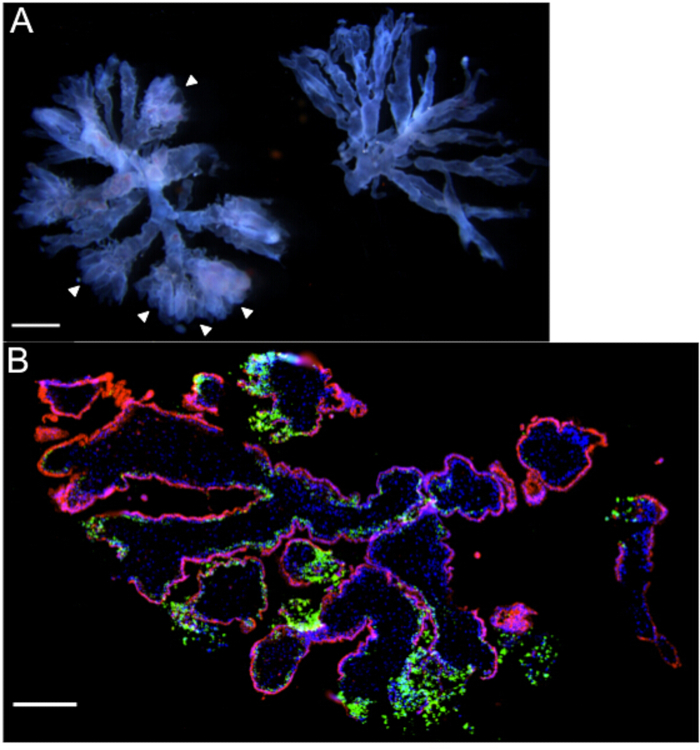
Figure 1: Villous organ cultures – Representative gross and microscopic images. (A) Two terminal villous trees with a gestational age of 6 weeks, as viewed under a dissecting microscope. Note the "fluffy" ends (arrowheads) and prominent fetal vasculature coursing through the branches of the tree on the left that make this piece suitable for organ culture. (B) Immunofluorescence microscopy of organ culture (gestational age 8.3 weeks) 72 hr post-infection with Listeria monocytogenes, highlighting heavy bacterial burden [green] in extravillous trophoblasts. DAPI is shown in blue, and βHCG+ syncytiotrophoblasts in red. Scale bars = 1 mm (A), 250 µm (B).
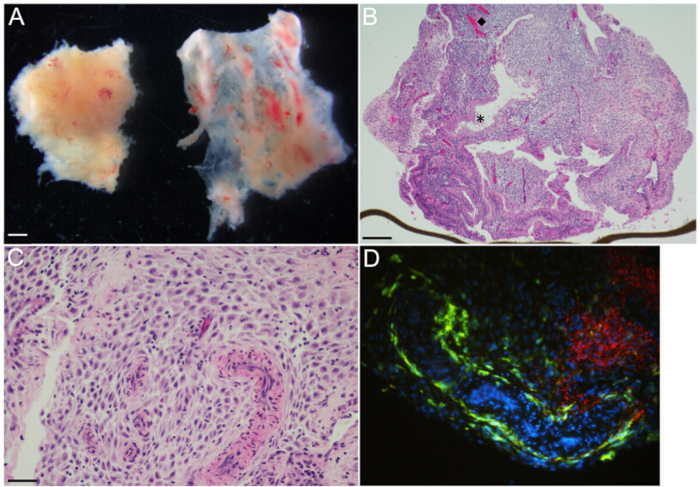
Figure 2: Decidual organ culture – Representative gross and microscopic images. (A) Decidua parietalis [left] and decidua capsularis [right] at gestational age 6 weeks, as viewed under a dissecting microscope. (B) H&E-stained section of 14.3 weeks gestational age decidua parietalis organ culture shows glands and vasculature organized heterogeneously throughout the decidual stroma. The (diamond, ◆) and the (asterisk, *) highlight representative vessels and glands, respectively. The brown line at the lower edge is the transwell membrane, on the edge. (C) H&E-stained section of 14.3 weeks gestational age decidua parietalis organ culture at higher magnification demonstrates muscular-walled spiral arterioles embedded in the decidual stroma. Maternal immune cells are small with dark round nuclei and are irregularly distributed throughout the stroma. (D) Immunofluorescence microscopy of organ culture 48 hr post-infection with L. monocytogenes, highlighting large zones of bacteria [red] in the decidual stroma, and maternal CD14+ macrophages [green] lining a tortuous vascular space and scattered in the stroma. Scale bars = 1 mm (A), 250 µm (B), 50 µm (C and D).
Offenlegungen
The authors have nothing to disclose.
Materials
| Sterilization pouches | Fisher Scientific | 01-812-54 | For autoclaving individual dissecting tools |
| Fine mesh strainer | Cuisinart (Amazon.com) | NA | Wrap completely in aluminum foil and autoclave prior to tissue collection. |
| Carboy with spigot | Fisher Scientific | 03-007-647 | For large volume preparation of Wash buffer. |
| Ice packs | Nortech labs | GB8818 | These do not have to be purchased, rather they can be recycled/reused from any routine laboratory shipment that includes them in the packaging. |
| 70% Ethanol | VWR | V1001 | 70% solution made by adding dH20 to 190 or 200 proof research grade alcohol |
| Micro dissecting forceps | Stoelting | 52102-43 | 4 inches, 1 x 2 x 0.5 mm3 , Slight Curve |
| Micro dissecting forceps | Stoelting | 52102-06 | 4 inches, Straight Fine, Sharp |
| Dissecting microscope | Leica Microsystems | MZ16 or M60 | We have had success with the listed models. External gooseneck flexible light sources are helpful but not necessary. |
| 50 ml conical tubes | Sigma-Aldrich (Corning) | CLS4558 | |
| Phosphate Buffered Saline | Gibco (ThermoFisher Scientific) | 10010023 | We purchase from our university Tissue Culture Core facility, alternate options such as this are available. |
| 10x Phosphate Buffered Saline | Teknova | P0195 | For preparation of Wash buffer we use 10x PBS |
| DMEM/F-12 nutrient mixture (Ham's) with GlutaMAX | Gibco (Life Technologies) | 10565-018 | We purchase this specific media formulation, containing 2.438 g/ L sodium bicarbonate, 55 mg/L sodium pyruvate, and 4.5 g/L glucose |
| 6-well tissue culture plate | BD Falcon | 353224 | Polystyrene, Tissue culture treated |
| 6-well transwells | Millipore | PICM03050 | Insert – 30 mm diameter, 0.4 μm pore size hydrophilic PTFE membrane |
| Extracellular Matrix (for example, Matrigel Matrix) | BD Biosciences | 354234 | We have utilized Matrigel Matrix in our studies. It is a solid at room temperature and at -20 °C. Avoid repeat freeze/thawing. Thaw bottle to viscous solution at 4 °C, and prepare ~ 300 μL aliquots in the cold room with chilled pipette tips. Store aliquots at -20 °C. |
| Paraformaldehyde, 16% w/v aqueous solution | Alfa Aesar | 30525-89-4 | For tissue fixation, a fresh preparation of 4% paraformaldehyde is made by diluting this stock in PBS. |
| Tissue culture incubator, maintained at 37 °C, 5% CO2, 3% oxygen (optional for villous organ cultures) | For some experiments, hypoxia may be preferred. This can be established multiple ways, including addition of exogenous nitrogen via gas cylinder, Tygon tubing, and a regulator. |

