In vitro Uncoating of HIV-1 Cores
Summary
Uncoating is an essential step in the early phase of the HIV-1 life cycle and is defined as the disassembly of the capsid shell and the release of the viral ribonucleoprotein complex (vRNP). Here, we demonstrate techniques for isolating intact cores from HIV-1 virions and for quantifying their uncoating in vitro.
Abstract
The genome of the retroviruses is encased in a capsid surrounded by a lipid envelope. For lentiviruses, such as HIV-1, the conical capsid shell is composed of CA protein arranged as a lattice of hexagon. The capsid is closed by 7 pentamers at the broad end and 5 at the narrow end of the cone1, 2. Encased in this capsid shell is the viral ribonucleoprotein complex, and together they comprise the core.
Following fusion of the viral membrane with the target cell membrane, the HIV-1 is released into the cytoplasm. The capsid then disassembles releasing free CA in the soluble form3 in a process referred to as uncoating. The intracellular location and timing of HIV-1 uncoating are poorly understood. Single amino-acid substitutions in CA that alter the stability of the capsid also impair the ability of HIV-1 to infect cells4. This indicates that the stability of the capsid is critical for HIV-1 infection.
HIV-1 uncoating has been difficult to study due to lack of availability of sensitive and reliable assays for this process. Here we describe a quantitative method for studying uncoating in vitro using cores isolated from infectious HIV-1 particles. The approach involves isolation of cores by sedimentation of concentrated virions through a layer of detergent and into a linear sucrose gradient, in the cold. To quantify uncoating, the isolated cores are incubated at 37°C for various timed intervals and subsequently pelleted by ultracentrifugation. The extent of uncoating is analyzed by quantifying the fraction of CA in the supernatant. This approach has been employed to analyze effects of viral mutations on HIV-1 capsid stability4, 5, 6. It should also be useful for studying the role of cellular factors in HIV-1 uncoating.
Protocol
1. Production of HIV-1 particles
Before proceeding, you must obtain permission and follow the guidelines from the Biosafety office of your institution to work in a biosafety facility approved for infectious HIV. You must ensure that proper care and safety precautions are followed during working in the biosafety laboratory.
Production of HIV-1 particles is typically performed by transient transfection of 293T cells with HIV-1 proviral DNA using a calcium phosphate transfection method7, 8. High titer virus stocks grown by culture of infected T cells are also suitable.
- Culture 293T cells in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and supplemented with antibiotics [Penicillin (100 IU/ml) and Streptomycin (100 μg/ml)] at 37°C, 5% CO2.
- Detach cells from nearly confluent dishes using 0.25% Trypsin-EDTA and seed 2×106 cells in 9 ml per 100 mm culture dish a day before transfection. Seed six 100 mm culture dishes for production of virions. If concentrated preparation of cores is required, more dishes could be seeded for transfection.
- On the next day, the cell monolayers should be about 25% confluent and ready for transfection. For six dishes of cells to be transfected, bring 120 μg of proviral DNA up to a volume of 2.7 ml with sterile water. To this mixture add 0.3 ml of 2.5 M CaCl2 and 3 ml of 2XBBS, and mix properly by pipetting up and down several times. Allow the mixture to stand at room temperature for 10-20 minutes.
- Add 1 ml of the resulting mixture dropwise to the center of each dish while swirling gently to mix. Place the dishes overnight (~16 hours) in an incubator set at 35°C and 3% CO2.
- Aspirate the culture medium and gently add 5 ml PBS.
- Aspirate PBS and add 5 ml of fresh medium. Culture the cells in incubator at 37°C and 5% CO2 for additional 24-48 hours.
- Collect the culture supernatant containing viral particles, transfer it to a 50 ml conical centrifuge tube. Combine the supernatants from all dishes and centrifuge at 1,500 x g for 5 min to pellet cells and debris. Filter the supernatant using 0.45 μm pore-size syringe filters. Precautions should be taken to minimize aerosol formation while working with the live virus. This includes use of conical tubes with O-rings or rotor bucket covers during the centrifugation step. If this is not possible, the screw caps of the tubes should be wrapped with parafilm.
2. Concentration of HIV-1 virions by ultracentrifugation
- Place the culture supernatant (30 ml) in a 38.5 ml polyallomer centrifuge tube (for Beckman SW32Ti rotor or equivalent). Gently underlay the virus containing supernatant with 5 ml solution of 20% sucrose in PBS using a 5 ml pipet.
- Centrifuge for 3 hours at 32,000 rpm at 4°C (175,000 x g at rmax) to pellet virus particles.
- Remove supernatant by aspiration, taking care not to disturb the pellet at the bottom of the tube. Add 0.5 ml of 1XSTE buffer (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA) to the tube and place at 4°C for 1 hour to loosen the pellet. Using a wide-bore 1 ml pipet tip gently pipet up and down a few times to detach the pellet from the bottom of the tube. Next, transfer the loosened pellet to a 1.5 ml microcentrifuge tube, taking care to avoid foaming. Incubate at 4°C for another 1-3 hours to allow the small clumps of virus to disperse. During this period, prepare the sucrose gradient as described in step 3.1.
- Gently pipet the virus suspension up and down several times and centrifuge at 8,000 rpm (6,000 x g) at 4°C in a refrigerated microfuge for 1 min to remove residual clumps. Keep the sample cold during this process.
3. Sucrose gradient centrifugation to isolate cores
HIV-1 cores are isolated using a “spin-thru” method9 which is a modification of the previously described method for the purification of HIV-2 cores10.
- Prepare a 12 ml linear 30% to 70% sucrose gradient in 1XSTE buffer in a 14.5 ml polyallomer centrifuge tube for SW32.1Ti rotor. To prepare gradient, use a 20 ml gradient former; place 6 ml of 70% sucrose solution on the near side (close to the outlet port) and 6 ml of 30% sucrose solution on the far side. Make sure that air bubbles are not trapped in the channel connecting the two chambers. Use the Auto Densi-Flow gradient former to pump the gradient from bottom to the top of the tube containing 1 ml of 85% sucrose solution at the bottom. Gently place the gradient at 4°C until cooled (2-4 hours).
- Using a wide-bore 1 ml pipet tip gently overlay the gradient with 0.25 ml of 15% sucrose solution in STE containing 1% Triton X-100.
- Gently overlay with 0.25 ml of 7.5% sucrose solution in STE using a wide-bore 1 ml pipet tip. Be careful not to mix the layers.
- Gently overlay with 0.5 ml virus suspension from step 2.4. This step should be performed carefully to avoid any disturbance in the gradient and mixing of the underlaid sucrose layers.
- Place the tubes in the pre-cooled SW32.1Ti bucket and centrifuge at 32,000 rpm (187,000 x g at rmax) overnight (16-20 hours) at 4°C. To avoid pausing of the centrifuge, do not start centrifugation until the vacuum in the centrifuge reaches 250 μm
- Collect 1 ml fractions from top to bottom of the gradient using the Auto-Densi-Flow gradient fractionator. Place the tubes on ice immediately upon collection. Mix the contents of the tubes by inverting few times, and withdraw 50 μl of each fraction for quantification by ELISA or reverse transcriptase activity assay.
4. Localization of HIV-1 cores and storage of cores
- Before performing uncoating assays, it is important to determine which fractions contain intact HIV-1 cores. This can be determined by p24 ELISA or reverse transcriptase activity assay using the 50 μl aliquots from step 3.6.
- The recovery of CA associated with the cores is often related to the stability of the cores. Measure the core associated CA by p24 ELISA9 using a sample from each fraction. Alternatively, measure the reverse transcriptase activity of each fraction. Using both methods the peak should be around fraction 10.
- Pool the fractions containing cores. If the cores are not to be used for uncoating assay immediately, aliquot the pooled cores into 0.2 to 0.3 ml aliquots, flash-freeze in liquid N2 and store at -80°C. Cores frozen in this manner are suitable for the uncoating assay. The yield of the core-associated CA is typically 1-2 μg of p24 per milliliter or about 15% of the total virion-associated p24.
5. Kinetic assay of HIV-1 uncoating
- The amount of HIV-1 cores required per uncoating reaction is approximately 50 ng. To perform the in vitro uncoating assay, predilute the aliquot of cores with an equal volume of cold 1XSTE buffer to reduce the viscosity of the suspension and to minimize pipetting errors.
- Further dilute 100 μl of the cores into 0.15 ml of cold 1XSTE buffer containing 10 μg/ml of BSA in a 1.5 ml microcentrifuge tube. We supplement the STE buffer with BSA (10 μg/ml) to minimize adsorption of CA to the walls of the tube. Mix by inverting the tube several times. Do not vortex the samples. Incubate the tubes in a water bath at 37°C for timed intervals (15, 30, 60 and 120 min). The tubes should be immersed in the water bath at least up to the level of the internal sample in order to ensure even warming.
- During incubation, gently mix the contents of the tube periodically (usually 5-10 min) by flicking the tube. For a zero minute control, dilute 100 μl of cores into 0.15 ml of cold 1XSTE buffer and incubate on ice for the entire length of the experiment (120 min). The zero minute control gives the basal uncoating value and is used to determine increase in uncoating of cores incubated at 37°C for different timed intervals.
- At the end of incubation period, stop the uncoating process by placing the tubes in ice for 10 minutes.
- Centrifuge the tubes at 45,000 rpm (TLA-55 rotor; 125,000 x g at rmax) for 20 minutes at 4°C. This will pellet the intact cores and the free CA released as a result of uncoating of cores will remain in the supernatant. The rotor should be precooled at 4°C prior to loading the samples.
- Transfer the supernatant to new precooled microfuge tubes preferably using gel-loading tips stored at room temperature. Please note that the pellet will not be visible to the naked eye, so take extreme care while pipetting out the supernatant. Resuspend the pellets in 250 μl of ELISA sample diluent (0.5% Triton X-100 and 5% Donor calf serum in PBS). To ensure efficient dissolution of the pellet, vortex after adding the ELISA sample diluent. Quantify the CA content of the pellet and supernatant using p24 ELISA as described in the following section.
- The extent of uncoating is obtained by calculating the fraction of CA present in the supernatant.
6. Assay for CA by p24 ELISA
- Many commercial ELISA kits are available and can be used to quantify CA. Use any commercial or “in-house” ELISA and include p24 standards to determine the linearity of the assay. Dilutions of the samples should be assayed to ensure accuracy.
- We have developed a homemade ELISA which we routinely use to assay for CA. The procedure is adapted from a previous report11 and is performed using capture and detector antibodies available from NIH AIDS Research and Reference Reagent Program.
7. Representative Results:
An example of a result from ELISA for determining fractions containing core is shown in Figure 1. After collecting fractions (1 ml each), 50 μl of sample from each fraction was used to make serial dilutions and analyzed by ELISA. The total p24 obtained by adding the values for twelve fractions was 14.5 μg. As shown in the figure fractions 8 to 10 contained cores. The p24 value for the core-containing fractions was 2.17 μg. The percentage of core-associated p24 (14.97%) was determined by taking the ratio of p24 value for the core fractions (2.17 μg) to the total p24 value (14.5 μg). Fraction 1 or 2 will always contain highest amount of p24; this represents free CA which is not associated with the cores. This is followed by a gradual reduction in p24 values with each subsequent fraction, then a sharp increase and finally a sharp drop in the p24 values. If proper care is taken while loading the gradient then the peak of core-associated CA is found around fraction 10.
A typical result obtained by assaying the kinetics of uncoating of HIV-1 cores is shown in Figure 2. The graph shows a time-dependent increase in uncoating of wild-type HIV-1 cores. The percent uncoating value was obtained by calculating the fraction of CA present in the supernatant. With practice, the assay is highly reproducible and is useful for determining the stability of viral cores in vitro. Please note that the reproducibility of the assay is highly dependent on the proper handling of the samples during the procedure. Since the cores are heat labile, the samples should be maintained at 4°C throughout the assay except during the incubation period.
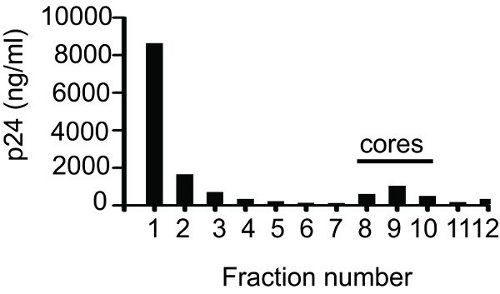
Figure 1 Equilibrium density gradient centrifugation of HIV-1 cores. A concentrated virus suspension was applied to the top of a 30 to 70% linear sucrose gradient overlaid with 1% Triton-X-100. Following overnight centrifugation at 187,000 x g and 4°C, 1 ml fractions were collected from top (Fraction 1) to bottom (Fraction 12) and p24 was quantified by ELISA.
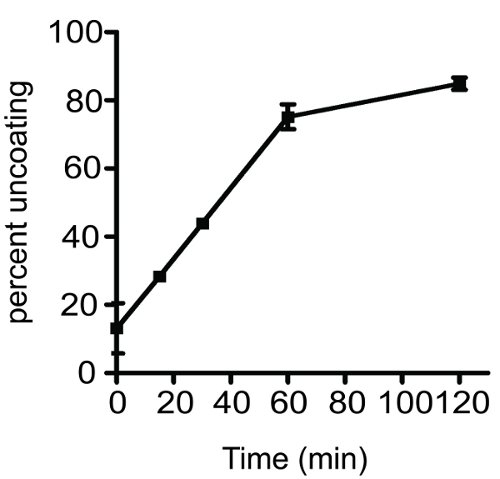
Figure 2 Time course of HIV-1 uncoating in vitro. Purified HIV-1 cores were diluted and incubated at 37°C for the indicated time intervals. The zero min sample was incubated on ice for the entire length of the experiment. Following incubation, samples were ultracentrifuged at 125,000 x g for 20 minutes at 4°C. Supernatant and pellets were separated and analyzed by p24 ELISA. The extent of uncoating was determined as described in the protocol text. Each uncoating reaction was performed in duplicate; error bars span the range of the two values.
Discussion
The method described here to purify HIV-1 cores to study uncoating in vitro is useful for studying this phase of the HIV-1 life cycle, especially due to unavailability of a validated method to analyze HIV-1 uncoating in target cells. Although this method uses equilibrium ultracentrifugation to purify cores, others have used direct pelleting after brief lysis with 1% Triton-X-10012, 13 or pelleting through a sucrose cushion overlaid with 0.1% Triton-X-10014, 15.
The recovery of cores obtained by this method is subject to experimental variation. A variable in the success of this protocol is the transfection efficiency. Typically we load a quantity of virus equivalent to at least 7 μg of p24 on the gradient. Our laboratory uses a calcium phosphate transfection method for virus production. While other transfection methods may also be suitable, we have not evaluated them for isolation of cores. Other important variables that influence core yield are the care taken in applying the layers to the gradient and in keeping the samples cold. Utmost care must be taken when preparing sucrose gradients to avoid mixing of gradient. Any disturbance while applying the concentrated virus suspension may lead to undesired and early contact of the virions with detergent beneath the barrier sucrose layer; such prolonged contact may break apart the fragile cores and lead to reduced capsid association. It is also equally important to keep the sucrose density gradient and the virus suspension cold throughout the procedure. The procedure has been mastered and successfully used by many different scientists in our laboratory.
HIV-1 cores are heat-labile4; therefore, it is very important to fractionate the sucrose gradient while it is cold and to place the collected fractions immediately on ice. Since uncoating of cores is also dependent on pH and salt concentration of the buffer4, it is highly advisable to measure the pH of STE buffer before proceeding with the assay. The rate of uncoating may slightly vary with each batch of cores. Uncoating reactions should be performed in duplicate, and if the values differ by more than 15%, then the reactions should be performed in triplicate until consistency is achieved. Free CA protein has a tendency to stick to the inside walls of the tubes; therefore it is imperative to include BSA at a final concentration of 10 μg/ml in the reaction mixture to minimize adsorption of CA to the walls of the tubes.
As mentioned previously, the uncoating assay described here can be used to identify and study cellular factors affecting uncoating. It may also be used to study the effect of capsid targeting compounds and cellular factors on reverse transcription in cores using an endogenous reverse transcription assay. Isolation of HIV-1 cores in sufficient quantities may also permit the analysis of interactions of the capsid with cellular factors by immunogold electron microscopy.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
HIV-1 uncoating studies in the Aiken laboratory were supported by NIH grants AI40364, AI50423 and AI076121. Several key materials, including reagents used in the p24 ELISA, were provided by the NIH AIDS Research and Reference Program, Division of AIDS, NIAID, NIH.
Materials
| Name of the reagent | Company | Catalog number | Comments (optional) |
| DMEM | Cellgro | 10-013-CV | |
| PBS | Cellgro | 21-031-CV | |
| Triton-X-100 | Mallinckrodt Baker Inc | 9002-93-1 | |
| Tween 20 | Acros | 9005-64-5 | |
| 0.25% Trypsin-EDTA | Cellgro | 25-053-CI | |
| 2XBBS | Composition: 50mM BES [pH 6.95], 280 mM NaCl and 1.5 mM Na2HPO4. Adjust pH to 6.95 at room temperature. Filter sterilize & store in aliquots at -20°C. | ||
| Coating antibody: Monoclonal antibody to p24 (183-H12-5C) | NIH AIDS Research and Reference Reagent Program | 3537 | |
| Primary antibody: HIV-Ig (hyperimmune human patient serum) |
NIH AIDS Research and Reference Reagent Program |
3957 | |
|
Secondary antibody: Goat anti-human IgG, peroxidase conjugated |
Pierce | 31130 | |
| HRP substrate | KPL Inc | 50-76-11 | |
| Immulon 2HB 96 well plates | Thermo Scientific | 3455 | |
| SW32Ti and SW32.1 Ti rotors and compatible ultracentrifuge | Beckman Coulter | ||
| TLA-55 rotor and tabletop ultracentrifuge | Beckman Coulter | ||
| High speed microfuge tubes | Beckman Coulter | 326823, 358123, 357448 | |
| Auto-Densi Flow density gradient fractionator | Labconco Corp. | 4517000 | |
| Gradient Former | CBS Scientific Co. Inc. | GM-20 |
Referenzen
- Ganser, B. K., Li, S., Klishko, V. Y., Finch, J. T., Sundquist, W. I. Assembly and analysis of conical models for the HIV-1 core. Science. 283, 80-83 (1999).
- Li, S., Hill, C. P., Sundquist, W. I., Finch, J. T. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature. 407, 409-413 (2000).
- Aiken, C. Viral and cellular factors that regulate HIV-1 uncoating. Curr. Opin. HIV. AIDS. 1, 194-199 (2006).
- Forshey, B. M., Schwedler, U. v. o. n., Sundquist, W. I., Aiken, C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 76, 5667-5677 (2002).
- Forshey, B. M., Aiken, C. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex. J. Virol. 77, 4409-4414 (2003).
- Wacharapornin, P., Lauhakirti, D., Auewarakul, P. The effect of capsid mutations on HIV-1 uncoating. Virology. 358, 48-54 (2007).
- Chen, C., Okayama, H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 7, 2745-2752 (1987).
- Aiken, C., Prasad, V. R., Ganjam, K. V. . Methods in Molecular Biology. 485, 41-53 (2009).
- Kotov, A., Zhou, J., Flicker, P., Aiken, C. Association of Nef with the human immunodeficiency virus type 1 core. J. Virol. 73, 8824-8830 (1999).
- Kewalramani, V. N., Emerman, M. Vpx association with mature core structures of HIV-2. Virology. 218, 159-168 (1996).
- Wehrly, K., Chesebro, B. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods. 12, 288-293 (1997).
- Welker, R., Hohenberg, H., Tessmer, U., Huckhagel, C., Kräusslich, H. G. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J. Virol. 74, 1168-1177 (2000).
- Briggs, J. A., Wilk, T., Welker, R., Kräusslich, H. G., Fuller, S. D. Structural organization of authentic, mature HIV-1 virions and cores. EMBO J. 22, 1707-1715 (2003).
- Auewarakul, P., Wacharapornin, P., Srichatrapimuk, S., Chutipongtanate, S., Puthavathana, P. Uncoating of HIV-1 requires cellular activation. Virology. 337, 93-101 (2005).
- Accola, M. A., Ohagen, A., Göttlinger, H. G. Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6(gag. J. Virol. 74, 6198-6202 (2000).

