The Mouse Cremaster Muscle Preparation for Intravital Imaging of the Microcirculation
Summary
A tissue preparation is described for visualization and experimental manipulation of the living microcirculation. In anesthetized male mice, the thin, highly vascularized cremaster muscle is prepared for intravital microscopy to study microvascular networks including arterioles, capillaries and venules. This preparation is readily adapted for rats and hamsters.
Abstract
Throughout the body, the maintenance of homeostasis requires the constant supply of oxygen and nutrients concomitant with removal of metabolic by-products. This balance is achieved by the movement of blood through the microcirculation, which encompasses the smallest branches of the vascular supply throughout all tissues and organs. Arterioles branch from arteries to form networks that control the distribution and magnitude of oxygenated blood flowing into the multitude of capillaries intimately associated with parenchymal cells. Capillaries provide a large surface area for diffusional exchange between tissue cells and the blood supply. Venules collect capillary effluent and converge as they return deoxygenated blood towards the heart. To observe these processes in real time requires an experimental approach for visualizing and manipulating the living microcirculation.
The cremaster muscle of rats was first used as a model for studying inflammation using histology and electron microscopy post mortem1,2. The first in vivo report of the exposed intact rat cremaster muscle investigated microvascular responses to vasoactive drugs using reflected light3. However curvature of the muscle and lack of focused illumination limited the usefulness of this preparation. The major breakthrough entailed opening the muscle, detaching it from the testicle and spreading it radially as a flat sheet for transillumination under a compound microscope4. While shown to be a valuable preparation to study the physiology of the microcirculation in rats5 and hamsters6, the cremaster muscle in mice7 has proven particularly useful in dissecting cellular pathways involved in regulating microvascular function8-11 and real-time imaging of intercellular signaling12.
The cremaster muscle is derived from the internal oblique and transverse abdominus muscles as the testes descend through the inguinal canal13. It serves to support (Greek: cremaster = suspender) and maintain temperature of the testes. As described here, the cremaster muscle is prepared as a thin flat sheet for outstanding optical resolution. With the mouse maintained at a stable body temperature and plane of anesthesia, surgical preparation involves freeing the muscle from surrounding tissue and the testes, spreading it onto transparent pedestal of silastic rubber and securing the edges with insect pins while irrigating it continuously with physiological salt solution. The present protocol utilizes transgenic mice expressing GCaMP2 in arteriolar endothelial cells. GCaMP2 is a genetically encoded fluorescent calcium indicator molecule12. Widefield imaging and an intensified charge-coupled device camera enable in vivo study of calcium signaling in the arteriolar endothelium.
Protocol
1. Mouse board, muscle pedestal, body wedge and superfusion solution
- Mouse board: A transparent rectangular Plexiglas board (6″ wide X 8″ long X 3/16″ thick) is made to fit on the stage of the microscope. The “mouse board” is where the anesthetized mouse is secured during surgical preparation of the cremaster muscle.
- Muscle pedestal: A transparent silastic rubber pedestal is prepared from Sylgard 184, which is mixed according to the manufacturer°s instructions. A convenient mold is a 15-mm thick x 50-mm diameter diameter disposable Petri dish. The Sylgard block is cut to the desired shape with a razor blade (see Figure 1C.). A thin layer of clear waterproof silicone adhesive is used to secure the Sylgard block to the mouse board. Helpful tips: After pouring the Sylgard into the Petri dish, degassing in a vacuum chamber for an hour removes air bubbles and improves clarity. Following degassing, curing time for Sylgard is shortened by placing the dish in a laboratory oven at 50 °C for several hours.
- Body wedge and heating platform: A wedge (2″ X 4″ X 15°) positioned underneath the mouse and against the pedestal tilts the body forward which facilitates extending the cremaster muscle over the pedestal from its origin. Incorporating an aluminum platform onto the surface provides conductive heat. The platform is heated by resistors connected to a DC power supply (Figure 1A). Calibrating the platform temperature to ˜40 °C maintains esophageal temperature at ˜37 °C. Otherwise, radiant heat from a lamp is used.
- Pins. To secure the cremaster muscle on the pedestal, pins are prepared from 0.15 mm insect pins that have been bent into an “L” shape.
- Physiological salt solution (PSS). Preparations are superfused (irrigated) continuously with bicarbonate-buffered PSS prepared in ultrapure (18.2 MΩ) H2O. Stock solutions are prepared at 20X final working concentration and sterilized through a 0.2 μm filter. Stock solutions remain good for several weeks when stored at 4°C. Preparing salts separately from the bicarbonate and mixing them with dilution on the day of the experiment prevents the precipitation of calcium bicarbonate. The stock salt solution is comprised of (in mmol/L): 2638 NaCl, 94 KCl, 40 MgS04, 23.4 CaCl2. The stock bicarbonate is 360 NaHCO3. On the day of an experiment, respective solutions are brought to a final volume (typically 2 L for a given experiment) in a volumetric flask and placed in a water bath at ˜37°C. Equilibrating the PSS with 5% CO2 / 95% N2 for ˜15 minutes using a gas disperser adjusts the pH to ˜7.4 and prevents precipitation. The PSS is bubbled continuously with 5% CO2 / 95% N2 throughout experiments. The final working solution composition (in mmol/L) is: 132 NaCl, 4.7 KCl, 1.2 MgS04, 2 CaCl2, 18 NaHCO3.
2. Anesthesia and preparation for surgery
- Following approval from the Institutional Animal Care and Use Committee, male mice at least 12 weeks old are used. The mouse is anesthetized with pentobarbital sodium (60 mg/kg) via intraperitoneal (i.p.) injection. A restraining tube proves useful for securing the mouse during the initial injection. Throughout surgical procedures and experimental protocols, anesthesia is maintained by supplements (10-20% of initial injection, i.p.) as needed (every 30-60 minutes; indicated by withdrawal response to toe or tail pinch). Tip: Diluting the pentobarbital 10 mg/ml in sterile saline before injection reduces the possibility of overdose.
- Anesthesia compromises temperature regulation so the mouse must be kept warm immediately after the first injection. Placing the mouse in a metal carrier (ventilated aluminum basket) on top of a heating plate (calibrated to ˜37 °C) works well. Alternatively, a heat lamp can be used taking care to position it at an appropriate distance from the mouse. The mouse should be monitored every 5-10 minutes until the appropriate level of anesthesia is achieved (lack of withdrawal to toe or tail pinch). A supplementary dose may be required after 15-20 minutes. It is best to be patient and proceed with caution to avoid overdose, especially with obese or aged animals.
- Hair is removed from the lower abdomen, lower back, scrotum and legs by carefully shaving respective regions. Particular attention should be taken to avoid trauma to the scrotum and testes, which will otherwise injure the cremaster muscle. Remove loose hair with a fresh alcohol swab.
- If the bladder is full it will feel like a small grape through the abdominal wall. Empty the bladder using gentle pressure to prevent the mouse from urinating on the cremaster preparation during experiments. A Kimwipe is an effect sponge to collect the urine.
- Position the mouse on its back, lying on the wedge with its legs straddling the pedestal. A silk suture (4-0 or 5-0) tied to each foot provides a tether for securing the mouse with its crotch against the pedestal. A piece of tape placed loosely across the chest and secured to the wedge maintains overall body position. Surgical procedures are performed while viewing through a stereomicroscope using microdissection scissors and angled forceps.
3. Surgical preparation of the open cremaster muscle
- A pin is placed through the apex of the scrotal sac (either the left or right side) and secured in the pedestal to place tension on the skin. Superfusion with PSS (34-35 °C) is initiated over the surgical field and maintained throughout dissection to keep the exposed tissue warm and moist. A wick (made from Kimwipe) positioned with one end next to the scrotal sac and the other next to a vacuum line removes the effluent PSS. A bead of silicone sealant adhered to the Plexiglas board and completely encircling the wedge and pedestal serves as “moat” to collect any PSS that is not aspirated, serving as an effective precaution to prevent PSS leakage onto the microscope.
- An incision is made along the ventral surface of the scrotal sac. If the testicle has been retracted by the cremaster muscle into the abdominal cavity, gentle pressure on the lower abdomen directs the testicle into the sac. The surface of the cremaster muscle overlying the testicle should be identified at this time. Connective tissue between the scrotal skin and the cremaster muscle is carefully removed to free the cremaster muscle from surrounding tissue.
- The scrotal skin is gently retracted behind the proximal edge of the pedestal and secured on each side with a pin. The exterior surface of the cremaster muscle surface is then cleared of connective tissue. Continuous superfusion during dissection hydrates the connective tissue, facilitating visibility and removal.
- A pin through the apex of the cremaster muscle is used to place it under longitudinal tension. A longitudinal incision is made through the ventral surface of the muscle with great care taken to minimize disruption of the vascular supply. Blood flow dynamics near the periphery of the preparation are altered by this tissue damage14. Therefore, to minimize these affects on data collection, blood vessels near the center of the preparation are used for imaging and physiological studies.
- The cremaster muscle is connected by a thin ligament to the epididymis underneath the testicle. Reflecting the testicle to one side exposes this ligament, which is carefully separated from the epididymis. Near the apex of the cremaster muscle is small artery and vein which connect it to the epididymis. Occluding these vessels between forceps and pulling them apart minimizes bleeding. The testicle, epididymis, testicular artery and vein are ligated proximally (4-0 or 5-0 silk suture), severed and discarded (orchiectomy) along with the associated inguinal fat pad. Alternatively, the testicle can be gently pushed back into the abdominal cavity and retained with a piece of cotton or Kimwipe. The orchiectomy serves to avoid possible trauma to the tissue when pushing the testes back into the abdominal cavity. We have not found noticeable differences in the quality of the cremaster microcirculation preparation (see Section 3.8) using either procedure7,15.
- The external surface of the cremaster muscle is cleared of remaining connective tissue and then spread radially on the surface of the pedestal. The edges are secured with pins (see Section 1.4) in 5-6 places to create a flat sheet of striated muscle with intact microcirculation.
- The completed preparation is transferred to the stage of an intravital microscope, superfused continuously (3-5 ml/min, 34 °C) and equilibrated for 30 minutes.
- Integrity of the cremaster preparation is evaluated according to several criteria. Spontaneous vasomotor tone with minimal surgical trauma is indicated by brisk flow in arterioles and lack of adherent leukocytes in venules. The most sensitive index of a responsive preparation is constriction of arterioles to raising the superfusion solution oxygen content for 5-10 minutes. This is done by equilibrating it with 21% O2 (vs. 5% O2; see Section 1.5; all with 5% CO2, balance N2). The presence of leukocytes in arterioles, the absence of red blood cells flowing through capillaries, and/or leukocytes accumulating in venules and surrounding tissue are signs of tissue damage and inflammation.
4. Intravital imaging of the cremaster muscle microcirculation
- Intravital imaging is performed on a custom microscope based upon an Olympus MVX10 Stereo Zoom platform. The MVX10 microscope body is mounted to a MVX10 hybrid stand equipped with transillumination (halogen lamp) for brightfield (Köhler) imaging and with epi-illumination (mercury lamp) for fluorescence imaging. Using epi-illumination, GCaMP2 is excited at 472/30 nm with emission collected at 520/35 nm. Images are acquired through a MV PLAPO 2XC lens (numerical aperture = 0.50; Olympus) using an XR/Mega-10 intensified digital camera (Stanford Photonics, Inc.; SPI) via Piper Control Software (SPI) on a personal computer. With this system, the observed field of view (FOV) can be varied from 553 μm X 442 μm to 22 mm X 18 mm.
- Upon completion of intravital imaging experiments, the anesthetized mouse is euthanized with overdose of pentobarbital followed by cervical dislocation.
5. Representative Results
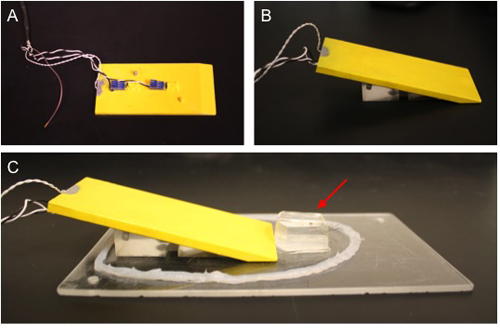
Figure 1. Mouse board for intravital imaging of the mouse cremaster preparation. A) Body wedge with aluminum platform (insulated with yellow plastic) viewed from the bottom. Heating resistors secured to the underside of the platform provide conducted heat. B) The heating platform rests on a plastic wedge. C) The body wedge is placed on a Plexiglas board for surgery and subsequent transfer to the stage of the intravital microscope. Sylgard pedestal is indicated with red arrow. A bead of waterproof silicone surrounds the entire preparation to contain any solution that may leak during the intravital procedure, preventing it from dripping onto the microscope.
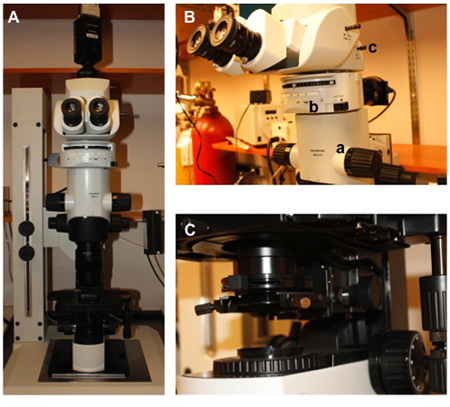
Figure 2. Custom MacroZoom microscope for widefield imaging. A) MVX10 microscope body (Olympus) with XR/Mega-10 ICCD camera (Stanford Photonics) mounted on trinocular port. B) Close-up view of the microscope body. (a) zoom control (0.63 to 6.3X); (b) filter wheel; (c) image doubler (NA = 0.50 with 25X optical magnification). C) Substage condenser for brightfield (Köhler) illumination (Condenser NA = 0.55, Working Distance = 27 mm).

Figure 3. Completed mouse cremaster preparation. Anesthetized mouse in supine position on warm plastic-coated aluminum platform (yellow). Body position is secured with tape. The cremaster muscle is spread radially on the transparent silastic rubber pedestal and pinned at the edges. Superfusion solution is introduced at the proximal end through a plastic dripper (white arrow). A vacuum line (white arrowhead) removes the solution via a Kimwipe wick. Two micropipettes are shown positioned with their tips in the tissue. A reference electrode (silver wire) is secured at the lower edge of the cremaster muscle.
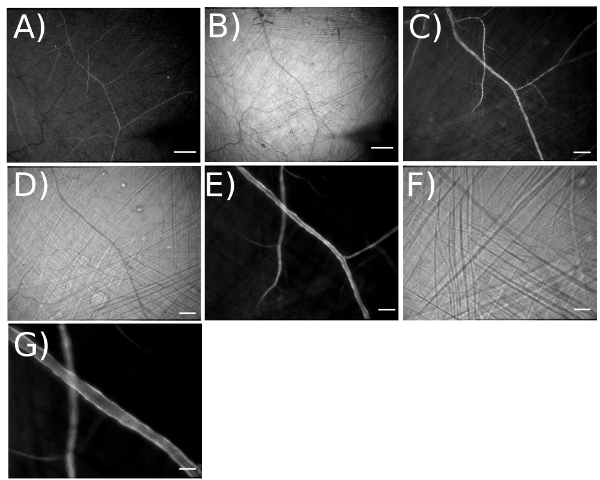
Figure 4. Illustrating the magnification range for visualizing arteriolar networks expressing GCaMP2 in endothelium. A) Fluorescent and B) brightfield image taken at an optical magnification of 3.2X for a total magnification = 42X on the video monitor. [Field of view (FOV) = 4,375 x 3,470 μm]. Scale bar = 500 μm. Working at this magnification facilitates placement of micropipettes at desired locations. C) Fluorescent and D) brightfield image taken at optical magnification of 6.4X for total magnification = 83X (FOV = 2,200 x 1,759 μm). Scale bar = 200 μm. E) Fluorescent and F) brightfield image taken at optical magnification of 12.6X for total magnification = 165X (FOV = 1,100 x 885 μm). Scale bar = 100 μm. G) With image doubler, optical magnification = 25.2X. Scale bar = 50 μm.
Discussion
Here we describe the open cremaster muscle preparation in the mouse for observing the microcirculation in vivo. This procedure is modeled after the “open” cremaster preparation first described in the rat4. With practice the entire surgical procedure can be completed in less than 1 hour. The versatility of this preparation allows for a variety of experimental manipulations and is readily adapted to hamsters as well as rats, enabling a variety of experimental models to be studied in similar fashion. The preparation is limited to male animals and measurements should be made towards the center of the tissue, avoiding damaged regions near the edges of the muscle14. It should also be recognized that, while pressure and flow distributions are altered when interconnecting vessels of the cremaster muscle are cut16, microvessels remain responsive and suitable for reproducible data collection. The primary limitation to visualizing the microcirculation in the cremaster muscle is the buildup of connective tissue as animals mature and increase in size, particularly in rats but also in hamsters. Further, as animals get fat, it becomes more difficult to control anesthesia because pentobarbital is lipophilic and can be absorbed in adipose tissue. The best way to proceed is by being patient while ensuring that the animal°s body temperature is maintained at ~37 °C while exposed tissue is irrigated continuously with PSS.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Research in the authors’ laboratory is supported by the National Institutes of Health grants R37-HL041026, R01-HL086483 and R01-HL056786 (SSS) and by F32-HL097463 and T32-AR048523 (PB) from the United States Public Health Service.
Materials
| Name of the reagent or device | Company | Catalogue number | Comments |
|---|---|---|---|
| Sodium Chloride | Fisher | S642-212 | |
| Potassium Chloride | Sigma | P9541 | |
| Magnesium Sulfate | Sigma | M2643 | |
| Calcium Chloride | Sigma | C1016 | |
| Sodium Bicarbonate | Fisher | S233 | |
| Nembutal Sodium Solution | Lundbeck | NDC 67386-501-55 | Also referred to as sodium pentobarbital |
| Temperature Controller | Warner Instruments | TC-344B | Alternate: adjustable 12V DC power supply |
| Series 20 platform heater kit RH-2 | Warner Instruments | 64-0274 | Requires custom-built aluminum plate |
| Mega-10 Camera | Stanford Photonics | XR/Mega-10 | |
| MVX10 | Olympus | ||
| MV PLAPO 2XC lens | Olympus | ||
| MVX10 hybrid stand | Leeds | LBX-Hybrid | |
| Piper Control Software | Stanford Photonics | Program for Mega-10 camera | |
| Stereo Microscope | Nikon | SMZ645 | |
| Waterproof Silicone Sealant (Clear) | General Electric | 47970-72643-LW5000 | Other clear silicone sealants also work |
| 60 x 15mm Petri Dish | Fisher | 08-757-13A | |
| Sylgard | Dow Corning | 184 | |
| Microdissection Scissors | Fine Science Tools | 15003-08 | |
| Dumont Forceps | Fine Science Tools | #5/45 | |
| GP Millipore Express PLUS Membrane | Millipore | SCGPT05RE | |
| Minutiens Insect Pins | Austerlitz | M size 0.15 mm | |
| Compact Pet Trimmer | Wahl Clipper Corp. | Model 9966 | Clean after each use |
Referenzen
- Majno, G., Palade, G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J. Biophys. Biochem. Cytol. 11, 571-605 (1961).
- Majno, G., Palade, G. E., Schoefl, G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J. Biophys. Biochem. Cytol. 11, 607-626 (1961).
- Grant, R. T. Direct Observation of Skeletal Muscle Blood Vessels (Rat Cremaster). J. Physiol. 172, 123-137 (1964).
- Baez, S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc. Res. 5, 384-394 (1973).
- Bohlen, H. G., Gore, R. W., Hutchins, P. M. Comparison of microvascular pressures in normal and spontaneously hypertensive rats. Microvasc. Res. 13, 125-130 (1977).
- Klitzman, B., Duling, B. R. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am. J. Physiol. 237, 481-490 (1979).
- Hungerford, J. E., Sessa, W. C., &, S. e. g. a. l., S, S. Vasomotor control in arterioles of the mouse cremaster muscle. FASEB J. 14, 197-207 (2000).
- Figueroa, X. F., Paul, D. L., Simon, A. M., Goodenough, D. A., Day, K. H., Damon, D. N., Duling, B. R. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ. Res. 92, 793-800 (2003).
- Wolfle, S. E., Schmidt, V. J., Hoepfl, B., Gebert, A., Alcolea, S., Gros, D., de Wit, C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ. Res. 101, 292-1299 (2007).
- Milkau, M., Kohler, R., de Wit, C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J. 24, 3572-3579 (2010).
- Bagher, P., Duan, D., Segal, S. S. Evidence for impaired neurovascular transmission in a murine model of Duchenne Muscular Dystrophy. J. Appl. Physiol. 110, 601-610 (2011).
- Tallini, Y. N., Brekke, J. F., Shui, B., Doran, R., Hwang, S. M., Nakai, J., Salama, G., Segal, S. S., Kotlikoff, M. I. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC-GCaMP2 transgenic mice. Circ. Res. 101, 1300-1309 (2007).
- Grant, R. T. The effects of denervation on skeletal muscle blood vessels (rat cremaster). J. Anat. 100, 305-316 (1966).
- Proctor, K. G., Busija, D. W. Relationships among arteriolar, regional, and whole organ blood flow in cremaster muscle. Am. J. Physiol. 249, H34-H41 (1985).
- Bagher, P., Segal, S. S. Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol. , (2011).
- Hill, M. A., Simpson, B. E., Meininger, G. A. Altered cremaster muscle hemodynamics due to disruption of the deferential feed vessels. Microvasc. Res. 39, 349-363 (1990).

