Real-Time Imaging of Macrophage Phagocytosis of IgG-Opsonized Red Blood Cells
Abstract
Source: Horsthemke, M., et al. Time-lapse 3D Imaging of Phagocytosis by Mouse Macrophages. J. Vis. Exp. (2018)
This video demonstrates an assay to monitor independent macrophage-mediated red blood cell (RBC) phagocytosis events. Mouse immunoglobulin G (IgG)-opsonized human RBCs are incubated with mouse macrophages and observed under a confocal microscope. The RBCs are engulfed by macrophages via a phagocytic cup formation and targeted for lysosomal degradation.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Seeding of Peritoneal Cells in Channel Slides
- After pipetting the peritoneal mouse macrophage cell suspension up and down to reduce clumping, pipette 100 µL suspension into the prefilled channel (volume, 100 µL) of a fibronectin-coated channel slide (a 100 µL channel has the dimensions (length x width x height): 50 mm x 5 mm 0.4 mm).
- Prefill the chamber by adding 1 mL serum-supplemented RPMI 1640 (Hepes) medium to one of the two reservoirs of the channel slide, tilting the slide, and then aspirating the medium from the downstream reservoir (Figure 1).
- Remove unwanted air bubbles, or long strips of air, in the channel by adding 1–2 mL medium to one of the two reservoirs, tightly applying a reservoir cap, and then pumping out the air through rhythmic thumb pressure on the cap and, where necessary, tilting the slide. After expelling air, tilt the slide with the uncapped reservoir (and containing medium) downward before removing the cap to avoid air being sucked back into the channel.
- Prefill the chamber by adding 1 mL serum-supplemented RPMI 1640 (Hepes) medium to one of the two reservoirs of the channel slide, tilting the slide, and then aspirating the medium from the downstream reservoir (Figure 1).
- Incubate the channel slide, seeded with cells, in a moist chamber for 2 h at 37 °C in the absence of CO2 (CO2 is not required since the HCO3-/CO2 buffer system is replaced by Hepes). The channel slides can be conveniently placed on a rack, which holds eight slides. Typically, 6–8 slides are prepared from one mouse, although up to 10 or more is possible.
- Remove the rack and exchange the RPMI 1640 (Hepes) medium in each slide for the RPMI 1640 medium containing bicarbonate, supplemented with 10% fetal bovine serum, as well as penicillin/streptomycin.
- Tilt the slide and aspirate medium first from the lower reservoir and then from the upper reservoir. Next, add 1 mL of the new medium to one of the reservoirs, tilt the slide, and aspirate the medium from the downstream reservoir, after it has flowed through the channel.
- After this wash (medium exchange) step, fill the channel slide by adding 1 mL medium to one of the reservoirs.
NOTE: Washing steps using channel slides is very simple and effective in terms of solution exchange and removing non-adherent cells. Note that the RPMI 1640 (bicarbonate) medium is normally pre-incubated with 5% CO2 overnight to ensure thermal and pH equilibrium.
- Incubate the cells overnight at 37 °C in a humidified incubator with 5% CO2. Perform experiments on the following day.
2. Isolation of Human Red Blood Cells
- On day 2 (the second day of experiments; after overnight incubation of the isolated peritoneal macrophages), collect 1–2 mL peripheral venous blood from a healthy donor, into a heparinized tube. Transfer 1 mL into a round bottom 2.0 mL (polypropylene) microcentrifuge tube, centrifuge at 300 x g for 5 min at 18 °C (empirical setting), and remove the supernatant (plasma and buffy coat).
- Gently aspirate 100 µL of the sedimented red blood cells and mix this volume 1:1 with modified RPMI 1640 (Hepes) medium (described below) in a round bottom 2.0 mL microcentrifuge tube. Label the tube "1:1".
NOTE: The RPMI 1640 (Hepes) medium used on day 2 (for assays) after isolating cells contains, in addition to 10% heat-inactivated fetal bovine serum, penicillin (100 units/mL), streptomycin (100 µg/mL), and 1 mM N-(2-mercaptopropionyl)glycine (to reduce phototoxicity). Hereafter, this medium is referred to as "modified" RPMI 1640 (Hepes) medium. - Pipette 4 µL of the 1:1 diluted red blood cell suspension into 2 mL modified RPMI 1640 (Hepes) medium, pre-pipetted into a 2.0 mL microcentrifuge tube (repeat this step, i.e. prepare 2 tubes). Label each tube "4:2000". Place the "1:1" and "4:2000" tubes on ice.
3. Labeling of the Macrophage Plasma Membrane
- Remove the seeded channel slides from the CO2 incubator and exchange the RPMI 1640 (bicarbonate) medium in each slide for a modified RPMI 1640 (Hepes) medium. Next, place the cells in a moist chamber at 37 °C in the absence of CO2.
NOTE: After overnight incubation and washing, most of the lymphocytes are removed from the channel. The majority of the remaining adherent cells are macrophages, which can be identified either morphologically or by immunofluorescence staining using anti-F4/80 antibody. Around 95% of mouse resident peritoneal macrophages are F4/80 positive. - Dilute fluorescently green labeled anti-mouse F4/80 antibody (stored at 4 °C) 1:40 in modified RPMI 1640 (Hepes) medium. Take a slide, tilt it, and pipette (drop by drop) 100 µL of the antibody mixture into the opening of the 100 µL channel of the slide (see Figure 1). Aspirate medium that flows into the downstream reservoir. Incubate the cells for 20 min at 37 °C (humidified environment, without CO2). During the incubation period, label the human red blood cells (see Section 5).
NOTE: As alluded to above, CO2 is not required since the HCO3-/CO2 buffer system is replaced by Hepes. - After 20 min incubation time (during which human red blood cells are stained and opsonized), wash the slide by adding 1 mL modified RPMI 1640 (Hepes) medium to one of the reservoirs and aspirating the medium after it has flowed through the channel, facilitated by tilting the slide. Add 1 mL medium for short-term storage or proceed to an experiment (i.e., pipetting in particles (opsonized red blood cells) and imaging phagocytosis by time-lapse spinning disk confocal microscopy).
4. Labeling the Plasma Membrane of Human Red Blood Cells
- Begin the labeling of human red blood cells immediately after incubating a slide of macrophages with green fluorescently labeled anti-F4/80 antibody (after Section 3.2). After gentle mixing, take 400 µL from one of the "4:2000" tubes (see Section 2.3) and dispense it into a (round bottom) 2.0 mL microcentrifuge tube. Allow time for the suspension to warm to around 37 °C (see paragraph below). Add 0.4 µL orange/red fluorescent plasma membrane stain (aliquots stored at -20 °C), mix and incubate at 37 °C for 5 min.
NOTE: A heated aluminum block, placed inside the laminar flow hood, is useful for minimizing heat loss while preparing slides. Tubes can be placed into bore wells of the heating block and a separate, or integrated, heated aluminum plate can serve as a working space. - After the 5-minute incubation period, prepare the first wash step by adding 1600 µL modified RPMI 1640 (Hepes) medium (to fill the 2.0 mL microcentrifuge tube).
- Centrifuge the tube at 300 x g for 5 min at 18 °C (empirical setting). A compact red pellet should be visible on the wall (i.e., off-center) at the bottom of the tube. Rotate the tube so that the pellet is facing upwards and carefully remove all of the supernatant successively (in two steps) using a 1–1.5 mL pipette tip.
- After aspirating the supernatant, add 2,000 µL modified RPMI 1640 (Hepes) medium, mix (to resuspend the cells), and repeat the above centrifugation and supernatant aspiration steps.
- Resuspend the pellet (of plasma membrane stained and 2x washed red blood cells) with 400 µL of modified RPMI 1640 (Hepes) medium. Label the tube PMS (abbreviation for plasma membrane stain).
NOTE: Alternatively, the succinimidyl ester of a pH-sensitive rhodamine derivative, which becomes more strongly fluorescent at acidic pH, could be used to label human red blood cells. In this case, the fluorescence intensity additionally serves as a measure of phagosome maturation after particles have been internalized.
5. Opsonization (Labeling) of Human Red Blood Cells with Mouse Immunoglobulin G (IgG)
- Add 1 µL mouse (IgG2b) monoclonal (clone HIR2) anti-human CD235a (1 mg/mL) antibody (stored at -20 °C) to the tube labeled PMS (see Sections 4.2–4.5), containing plasma membrane stained human red blood cells suspended in 400 µL medium. CD235a (also known as glycophorin A) is an erythroid lineage-specific membrane sialoglycoprotein.
- Incubate at 37 °C for 8 min. Note that opsonization of human red blood cells with IgG causes agglutination (cell clumping). Although agglutination can serve as a visual indicator of opsonization, it is undesirable for the imaging of single phagocytic effects. To circumvent agglutination, repeatedly mix the cell suspension (every 1 min) using, for example, a variable 20–200 µL volume pipette set to 200 µL.
- Towards the end of the 8-minute incubation period, if desired, wash the macrophage slide, incubated with green fluorescently labeled anti-F4/80 antibody, with 1 mL modified RPMI 1640 (Hepes) medium. Ensure that both reservoirs of the channel slide are free of the medium after the washing steps.
6. Imaging the Phagocytosis of Plasma Membrane Stained and IgG-coated Human Red Blood Cells
- After the 8 min incubation with anti-CD235a antibody (Section 5.2), pipette 100 µL suspension containing plasma membrane-stained and IgG-opsonized human red blood cells into the channel of a slide containing macrophages labeled with green fluorescent anti-F4/80 antibody (Step 3). Thus, red fluorescent, IgG-opsonized human red blood cells are added to green fluorescent phagocytes (macrophages).
- As soon as human red blood cells have been added, mount the channel slide on a spinning disk confocal microscope (with the stage incubator set to 37 °C) for imaging, for example, via a 60X/1.49 oil-immersion objective lens. Start imaging as soon as possible, since particles begin to settle within 1 min.
NOTE: Using fluorescent beads with a diameter of 100 nm, we measured, by analysis of point spread functions, XY resolutions of x = 0.22 µm, y = 0.23 µm (488 nm laser) and x = 0.27 µm, y = 0.27 µm (561 nm laser). However, to reduce photobleaching and phototoxicity during time-lapse 3D imaging of living cells, we compromise spatial resolution using 2 x 2 binning. Binning increases sensitivity (improves the signal-to-noise ratio) and the allowable frame rate, but at the expense of spatial resolution. - Use a perfect focus (or similar) system to prevent focus drift during recordings. After activating the perfect focus system, use the offset control to focus on macrophage lamellipodial protrusions immediately above the coverslip (see note below) of the channel slide. This focus level corresponds to Z = 0 µm. Obtain Z-stacks from -1 µm to +16 µm at 0.8 µm steps, which amounts to 22 Z-slices. Z-stacks can, for example, be obtained at a rate of 1 stack (for each channel) every 15 s for a total of 16 min.
NOTE: Longer recording periods suffer from losses of image quality, mainly due to photobleaching. Z-stacks are acquired for each of the two channels: macrophages are imaged using a 488 nm laser (green channel) and human red blood cells are imaged using a 561 nm laser (red channel). Using this approach, various views of macrophages ingesting IgG-opsonized human red blood cells at different time points can be obtained (for example, see Figure 2).
NOTE: Typical settings of the system (with 2 x 2 binning) are: green channel (20.5% laser (50 mW; 488 nm) power, 101 sensitivity (range, 0–255), and 200 ms exposure time); red channel (10.5% laser (50 mW; 561 nm) power, 101 sensitivity (range, 0-255) and 98 ms exposure time).
NOTE: The bottom of the channel slide is a polymer with the same thickness as a #1.5 glass coverslip and the same optical properties of glass, but, notably, the material has the advantage of gas permeability.
Representative Results
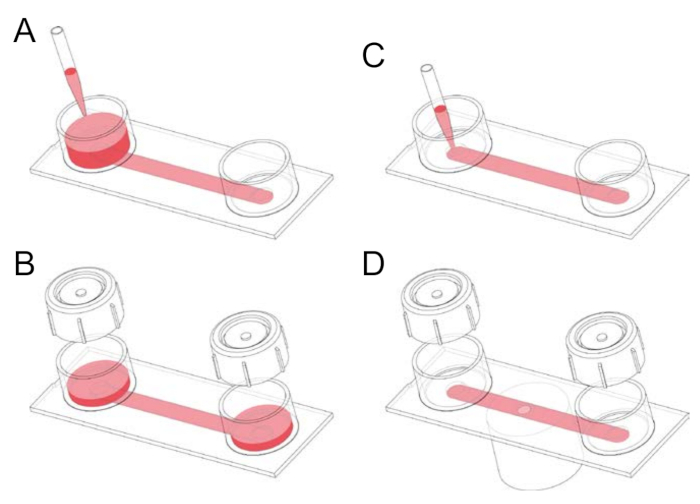
Figure 1: Handling of fibronectin-coated channel slides. (A) A channel slide consists of two reservoirs connected by a channel with the dimensions 50 mm x 5 mm 0.4 mm. Channel slides are initially prefilled by applying 1-2 mL medium to one of the two reservoirs and tilting the slide. (B) Caps can be placed onto the reservoirs prior to incubation. The caps can be conveniently used to pump out unwanted air bubbles prior to seeding the channel with cells. (C) The air bubble-free 100 µL channel can be filled by directly pipetting the medium into the mouth of a channel. This step is used, for example, to seed macrophages into a slide or to add gfluorophore (green fluorescent)-conjugated anti-F4/80 antibody, which serves as a membrane label, as well as a mouse macrophage marker. (D) After pipetting particles, such as opsonized human red blood cells, into a channel seeded with fluorescently stained macrophages, the slide can be placed on the stage of an inverted microscope, and time-lapse spinning disk confocal microscopy can be performed
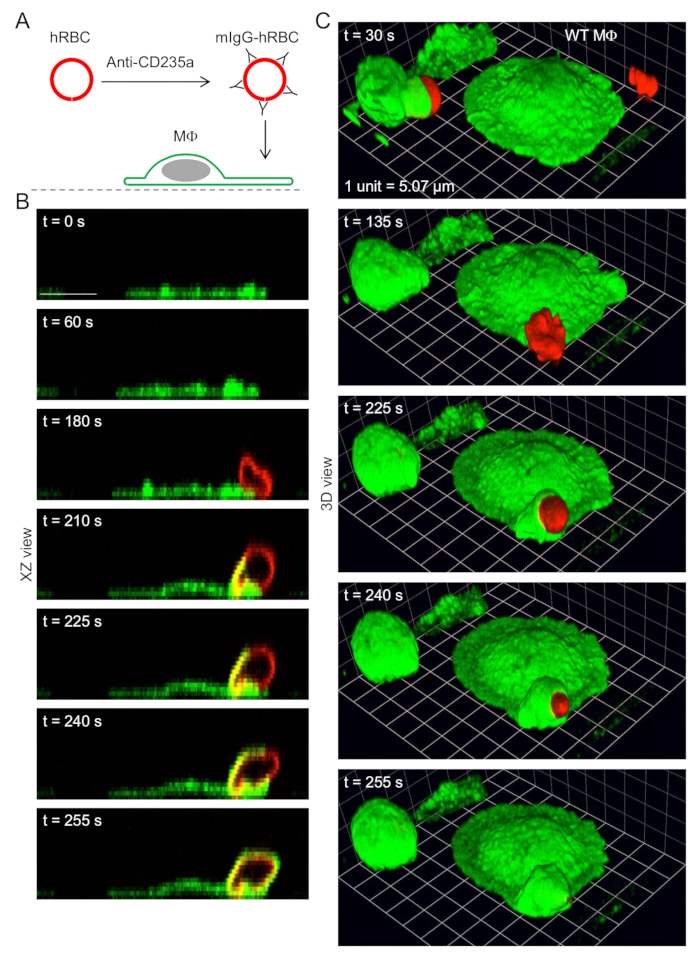
Figure 2: Time-lapse 3D imaging of phagocytosis. (A) Schematic diagram showing the opsonization of plasma membrane stained (red fluorescent) human red blood cells (hRBCs) with mouse (m) anti-CD235a immunoglobulin G (mIgG) antibody, and presentation of labeled hRBCs to mouse macrophages (Ms), labeled (green fluorescent) with green fluorescent fluorophore-conjugated anti-F4/80 antibody. (B)Time-lapse images (XZ views), obtained by spinning disk confocal microscopy, showing phagocytic cup formation and ingestion of mIgG-opsonized hRBCs. Scale bar = 10 µm. (C) 3D reconstructions showing macrophages ingesting mIgG-opsonized hRBCs. Corresponding XZ views (for 3 of the time points) are shown in B. Grid spacings represent 5.07 µm.
Offenlegungen
The authors have nothing to disclose.
Materials
| 24 G plastic catheter | B Braun Mesungen AG, Germany | 4254503-01 | Used for peritoneal lavage |
| Hank's buffered salt solution without Ca2+ and Mg2+ | Thermo Fisher Scientific | 14170120 | Used for peritoneal lavage |
| 14 mL polypropylene round bottom tubes | BD Falcon | 352059 | Used to collect peritoneal cells |
| RPMI 1640 medium containing 20 mM Hepes | Sigma-Aldrich | R7388 | Basis medium for assays |
| Heat-inactivated fetal bovine serum | Thermo Fisher Scientific | 10082139 | Used as supplement for RPMI 1640 media |
| 100x penicillin/streptomycin | Thermo Fisher Scientific | 15140122 | Used as supplement for RPMI 1640 media |
| Fibronectin-coated µ-Slide I chambers | Ibidi, Martinsried, Germany | 80102 | Channel slides used for assays |
| µ-Slide (anodized aluminum) rack | Ibidi, Martinsried, Germany | 80003 | Autoclavable stackable rack for channel slides |
| RPMI 1640 medium containing bicarbonate | Sigma-Aldrich | R8758 | Medium for overnight culture |
| N-(2-mercaptopropionyl)glycine | Sigma-Aldrich | M6635 | Scavenger of reactive oxygen species |
| Alexa Fluor 488-conjugated rat (IgG2a) monoclonal (clone BM8) anti-mouse F4/80 antibody | Thermo Fisher Scientific | MF48020 | Mouse macrophage marker and plasma membrane label |
| CellMask Orange | Thermo Fisher Scientific | C10045 | Red fluorescent plasma membrane stain |
| Succinimidyl ester of pHrodo | Thermo Fisher Scientific | P36600 | Amine-reactive succinimidyl ester of pHrodo |
| Mouse (IgG2b) monoclonal (clone HIR2) anti-human CD235a | Thermo Fisher Scientific | MA1-20893 | Used to opsonize human red blood cells with IgG |
| Alexa Fluor 594-conjugated goat anti-mouse (secondary) IgG antibody | Abcam | Ab150116 | Used to confirm opsonization of human red blood cells with mouse IgG |
| Rat anti-mouse C3b/iC3b/C3c antibody | Hycult Biotech | HM1065 | Used to confirm C3b/iC3b opsonization of human red blood cells |
| Alexa Fluor 488-conjugated goat anti-rat IgG antibody | Thermo Fisher Scientific | A-11006 | Used as secondary antibody to confirm C3b/iC3b opsonization |
| UltraVIEW Vox 3D live cell imaging system | Perkin Elmer, Rodgau, Germany | Spinning disk confocal microscope system | |
| Nikon Eclipse Ti inverse microscope | Nikon, Japan | Inverted microscope | |
| CSU-X1 spinning disk scanner | Yokogawa Electric Corporation, Japan | Nipkow spinning disk unit | |
| 14-bit Hamamatsu C9100-50 Electron Multiplying-Charged Couple Device (EM-CCD) peltier-cooled camera | Hamamatsu Photonics Inc., Japan | EM-CCD camera of the spinning disk confocal microscope system | |
| 488 nm solid state laser, 50 mW | Perkin Elmer, Rodgau, Germany | Laser (488 nm) source of spinning disk confocal microscope system | |
| 561 nm solid state laser, 50 mW | Perkin Elmer, Rodgau, Germany | Laser (561 nm) source of spinning disk confocal microscope system |

