Seeded Synthesis of CdSe/CdS Rod and Tetrapod Nanocrystals
Summary
A protocol for the seeded synthesis of rod-shaped and tetrapod-shaped multicomponent nanostructures consisting of CdS and CdSe is presented.
Abstract
We demonstrate a method for the synthesis of multicomponent nanostructures consisting of CdS and CdSe with rod and tetrapod morphologies. A seeded synthesis strategy is used in which spherical seeds of CdSe are prepared first using a hot-injection technique. By controlling the crystal structure of the seed to be either wurtzite or zinc-blende, the subsequent hot-injection growth of CdS off of the seed results in either a rod-shaped or tetrapod-shaped nanocrystal, respectively. The phase and morphology of the synthesized nanocrystals are confirmed using X-ray diffraction and transmission electron microscopy, demonstrating that the nanocrystals are phase-pure and have a consistent morphology. The extinction coefficient and quantum yield of the synthesized nanocrystals are calculated using UV-Vis absorption spectroscopy and photoluminescence spectroscopy. The rods and tetrapods exhibit extinction coefficients and quantum yields that are higher than that of the bare seeds. This synthesis demonstrates the precise arrangement of materials that can be achieved at the nanoscale by using a seeded synthetic approach.
Introduction
The synthesis of multicomponent nanostructures has developed rapidly over recent years1. A range of II-VI semiconductor nanoheterostructures has been synthesized, including spherical core-shell particles2,3, seeded nanorods4,5, and seeded tetrapods6. These heterostructures allow for sophisticated manipulation of photons and carriers at the nanoscale and exhibit many interesting phenomena, including extremely high extinction coefficients, polarized emission4 and high quantum yields2,7.
The synthesis of multicomponent nanostructures requires precise control over the composition, phase, shape, size, and connectivity of each component. Control over these various factors relies largely on the ability to independently modulate the rate of nucleation and growth8,9,10. One strategy to achieve independent control over nucleation and growth is to separate these two processes into separate synthetic steps. Here, we will show how small seeds of CdSe generated under reaction conditions optimized for nucleation and growth of spherical nanocrystals can then be transferred to a second flask where the growth of CdS occurs under reaction conditions optimized for anisotropic growth of rod-shaped arms6. By tuning the crystal structure of the CdSe seed from wurtzite to zinc-blende, we will demonstrate how the morphology of CdSe/CdS heterostructures grown under similar conditions can be changed from a nanorod to a tetrapod, highlighting the precise control over phase, shape, and size afforded by state-of-the-art nanoparticle synthesis methods. Nanostructures of the cadmium chalcogenides have served as a useful model-system for the development of new synthetic techniques, as this material system has been intensively explored; although the synthesis of a particular nanostructure is shown in this video protocol, the same techniques are applicable to the synthesis of numerous other nanostructures.
Small differences in the techniques and reagents employed in nanoparticle syntheses have been shown to create dramatic differences in the final product. For instance, the temperature ramp rate11 and impurities12-14 present during the synthesis have been shown to dramatically impact the size and shape of the nanocrystals. With identification of the specific factors to which the synthetic procedures are highly sensitive, the reproducibility of nanocrystal syntheses continues to improve. This detailed video protocol is intended to help new practitioners in the field avoid many common pitfalls associated with the synthesis of nanoparticles.
Protocol
Please consult all relevant material safety data sheets (MSDS) before use. Several of the chemicals used in these syntheses are acutely toxic and carcinogenic. Nanomaterials may have additional hazards compared to their bulk counterpart. Please use all appropriate safety practices when performing a nanocrystal reaction including the use of engineering controls (fume hood, glovebox) and personal protective equipment (safety glasses, gloves, lab coat, full-length pants, closed-toe shoes). Portions of the following procedures involve standard air-free handling techniques15.
1. Preparation of Molecular Precursors
- Preparation of cadmium (II) myristate
Cadmium myristate can be synthesized in either an ex situ or in situ method, achieving similar results.- Ex situ preparation of cadmium myristate16
- Dissolve 0.240 g sodium hydroxide and 1.370 g myristic acid in 240 ml methanol.
- Separately, dissolve 0.617 g cadmium nitrate tetrahydrate in 40 ml methanol.
- Add the cadmium nitrate solution drop-wise (~1 drop/sec) to the stirring (800 rpm) sodium myristate solution using an addition funnel.
- Filter the solution. Wash the resulting white precipitate with methanol three times. Dry the precipitate under vacuum overnight.
- In situ preparation of cadmium myristate6
- Combine 39 mg cadmium oxide (CdO) with 0.137 g myristic acid in 5 ml 1-octadecene (ODE) in a 50 ml 3-neck round bottom flask with a stir bar.
- Heat the solution to 250 °C under inert gas while stirring (800 rpm) until the solution becomes a pale yellow color. Carefully swirl the solution as necessary to remove excess CdO from the sidewalls of the flask. Leaving this solution at elevated temperature beyond this point will cause it to become an undesired black color.
- Cool the solution to room temperature for use in subsequent reactions.
- Ex situ preparation of cadmium myristate16
- Preparation of TOP:Se precursor
- Combine 58 mg selenium powder with 0.360 g tri-n-octylphosphine (TOP) under an inert atmosphere.
- Sonicate until the selenium powder is fully dissolved, yielding an optically clear solution. Alternatively, the solution can also be heated to 100 °C in order to fully dissolve the selenium. Use proper glassware or a glove box to prevent exposure of the solution to air during sonication or heating.
- Preparation of TOP:S precursor
- Combine 0.160 g elemental sulfur with 1.853 g TOP.
- Heat to 50 °C under an inert atmosphere until the solution is optically clear. Cool to room temperature for future use.
2. Synthesis of CdSe Seeds
- Synthesis of wurtzite CdSe (w-CdSe) nanocrystals5
- Combine 3.0 g trioctylphosphine oxide (TOPO), 280 mg n-octadecylphosphonic acid (ODPA), and 60 mg CdO with a stir bar in a 25 ml 3-neck round bottom flask equipped with a thermocouple (inserted in a custom glass adapter or punctured through a rubber septum), a reflux condenser, and a rubber septum. Inspect all glassware for defects prior to use due to the high temperature of reaction and potential need for rapid cooling. Assemble all glass-to-glass joints with high temperature vacuum grease.
- Degas the mixture at 150 °C under vacuum for 1 hr while stirring (800 rpm).
- Heat the solution to 300 °C under inert gas to form an optically clear solution. Carefully swirl the solution to remove excess CdO from the sidewalls of the flask. Allow the solution to become optically clear again.
- Add 1.5 g TOP drop-wise to the solution and raise the temperature to 370 °C.
- Rapidly inject TOP:Se solution using a syringe with a large diameter (14 G) needle. The rapid injection will create a sharp nucleation event, promoting a narrow size distribution.
- Let the reaction proceed for the desired duration, as the size of the w-CdSe nanocrystal can be tuned by adjusting the reaction time. Immediately cooling after the injection will yield nanocrystals of ~2-2.5 nm in diameter whereas reacting the solution for 3 and 5 min will yield ~3 nm and ~4 nm diameter nanocrystals, respectively.
- Remove the heating mantle and rapidly cool the flask using a stream of compressed air. Care should be taken when rapidly cooling a flask from elevated temperatures as the thermal shock may break the glassware. For this reason, the use of a water bath for rapid cooling is strongly discouraged.
- Add 10 ml of anhydrous methanol to the solution when the temperature is below 60 °C to flocculate the nanocrystals. Transfer the solution to a vial under inert gas and centrifuge.
- Decant the solution. Discard the supernatant and redisperse the precipitate in anhydrous toluene. Add anhydrous methanol until the solution becomes turbid and centrifuge.
- Repeat a similar cleaning procedure (step 2.1.9) using a different solvent/nonsolvent pair: hexane/isopropanol. This helps prevent situations in which the nanocrystals and precursors precipitate simultaneously.
- Disperse the cleaned precipitate in a small volume (a few milliliters) of anhydrous toluene. The use of a small volume of solvent here will avoid the need to concentrate the particles as an additional step prior to their use in the seeded growth of CdS.
- Confirm the wurtzite crystal structure of the CdSe nanocrystals by X-ray diffraction. This step is strongly encouraged due to the polymorphism of CdSe and the strong dependence on the structure of the CdSe seed for the resulting heterostructures.
- Dilute a small aliquot of the w-CdSe stock solution by a known dilution factor and acquire a UV-Vis absorption spectrum.
- Determine the w-CdSe nanocrystal diameter and extinction coefficient based upon the first exciton absorbance according to the following formulas17:
D = (1.6122×10-9)λ4 – (2.6575×10-6)λ3 + (1.6242×10-3)λ2 – (0.4277)λ + 41.57
ε = 5857(D)2.65
where D is the diameter (nm) of the w-CdSe nanocrystal, λ is the wavelength (nm) of the first excitonic absorption peak, and ε is the extinction coefficient (L/mol•cm) of nanocrystals at the first exciton. - Determine the concentration of the w-CdSe nanocrystal stock solution using the absorbance at the first excitonic absorption peak in accordance with Beer-Lambert’s Law and the appropriate dilution factor.
- To prepare w-CdSe for the growth of seeded rods, combine 0.5 g TOP with a volume of the w-CdSe nanocrystal stock solution corresponding to 10-8 mol of w-CdSe nanocrystals. Sonicate briefly if needed to redisperse the w-CdSe nanocrystals in TOP. The use of a small volume of toluene for the w-CdSe stock solution in a prior step (2.1.11) should lead to only a small amount of toluene, on the order of microliters, in the seed solution used for the injection. The injection of larger volumes of volatile organic solvents, such as toluene, at temperatures substantially above their boiling point poses a safety hazard and will adversely affect the nanocrystal product due to large drops in reaction temperatures.
- Synthesis of zinc-blende CdSe (zb-CdSe) nanocrystals16,18,19
- Combine 0.17 g cadmium myristate and 37 ml ODE with a stir bar in a 50 ml 3-neck round bottom flask equipped with a thermocouple (inserted in a custom glass adapter or punctured through a rubber septum), a reflux condenser, and a rubber septum. Inspect all glassware for defects prior to use due to the high temperature of reaction. Assemble all glass-to-glass joints with high temperature vacuum grease.
- Degas the solution under vacuum at 90 °C for 1 hr while stirring (800 rpm). Subsequently, cool the solution to room temperature under inert gas.
- Briefly remove the rubber septum and add 33 mg selenium dioxide powder to the flask. Replace the septum. Degas the solution under vacuum at 50 °C for 15 min.
- Heat to 240 °C at a ramp rate of approximately 10 °C/min under inert gas. As the temperature rises, the solution will begin to change color, progressing from yellow to red.
- Add a previously degassed solution of 1 ml oleic acid and 1 ml oleylamine in 4 ml ODE drop-wise when the solution reaches 240 °C.
- React the solution for 30 min at 240 °C. The size of the zb-CdSe nanocrystals can be tuned by modifying the reaction time. Take aliquots for absorption and photoluminescence spectra to monitor particle growth if desired.
- Remove the heating mantle and cool to room temperature. Transfer the crude solution to an inert atmosphere glove box and split the reaction mixture into 20 ml fractions in centrifuge vials.
- Add 10 ml anhydrous acetone to each vial and centrifuge solutions to isolate the nanocrystals.
- Pour off the supernatant and redisperse the precipitate in anhydrous toluene. Add acetone until the solution becomes turbid and centrifuge.
- Repeat a similar cleaning procedure (step 2.2.9) using a different solvent/nonsolvent pair: chloroform/methanol. This helps prevent situations in which the nanocrystals and precursors precipitate simultaneously.
- Disperse the cleaned precipitate in a small volume (a few milliliters) of anhydrous chloroform. The use of a small volume of solvent here will avoid the need to concentrate the particles as an additional step prior to their use in the seeded growth of CdS.
- After cleaning the particles, confirm the zinc-blende crystal structure of the CdSe nanocrystals by X-ray diffraction. This step is strongly encouraged due to the polymorphism of CdSe and the strong dependence on the structure of the CdSe seed for the resulting heterostructures.
- Dilute a small aliquot of the zb-CdSe stock solution by a known dilution factor and acquire a UV-Vis absorption spectrum.
- Determine the zb-CdSe nanocrystal diameter and extinction coefficient based upon the first exciton absorbance according to the following formulas20:

ε = 19300 (D)3
where D is the diameter (nm) of the zb-CdSe nanocrystal, Eg the band gap energy (eV) corresponding to the first excitonic absorption peak, and ε is the extinction coefficient (L/mol•cm) of nanocrystals at 340 nm. - Determine the concentration of the zb-CdSe nanocrystal stock solution using the absorbance at 340 nm in accordance with Beer-Lambert’s Law and the appropriate dilution factor.
- Combine 0.5 g TOP with a volume of the zb-CdSe nanocrystal stock solution corresponding to 10-8 mol of zb-CdSe nanocrystals to prepare zb-CdSe injection for the growth of seeded tetrapods. Sonicate briefly if needed to redisperse the zb-CdSe nanocrystals in TOP. The use of a small volume of chloroform for the zb-CdSe stock solution in a prior step (2.2.11) should lead to only a small amount of chloroform, on the order of microliters, in the seed solution used for the injection. The injection of larger volumes of volatile organic solvents, such as chloroform, at temperatures substantially above their boiling point poses a safety hazard and will adversely affect the nanocrystal product due to large drops in reaction temperatures.
3. Synthesis of CdSe/CdS Heterostructures
- Synthesis of CdSe/CdS seeded nanorods6
- Combine 207 mg CdO, 1.08 g ODPA, 3.35 g TOPO, and 15 mg propylphosphonic acid (PPA) with a stir bar in a 25 ml 3-neck round bottom flask equipped with a thermocouple (inserted in a custom glass adapter or punctured through a rubber septum), a reflux condenser, and a rubber septum. Inspect all glassware for defects prior to use due to the high temperature of reaction. Assemble all glass-to-glass joints with high temperature vacuum grease.
- Degas the solution under vacuum at 120 °C for 30 min while stirring (800 rpm).
- Heat the solution to 320 °C under inert gas to form an optically clear solution. Carefully swirl the solution to remove excess CdO from the sidewalls of the flask. Allow the solution to become optically clear again.
- Cool the solution to 120 °C. Degas the solution under vacuum for 1 hr at 120 °C.
- After degassing and complexing the reaction solution, heat the mixture to 340 °C under inert gas. Inject 1.5 g TOP drop-wise and let the temperature recover to 340°C.
- Rapidly inject 0.65 g TOP:S using a syringe with a large diameter (14 G) needle.
- 20 seconds after the TOP:S injection, rapidly inject w-CdSe solution (10-8 mol w-CdSe nanocrystals in 0.5 g TOP) using a syringe with a large diameter (14 G) needle.
- Adjust the reaction temperature to 320 °C. After 10 min at 320 °C, remove the heating mantle and cool the solution.
- Add ~10 ml anhydrous toluene to the flask when the temperature is around 100 °C to prevent solidification of the TOPO.
- Transfer the crude solution to a centrifuge vial. The purification of the crude solution of seeded rods can be performed in an inert atmosphere glovebox or ambient conditions, if desired, as the overgrowth of CdS enhances the environmental stability of the nanocrystals. Add an equal volume of anhydrous ethanol to flocculate the nanocrystals and centrifuge.
- Pour off the supernatant and redispersed the precipitate in hexane/octylamine (8:1 by volume). Add anhydrous ethanol until the solution appears turbid and centrifuge. Redisperse precipitate in anhydrous toluene.
- Occasionally, solutions of seeded rods will form a gel during the cleaning process. If this occurs, add a few drops of octylamine to dissolve the gel and continue the cleaning procedure.
Note: The length of the nanorods can be tuned by changing the concentration of injected CdSe seeds; higher seed concentrations lead to shorter rods. Adjusting the TOP:S concentration will vary the rod diameter and length; higher TOP:S concentration produces longer, thinner rods. See reference6 for further details.
- Synthesis of CdSe/CdS seeded tetrapods6,17
- Combine 207 mg CdO, 1.08 g ODPA, 3.35 g TOPO, and 50 mg PPA with a stir bar in a 25 ml 3-neck round bottom flask equipped with a thermocouple (inserted in a custom glass adapter or punctured through a rubber septum), a reflux condenser, and a rubber septum. Inspect all glassware for defects prior to use due to the high temperature of reaction. Assemble all glass-to-glass joints with high temperature vacuum grease.
- Degas the solution under vacuum at 120 °C for 1 hr while stirring (800 rpm).
- Heat the solution to 280 °C under inert gas to form an optically clear solution. Carefully swirl the solution to remove excess CdO from the sidewalls of the flask. Allow the solution to become optically clear again.
- Cool the solution to 120 °C. Degas the solution under vacuum for 1 hr at 120 °C.
- After degassing and complexing the reaction solution, heat the mixture to 300 °C under inert gas. Inject 1.5 g TOP drop-wise and let the temperature recover to its initial value.
- Rapidly inject 0.65 g TOP:S using a syringe with a large diameter (14 G) needle.
- 40 seconds after the TOP:S injection, rapidly inject zb-CdSe solution (10-8 mol zb-CdSe nanocrystals in 0.5 g TOP) using a syringe with a large diameter (14 G) needle.
- Raise the reaction temperature to 315 °C at a rate of 1 °C/min. After 20 min at 315 °C, remove the heating mantle and cool.
- Add anhydrous toluene to the flask when the temperature is around 100 °C to prevent solidification of the TOPO.
- Transfer the crude solution to a centrifuge vial. The purification of the crude solution of seeded tetrapods can be performed in an inert atmosphere glovebox or ambient conditions, if desired, as the overgrowth of CdS enhances the environmental stability of the nanocrystals. Add anhydrous acetone until the solution becomes turbid and centrifuge.
- Pour off the supernatant and redisperse the precipitate in hexane/octylamine (8:1 by volume). Add anhydrous acetone until the solution becomes turbid and centrifuge.
- Repeat the purification of the tetrapods twice more using toluene and acetone as the solvent and nonsolvent, respectively. Store the tetrapods in toluene.
- Separate tetrapods from undesired nanorod byproducts by centrifuging toluene solutions at 12,000 x g for 30 min to develop a concentration gradient due to the mass difference between rods and tetrapods. Carefully remove and discard the upper layers, which contain mainly rods. Combine and dissolve the lower layers, which contain mostly tetrapods, in toluene.
Note: The length of the tetrapod arms can be tuned by changing the injection seed concentration; higher seed concentrations produce shorter arms. The growth time can be used to adjust the arm diameter; longer growth times increase the diameter of the arms. See reference6 for details. The yield of tetrapods can be varied by using different amounts of PPA in the initial reaction mixture. See reference19 for further details.
Representative Results
Absorption spectra, photoluminescence (PL) spectra, X-ray diffraction (XRD) patterns, and transmission electron microscopy (TEM) images were collected for the seeds (Figure 1) and the final heterostructures (Figure 2). The absorption and PL spectra are used to calculate the extinction coefficient and quantum yield of the nanostructures. XRD and TEM are used to determine the phase and morphology of the nanostructures.
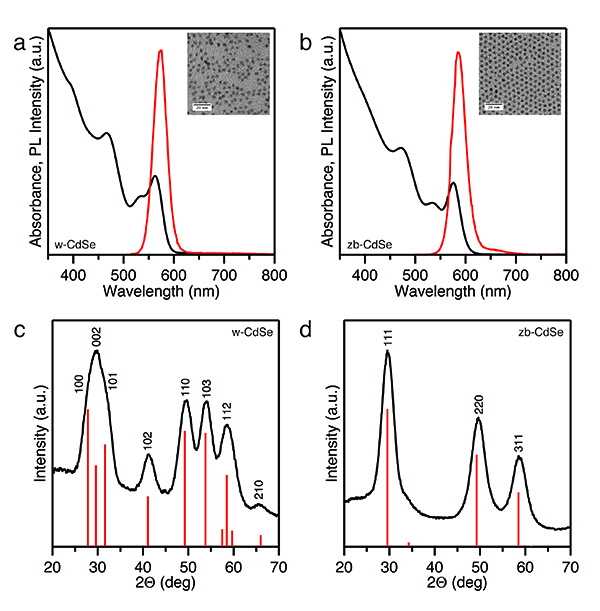
Figure 1. CdSe nanocrystal seeds of wurtzite and zinc-blende crystal structures. (a, b) Absorption (black) and PL (red) spectra with inset of TEM images of wurtzite and zinc-blende CdSe nanocrystals, respectively. (c, d) X-ray diffraction patterns of wurtzite and zinc-blende CdSe nanocrystals with the line patterns of corresponding bulk materials provided for reference. Click here to view larger image.
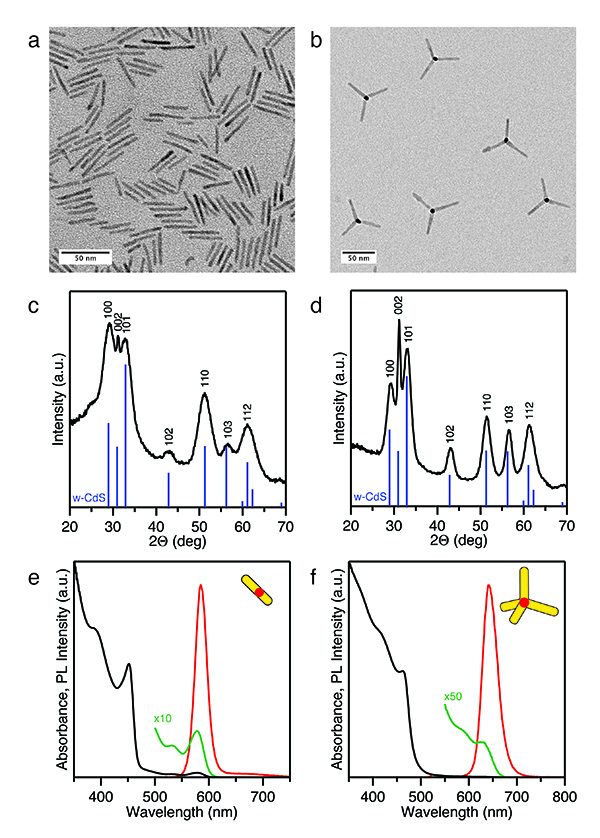
Figure 2. CdSe/CdS seeded heterostructures of rod and tetrapod morphologies. (a, b) TEM images of CdSe/CdS nanorods and CdSe/CdS tetrapods, respectively. (c, d) XRD patterns of CdSe/CdS nanorods and tetrapods. Only reflections from wurtzite CdS are apparent. (e, f) Absorption (black) and PL (red) spectra of CdSe/CdS nanorods and tetrapods. Green line shows magnified absorption spectra to highlight absorption due to the CdSe seed. Click here to view larger image.
Discussion
The optical spectra for the w-CdSe and zb-CdSe seeds exhibit clear excitonic absorption (Figures 1a and 1b) as the seeds are highly monodisperse; the energy of the first exciton corresponds to diameters of 3.3 nm and 3.6 nm for w-CdSe and zb-CdSe, respectively. The seeds also exhibit strong photoluminescence, with quantum yields ranging from approximately 3-30%, depending on the method of preparation of the sample; for instance, a greater number of cleaning steps tends to remove the surface ligands and lead to lower quantum yields6. The narrow full width at half-maximum of the PL peak (31 nm and 33 nm for the w-CdSe and zb-CdSe nanocrystals, respectively) is consistent with size distributions of 5-10%21.
The X-ray diffraction patterns collected for the seeds (Figures 1c and 1d) confirm that they are of the desired phase. The wurtzite phase has clear reflections at 41.1° and 53.8° that are absent for the zinc-blende phase. The peak broadening arises from Scherrer broadening due to finite crystal size.
The differences in the crystal structure of the CdSe seeds lead to dramatically different morphologies of the final heterostructure (Figures 2a and 2b)6. w-CdS nucleates and grows from the (001) and (00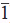 ) facets of the w-CdSe, leading to a final rod-shaped particle since there is uniaxial growth. In contrast, w-CdS nucleates and grows off the {111} facets of zb-CdSe. Of the eight {111} facets, four exhibit lower coordinative saturation than the others; as a result, we observe more rapid growth of w-CdS off of these four facets, which leads to a tetrapod morphology22. The seeded rods and tetrapods display high crystallinity, corresponding to wurtzite CdS as confirmed by XRD (Figures 2c and 2d).
) facets of the w-CdSe, leading to a final rod-shaped particle since there is uniaxial growth. In contrast, w-CdS nucleates and grows off the {111} facets of zb-CdSe. Of the eight {111} facets, four exhibit lower coordinative saturation than the others; as a result, we observe more rapid growth of w-CdS off of these four facets, which leads to a tetrapod morphology22. The seeded rods and tetrapods display high crystallinity, corresponding to wurtzite CdS as confirmed by XRD (Figures 2c and 2d).
Because the CdS portion of the heterostructures is much larger compared to the CdSe seed, the absorption due to the seeds is a small percentage of the overall absorption. As a result, the heterostructures absorb weakly at wavelengths longer than 500 nm, where the majority of absorption is due to the CdSe seed (Figures 2e and 2f). At wavelengths shorter than 500 nm, absorption is dramatically enhanced since the larger bandgap CdS begins to absorb significantly. The extinction coefficients for the seeds are on the order of 105 M-1cm-1, while the rods and tetrapods have significantly enhanced extinction coefficients6 of 107 M-1cm-1 and 108 M-1cm-1, respectively. For both the rods and tetrapods, the first excitonic transition for the heterostructures is red-shifted compared to the bare seeds (Figures 2e and 2f) since the electrons and holes are spread out over a larger particle.
Depending on the particular size of the components of the heterostructure and the resulting band structure, the hole tends to be somewhat confined to the seed while the electron is more free to move throughout the entire particle23. As a result, recombination occurs away from the particle surface, leading to quantum yields for rods above 75% and for tetrapods between 35-70%, which are significantly higher than that of the bare CdSe seeds6. Furthermore, the inorganic passivation from the CdS on the CdSe seeds provides enhanced environmental stability.
We have demonstrated a method for controlling the final morphology of a nanoscale heterostructure via control over the crystal structure of the seed. This same seeded-growth approach has already been applied to a wide range of structures, and we expect that the types of heterostructures that can be synthesized by this approach will continue to expand.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Physical Chemistry of Inorganic Nanostructures Program, KC3103, Director, Office of Science, Office of Basic Energy Sciences, of the United States Department of Energy under contract DE-AC02-05CH11231. The Center for Nanoscale Materials at Argonne National Laboratory is supported by a U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences User Facility under Contract No. DE-AC02-06CH11357. K.M. and B.J.B. gratefully acknowledge the support of the Department of Energy Office of Science Graduate Fellowship Program (DOE SCGF), made possible in part by the American Recovery and Reinvestment Act of 2009 and administered by ORISE-ORAU under contract no. DE-AC05-06OR23100.
Materials
| Sodium hydroxide, 98% | Fisher | BP359 | |
| Myristic acid, 99% | Sigma Aldrich | M3128 | |
| Methanol, 99.8% | Fisher | A412 | |
| Cadmium nitrate tetrahydrate, 99% | Sigma Aldrich | 20911 | Highly toxic |
| Cadmium oxide (CdO), 99.99% | Sigma Aldrich | 202894 | Highly toxic |
| 1-Octadecene (ODE), 90% | Sigma Aldrich | O806 | Technical grade |
| Selenium (Se), 100 mesh, 99.99% | Sigma Aldrich | 229865 | |
| Tri-n-octylphosphine (TOP), 97% | Strem | 15-6655 | Air sensitive |
| Sulfur (S), 99.9995% | Alfa Aesar | 10343 | |
| Trioctylphosphine oxide (TOPO), 99% | Strem | 15-6661 | |
| N-Octadecylphosphonic acid (ODPA), 99% | PCI Synthesis | 104224 | |
| Methanol, anhydrous, 99.8% | Sigma Aldrich | 322415 | |
| Toluene, anhydrous, 99.8% | Sigma Aldrich | 244511 | |
| Chloroform, anhydrous, 99% | Sigma Aldrich | 288306 | |
| Acetone, 99.9% | Fisher | A949 | Dried over 4Å sieves |
| Hexane, anhydrous, 95% | Sigma Aldrich | 296090 | |
| 2-Propanol, anhydrous, 99.5% | Sigma Aldrich | 278475 | |
| Selenium dioxide, 99.999% | Sigma Aldrich | 204315 | |
| Oleic acid, 90% | Sigma Aldrich | 364525 | Technical grade |
| Oleylamine, 70% | Sigma Aldrich | O7805 | Technical grade |
| Propylphosphonic acid (PPA), 95% | Sigma Aldrich | 305685 | |
| Ethanol, anhydrous, 99.5% | Sigma Aldrich | 459836 | |
| Octylamine, 99% | Sigma Aldrich | O5802 |
Referenzen
- Talapin, D. V., Lee, J. -. S., Kovalenko, M. V., Shevchenko, E. V. Propects of Colloidal Nanoscrystals for Electronic and Optoelectronic Applications. Chem. Rev. 110, 389-458 (2010).
- Hines, M. A., Guyot-Sionnest, P. Synthesis and Characterization of Strongly Luminescing ZnS-Capped CdSe Nanocrystals. J. Phys. Chem. 100, 468-471 (1996).
- Peng, X., Schlamp, M. C., Kadavanich, A., Alivisatos, A. P. Epitaxial Growth of Highly Luminescent CdSe/CdS Core/Shell Nanocrystals with Photostability and Electronic Accessibility. J. Am. Chem. Soc. 119, 7019-7029 (1997).
- Talapin, D. V., et al. Highly Emissive Colloidal CdSe/CdS Heterostructures of Mixed Dimensionality.. Nano Lett. 3, 1677-1681 (2003).
- Carbone, L., et al. Synthesis and Micrometer-Scale Assembly of Colloidal CdSe/CdS Nanorods Prepared by a Seeded Growth Approach. Nano Lett. 7, 2942-2950 (2007).
- Talapin, D. V., Nelson, J. H., Shevchenko, E. V., Aloni, S., Sadtler, B., Alivisatos, A. P. Seeded Growth of Highly Luminscent CdSe/CdS Nanoheterostructures with Rod and Tetrapod Morphologies. Nano Lett. 7, 2951-2959 (2007).
- Dabbousi, B. O., et al. (CdSe)ZnS Core-Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B. 101, 9463-9475 (1997).
- Sugimoto, T. Preparation of Monodispersed Colloidal Particles. Adv. Colloid Interface Sci. 28, 65-108 (1987).
- Shevchenko, E. V., et al. Study of Nucleation and Growth in the Organometallic Synthesis of Magnetic Alloy Particles: The Role of Nucleation and Rate in Size Control of CoPt3 Nanocrystals. J. Am. Chem. Soc. 125, 9090-9101 (2003).
- Carbone, L., Cozzoli, P. D. Colloidal Heterostructured Nanocrystals: Synthesis and Growth Mechanisms. Nanotoday. 5, 449-493 (2010).
- Baumgardner, W. J., Quan, Z., Fang, J., Hanrath, T. Timing Matters: The Underappreciated Role of Temperature Ramp Rate for Shape Control and Reproducibility of Quantum Dot Synthesis. Nanoscale. 4, 3625-3528 (2012).
- Evans, C. M., Evans, M. E., Krauss, T. D. Mysteries of TOPSe Revealed: Insights into Quantum Dot Nucleation. J. Am. Chem. Soc. 132, 10973-10975 (2010).
- Steckel, J. S., Yen, B. K. H., Oertel, D. C., Bawendi, M. G. On the Mechanism of Lead Chalcogenide Nanocrystal Formation. J. Am. Chem. Soc. 128, 13031-13033 (2006).
- Wang, F., Tang, R., Buhro, W. E. The Trouble with TOPO; Identification of Adventitious Impurities Beneficial to the Growth of Cadmium Selenide Quantum Dots, Rods, and Wires. Nano Lett. 8, 3521-3524 (2008).
- Shriver, D. F., Drezdzon, M. A. . The Manipulation of Air-Sensitive Compounds. , (1986).
- Yang, Y. A., Wu, H., Williams, K. R., Cao, Y. C. Synthesis of CdSe and CdTe Nanocrystals without Precursor Injection. Angew. Chem. Int. Ed. 44, 6712-6715 (2005).
- Yu, W. W., Qu, L., Guo, W., Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 15, 2854-2860 (2003).
- Chen, O., et al. Synthesis of Metal-Selenide Nanocrystals Using Selenium Dioxide as the Selenium Precursor. Angew. Chem. Int. Ed. 47, 8638-8641 (2008).
- Huang, J., Kovalenko, M. V., Talapin, D. V. Alkyl Chains of Surface Ligands Affect Polytypism of CdSe Nanocrystals and Play an Important Role in the Synthesis of Anisotropic Nanoheterostructures. J. Am. Chem. Soc. 132, 15866-15868 (2010).
- Čapek, R. K., et al. Optical Properties of Zincblende Cadmium Selenide Quantum Dots. J. Phys. Chem. C. 114, 6371-6376 (2010).
- Qu, L., Peng, X. Control of Photoluminescence Properties of CdSe Nanocrystals in. Growth. J. Am. Chem. Soc. 124, 2049-2055 (2002).
- Manna, L., Scher, E. C., Alivisatos, A. P. Shape Control of Colloidal Semiconductor Nanocrystals. J. Cluster Sci. 13, 521-532 (2002).
- Sitt, A., Sala, F. D., Menagen, G., Banin, U. Multiexciton Engineering in Seeded Core/Shell Nanorods: Transfer from Type-I to Quasi-type-II Regimes. Nano Lett. 9, 3470-3476 (2009).

