Paired Patch Clamp Recordings from Motor-neuron and Target Skeletal Muscle in Zebrafish
Summary
Larval zebrafish represent the first vertebrate model system to allow simultaneous patch clamp recording from a spinal motor-neuron and target skeletal muscle. This video demonstrates the microscopic methods used to identify a segmental CaP motor-neuron and target muscle cells as well as the methodologies for recording from each cell type.
Abstract
Larval zebrafish represent the first vertebrate model system to allow simultaneous patch clamp recording from a spinal motor-neuron and target muscle. This is a direct consequence of the accessibility to both cell types and ability to visually distinguish the single segmental CaP motor-neuron on the basis of morphology and location. This video demonstrates the microscopic methods used to identify a CaP motor-neuron and target muscle cells as well as the methodologies for recording from each cell type. Identification of the CaP motor-neuron type is confirmed by either dye filling or by the biophysical features such as action potential waveform and cell input resistance. Motor-neuron recordings routinely last for one hour permitting long-term recordings from multiple different target muscle cells. Control over the motor-neuron firing pattern enables measurements of the frequency-dependence of synaptic transmission at the neuromuscular junction. Owing to a large quantal size and the low noise provided by whole cell voltage clamp, all of the unitary events can be resolved in muscle. This feature permits study of basic synaptic properties such as release properties, vesicle recycling, as well as synaptic depression and facilitation. The advantages offered by this in vivo preparation eclipse previous neuromuscular model systems studied wherein the motor-neurons are usually stimulated by extracellular electrodes and the muscles are too large for whole cell patch clamp. The zebrafish preparation is amenable to combining electrophysiological analysis with a wide range of approaches including transgenic lines, morpholino knockdown, pharmacological intervention and in vivo imaging. These approaches, coupled with the growing number of neuromuscular disease models provided by mutant lines of zebrafish, open the door for new understanding of human neuromuscular disorders.
Protocol
1. Preparation of Fish for Paired Recordings
- Prior to beginning the procedure, prepare five key solutions, which include: Bath Solution, Bath Solution with Tricaine (MS222), Bath Solution with Formamide, Neuron Internal Solution and Muscle Internal Solution.
- Next, collect a single larval zebrafish between the ages of 72 to 96 hours and place in Bath Solution with Tricaine. Within one minute of exposure to the anesthetic, the fish will not respond to touch.
- Place the fish on top of a plastic dish and, using a stereomicroscope, decapitate with a double edge razor blade.
- Transfer the fish to a glass recording chamber coated with sylgard and filled with Bath Solution. Fasten at each end by inserting electrolytically sharpened tungsten pins through the notochord.
- Create a skin flap near the rostral end of the fish with a pin that will provide a adequate grip for a pair of fine forceps. Remove the overlying skin by gripping it near the rostral pin with a pair of fine forceps. Peel slowly in the caudal direction to remove the skin.
- Treat the fish with 3 mls of Bath Solution with Formamide for 3-5 minutes to block muscle contractions that might interfere with recordings. Wash 5 times with normal Bath Solution. Remove part of the superficial layer of muscle over 5 to 6 segments by gently scrapping with the side of a tungsten pin.
- Transfer the fish to an upright microscope located in a Faraday cage to shield electrical noise. The microscope is equipped with a fixed stage, long working distance 40x objective and zoom of .5 to 4x. The microscope is mounted on a motorized X-Y translation stage in order to move the preparation between nerve and muscle under high power. The use of differential interference contrast optics helps to visualize the neurons.
- Under 0.5 x zoom, use a wide bore 15-micron pipette to remove overlying muscle and expose the spinal cord. Negative pressure is applied through a 3 cc disposable syringe and muscle cells are removed one by one. This procedure is repeated for 5 to 6 dorsal segments of skeletal muscle. The same wide bore pipette is used to also remove the upper two layers of ventral muscle to obtain target fast skeletal muscle.
- This completes the preparation for paired recordings and the microscope zoom is increased to approximately 1.6x.
2. Paired Recordings of CaP Motor-neuron and Target Muscle Cells
- The cleaned segments are scanned to locate the CaP neurons. Each hemi-segment of spinal cord contains one large, 10 micron diameter, CaP motor-neuron. This teardrop shaped soma is located superficially under the spinal dura near the caudal most end of the segmental boundary. A CaP neuron is chosen for recording and the ventral target muscle in the adjacent caudal segment is scanned to check integrity.
- Two patch electrodes are positioned for recording, one for the neuron and a second for muscle. The upper electrode has a shallow taper for penetration of the tough spinal dura and tip opening of approximately 2 microns. The lower electrode has a steeper taper for low resistance voltage clamp recording of skeletal muscle
- Position the neuron electrode at an angle of approximately 30 degrees to penetrate the spinal dura, about one half-segment rostral to the selected CaP neuron. Advance the electrode using an axial drive manipulator while applying a gentle continuous positive pressure of approximately 60 millimeters mercury. As the electrode breaks through the dura, the CaP neuron may be displaced by the perfusion from the electrode, requiring a readjustment of the focus.
- When the electrode touches the CaP neuron soma positive pressure is released and usually results in immediate formation of a gigaohm seal. However, in some cases where a seal fails to form, it becomes necessary to apply a gentle negative pressure. After seal formation, the electrode potential is adjusted to -80mV and application of negative pressure ruptures the cell membrane. The CaP neuron is characterized by an input resistance of 140 to 180 Mega-ohms and an action potential that overshoots +40 millivolts. This is tested by injecting incrementally increasing depolarizing steps under current clamp until the action potential is elicited.
- Next, the microscope is relocated to ventral muscle using the X-Y motorized translator under high power. A fast muscle cell is chosen from the target field.
- The muscle electrode is advanced toward the target muscle cell under positive pressure, which when released, forms a gigaohm seal. The electrode potential is adjusted to -50 millivolts to inactivate sodium channels and under gentle suck the cell membrane is ruptured. A drop in resistance to 100 to 200 Mega-ohms and the sudden appearance of the large capacitive transients signals entry.
- To test for paired recording, the motor-neuron is depolarized by incrementally larger injections of current until an action potential is elicited. At this point an endplate current should be elicited in muscle. Continued stimulation of the motorneuron at 1 Hertz results in endplate currents without failure. As the stimulus frequency is increased to 100 Hertz the endplate currents fluctuate in amplitude and undergo functional depletion within 10 seconds of stimulation.
3. Representative Results
Typically, the neuron recording is stable for up to one hour but the muscle cells deteriorate as reflected as an increase in the series resistance. When this occurs the experiment is either terminated or another muscle cell is patch clamped. All recordings should be completed within one hour.
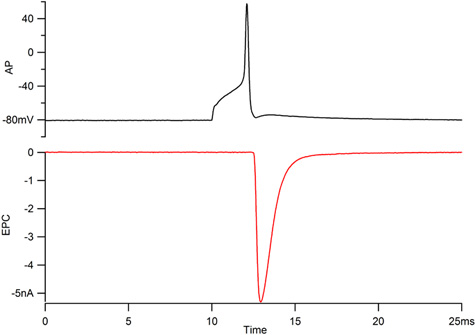
Figure 1. Recordings of the motorneuron action potential (AP, top) and the associated muscle endplate current (EPC, bottom). The action potential for a CaP neuron should overshoot +40 mV and the EPC should rise within 300 μsec and decay along an exponential time course with a time constant under 1 msec.
| Name | Recipe |
| Bath Solution (in mM) | 134 NaCl, 2.9 KCl, 2.1 CaCl2, 1.2 MgCl2, 10 Glucose, 10 Na-HEPES, pH 7.8 |
| Bath Solution with Tricaine | Bath Solution containing 0.02% tricaine |
| Bath Solution with Formamide | Bath Solution containing 2M formamide |
| Neuron Internal Solution (in mM) | 115 K-gluconate, 15 KCl, 2 MgCl2, 10 K-HEPES, 5 K-EGTA, 4 Mg-ATP, pH 7.2 |
| Muscle Internal Solution (in mM) | 120 KCl, 10 K-HEPES, 5 BAPTA, pH 7.4 |
Table 1. Solutions.
Discussion
We have been using zebrafish paired recordings (Wen and Brehm, 2005) principally to study the processes of vesicle release and recycling during high frequency stimulation. This process is disrupted in motility mutants wherein normal synaptic transmission is compromised due to point mutations in key synaptic components. For example, mutations that disrupt postsynaptic receptor aggregates (Ono et al., 2001, 2002, 2004) and synthesis of presynaptic transmitter (Wang et al., 2008) result in un-coordinated swimming. Paired recordings provide the information that can pinpoint the source of the functional defect, thus linking behavior, genetics and physiology. It should now be possible to further dissect the processes underlying synaptic transmission by means of transient expression in the CaP motor-neurons using judicious promoters. In this way dominant negative or mutated genes can be specifically expressed in these neurons and both behavioral and electrophysiological consequences determined. The additional advantage offered by the whole cell configuration allows dialysis of the CaP soma with agents that can potentially alter vesicle recycling, such as clostridial toxins and calcium buffers. Due to the short distance between the soma and muscle, diffusion should occur well within the time frame of recordings. The potential of this preparation has only begun to be tapped. The transparency of the fish at this age and superficial location of synapses also facilitates use of optical tools (Fetcho and O’Malley, 1997; Fetcho and Higashijima, 2004). We have been very successful in measuring of endo and exocytosis in living fish using FM dyes and genetic indicators such as Synaptophluorin (Li et al., 2003). Additionally, fluorescent conjugated toxins can be used to label synaptic proteins for imaging of live fish (Ibanez-Tallon et al., 2004). Given the simplicity of this preparation it holds great promise for future study.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Funded by the NIH (NS-18205).
Materials
| Material Name | Typ | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Pneumatic transducer tester | Fluke Biomedical Instruments | DPM1B | ||
| 0.002 x 3 inch tungsten rod | A-M Systems Inc | 715000 | ||
| Sylgard 184 Elastomer | Dow Corning Corp. | The thickness of application will affect the DIC optics |
Referenzen
- Fetcho, J. R., O’Malley, D. Imaging neuronal networks in behaving animals. Current Opinion in Neurobiology. 7, 832-838 (1997).
- Fetcho, J. R., S, H. i. g. a. s. h. i. j. i. m. a. Optical and genetic approaches toward understanding neuronal circuits in zebrafish. Integr Comp Biol. 44, 57-70 (2004).
- Ibañez-Tallon, I., Wen, H., Miwa, J., Xing, J., Aslantas-Tekinay, A., Ono, F., Brehm, P., Heintz, N. Tethering naturally occurring peptide toxins for cell autonomous modulation of ion channels and receptors in vivo. Neuron. 43, 305-311 (2004).
- Li, W., Ono, F., Brehm, P. Optical measurements of presynaptic release in mutant zebrafish lacking postsynaptic receptors. J. Neuroscience. , 23-10467 (2003).
- Ono, F., Mandel, G., Brehm, P. Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. J. Neuroscience. 24, 475-5481 (2004).
- Ono, F., Shcherbatko, A., Higashijima, S., Fetcho, J., Mandel, G., Brehm, P. Paralytic zebrafish lacking ACh receptors fail to localize rapsyn clusters to the synapse. J. Neuroscience. 21, 5439-5448 (2001).
- Ono, F., Shcherbatko, A., Higashijima, S., Mandel, G., Brehm, P. The zebrafish motility mutant twitch once reveals new roles for rapsyn in synaptic function. J. Neuroscience. 22, 6491-6498 (2002).
- Wang, M., Wen, H., Brehm, P. Function of neuromuscular synapses in the zebrafish choline-acetyltransferase mutant bajan. J Neurophysiol. , 100-104 (2008).
- Wen, H., Brehm, P. Paired motor-neuron muscle recordings in zebrafish test the receptor blockade model for shaping synaptic current. J. Neuroscience. 25, 8104-8111 (2005).

