Drosophila Pupal Abdomen Immunohistochemistry
Summary
Antibody staining of the Drosophila pupae can enhance genetic analyses of adult abdominal developmental genetics. We present our protocol for dissection, fixation and antibody staining of staged Drosophila pupal abdomen.
Abstract
The Drosophila pupal abdomen is an established model system for the study of epithelial morphogenesis and the development of sexually dimorphic morphologies 1-3. During pupation, which spans approximately 96 hours (at 25 °C), proliferating populations of imaginal cells replace the larval epidermis to generate the adult abdominal segments. These imaginal cells, born during embryogenesis, exist as lateral pairs of histoblast nests in each abdominal segment of the larvae. Four pairs of histoblast nests give rise to the adult dorsal cuticle (anterior and posterior dorsal nests), the ventral cuticle (ventral nests) and the spiracles associated with each segment (spiracle nests) 4. Upon puparation, these diploid cells (distinguishable by size from the larger polyploid larval epidermal cells- LECs) begin a stereotypical process of proliferation, migration and replacement of the LECs. Various molecular and genetic tools can be employed to investigate the contributions of genetic pathways involved in morphogenesis of the adult abdomen. Ultimate adult phenotypes are typically analyzed following dissection of adult abdominal cuticles. However, investigation of the underlying molecular processes requires immunohistochemical analyses of the pupal epithelium, which present unique challenges. Temporally dynamic morphogenesis and the interactions of two distinct epithelial populations (larval and imaginal) generate a fragile tissue prone to excessive cell loss during dissection and subsequent processing. We have developed methods of dissection, fixation, mounting and imaging of the Drosophila pupal abdominem epithelium for immunohistochemical studies that generate consistent high quality samples suitable for confocal or standard fluorescent microscopy.
Protocol
1. Day 1
Before you start:
A healthy population of flies should be maintained using standard culturing protocols: remove adults from bottles or vials after 3-4 days of egg-lay and allow development to proceed at a constant temperature until wandering 3rd instar larvae initiate pupariation. The larval/pupal transition is marked by the formation of the prepupae (considered 0 hours after puparium formation-APF). Immobile pupae are distinguished from older pupae by their white coloration and from larvae that have not yet begun pupariation by their oblong,rounded shape and protrusion of the anterior spiracles.
You will need:
- A paint brush for collecting pupae
- A humid chamber for culturing pupae: A Petri dish lined with wetted paper towel and covered with filter paper marked for the various time points of collection, genotype or sex of the pupae
- 1X Phosphate buffered saline (PBS) for washing pupae
Collection, culturing and staging pupae
- Using a wetted paintbrush gently remove 0hr APF pupae from culture bottles/vials and place them in the lid of the humid chamber.
- Using the paintbrush and 1X PBS gently wash the pupae to remove debris from the pupa case
- If necessary sort the pupae by sex. The gonad primordia of pupae can be used to sort the sexes. The male gonad primordia are a lateral pair of translucent discs easily seen through the pupal cuticle approximately 2/3 down the length of the body 5. The female gonad primordia are smaller and not as easily identified.
- Place the pupae in the appropriately labeled position in the humid chamber and return to constant temperature. Culture to the appropriate developmental time point.
2. Day 2: Dissection, fixation and primary antibody incubation
Before you start:
You will need:
- Dissection stereomicroscope
- Surgical forceps
- Surgical scalpel with number 11 blades
- Two nine-well glass depression dishes (Corning product # 7220-85)
- Fill wells of one dish with 1X PBS: This is the rinsing dish for cleaning dissected pupae
- Fill wells of second dish with fixation buffer
- Humid chamber for antibody incubation. We use plastic sealable sandwich boxes lined with wetted paper towels.
- Fixation buffer: 1x PBS, 4% paraformaldehyde, 0.2% deoxycholic acid (for permeabilization)
- Dissection platform: Clear microscope slide with a piece of double-sided tape adhered to one side
- 1X PBS
- p100 or p200 pipettor
Dissection
- Using forceps gently remove pupae one at a time and place them on the dissection platform with the anterior end of the pupae facing the broadest width of the tape. If pupae are excessively wet, first blot them dry using a paper towel.
- Before the pupae have completely adhered to the tape, use a paintbrush to position them.
- Allow the pupae to air dry and adhere to the tape (5-10 minutes)
- With the surgical scalpel dissect each pupae bilaterally. This is best accomplished with a single rapid cut from the anterior to the posterior end of the pupae. If properly done, the cut will bisect both anterior and posterior spiracles pairs. Dissect no more than 10 pupae at a time as washing and cleaning the samples quickly is necessary to prevent proteolytic damage to the tissue.
- Using a paintbrush, transfer a small amount of 1X PBS to each dissected pupae to loosen them from the tape.
Cleaning, fixation and primary antibody incubation
- With a pair of surgical forceps grasp an individual pupa half anteriorly and immediately immerse it in a well of the rinsing dish. The dissection platform should be replaced by the rinsing dish on the microscope stage. The pupae case should be left on the sample until processing is complete and samples are ready for mounting.
- While still grasping the pupa half use a pipettor to gently wash away the internal tissue of the abdomen. Too much pressure during washing can lead to loss of epithelial cells.
- Immediately transfer the clean pupa half to fixation buffer at room temperature and process the remaining pupa halves similarly. With practice, dissection and washing of 20 pupa halves should take less than 5 minutes.
- Allow pupae to incubate in fixation buffer at room temperature for 1 (one) hour.
- Rinse fixed pupae 3x five minutes in 1X PBS
- Samples may immediately be processed for immunohistochemistry or may be stored in 100% ethanol at -20°C for up to 3 months without loss of cells or epitope reactivity.
- To store samples, equilibrate samples through a dilution series of PBS:EtOH (3:1, 1:1, 1:3) at room temperature for 20 minutes in each dilution prior to transferring to 100% EtOH.
- Samples must be re-equilibrated in PBS through the reverse series before processing for immunohistochemistry.
- Block samples in 1x PBS (supplemented with 2% bovine serum albumin) for 1 (one) hour before addition of primary antibody.
- Incubate with primary antibody diluted to appropriate concentration in 1X PBS over night at 4°C without rocking.
3. Day 3: Secondary antibody incubation, mounting and imaging
Before you start:
You will need:
- Two surgical forceps
- Mounting media (glycerol, Vectashield [Vector Labs], or Slowfade Gold [Invitrogen])
- Depression well microscope slides (0.8 mm)
- Coverslips (24 x 40 mm)
Secondary antibody incubation and mounting:
- Wash samples 3x 10 minute each with 1x PBS
- Block samples in 1x PBS (supplemented with 2% bovine serum albumin) for 1 (one) hour before addition of secondary antibody.
- Incubate with secondary antibody diluted to appropriate concentration in 1X PBS at room temperature in the dark without rocking for 3 (three) hours.
- Wash samples 3x 10 minute each with 1x PBS
- If appropriate, counterstain pupae (Example we stain nuclei with DAPI [4′,6-diamidino-2-phenylindole] diluted in PBS for 10 minutes).
- Equilibrate samples (minimum of 30 minutes) in appropriate media before mounting.
- Prepare a sample slide. The morphology of the pupae prevents flat mounting. Samples are mounted in the well of a depression slide (drop slide) for imaging. Place 75-100ul of mounting media in the depression well. Several samples can be mounted on a single slide.
- The pupa case must be removed from the samples before mounting. Grasp an individual pupa case anteriorly being sure not to grasp the internal pupal membrane.
- With a second pair of forceps gently grasp the internal pupal membrane by the head and remove it from the pupal case.
- Immediately transfer the sample to the depression well and continue processing additional samples.
- Using a probe or forceps position the samples uniformly in the depression well with the lateral surface facing up. Occasional air bubbles can be removed with a probe.
- Gently lower the coverslip onto the samples taking care not to introduce air bubbles
4. Representative results:
Samples prepared using this protocol retain the gross morphology of the adult abdomen. Image stacks may be projected to generate a two-dimensional image or 3-D rendering may be applied to investigate abdomen topology.
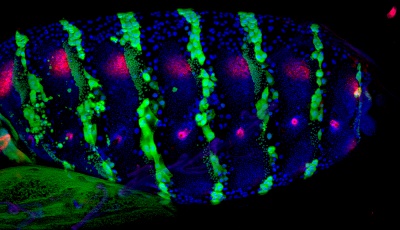
Figure 1. Segmentation gene products in Drosophila pupae. Wingless protein and Engrailed expression (En-gal4:uas-GFP) were visualized at 26 hour after puparium formation (APF) with mouse anti-Wg (4D4: Iowa Hybridoma Bank) and anti-GFP. 10x magnification, anterior is left and dorsal is up. Nucleii were counterstained with DAPI.
Stacks of approximately 50 images (ΔZ between slices is 2.5μm) were projected.
Discussion
The techniques presented in this video can be used to prepare Drosophila pupae from a variety of developmental time points. Pupae processed during the period of 24 hours APF to 32 hours APF are most prone to cell loss from the epithelium. The use of detergents (such as Triton X-100 and Tween-20) during extended incubation steps increases the likelihood of cell loss and is therefore not recommended. Rather, deoxycholic acid is used as a detergent during the initial fixation step. All subsequent steps are performed in 1X PBS without detergent. Additionally, rocking samples during extended incubation steps increases cell loss and should be avoided.
Samples can be imaged using confocal microscopy techniques. However, Drosophila pupae processed as described can also be imaged using a structured illumination microscopy system yielding image quality comparable to confocal techniques.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the National Science Foundation.
Materials
- Dissection stereomicroscope
- Surgical forceps
- Surgical scalpel with number 11 blades
- Nine-well glass depression dishes (Corning product # 7220-85)
- Humid chamber for culturing pupae
- 1X Phosphate buffered saline (PBS)
- #11 surgical scalpel
- double-sided tape
- Fixation buffer: 1x PBS, 4% paraformaldehyde, 0.2% deoxycholic acid (for permeabilization)
- p100 or p200 pipettor
- Depression well microscope slides (0.8 mm)
Referenzen
- Kopp, A., Duncan, I. Anteroposterior patterning in adult abdominal segments of Drosophila. Dev. Biol. 242, 15-30 (2002).
- Ninov, N., Chiarelli, D. A., Martin-Blanco, E. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development. 134, 367-379 (2007).
- Bischoff, M., Cseresnyes, Z. Cell rearrangements, cell divisions and cell death in a migrating epithelial sheet in the abdomen of Drosophila. Development. 136, 2403-2411 (2009).
- Matsuda, R. in Morphology and evolution of the insect abdomen. , (1976).
- Kerkis, J. The Growth of the Gonads in Drosophila Melanogaster. Genetik. 16, 212-224 (1931).

