Retrograde Labeling of Retinal Ganglion Cells in Adult Zebrafish with Fluorescent Dyes
Summary
We introduce an efficient method to retrograde label retinal ganglion cells (RGCs) in adult zebrafish.
Abstract
As retrograde labeling retinal ganglion cells (RGCs) can isolate RGCs somata from dying sites, it has become the gold standard for counting RGCs in RGCs survival and regeneration experiments. Many studies have been performed in mammalian animals to research RGCs survival after optic nerve injury. However, retrograde labeling of RGCs in adult zebrafish has not yet been reported, though some alternative methods can count cell numbers in retinal ganglion cell layers (RGCL). Considering the small size of the adult zebrafish skull and the high risk of death after drilling on the skull, we open the skull with the help of acid-etching and seal the hole with a light curing bond, which could significantly improve the survival rate. After absorbing the dyes for 5 days, almost all the RGCs are labeled. As this method does not need to transect the optic nerve, it is irreplaceable in the research of RGCs survival after optic nerve crush in adult zebrafish. Here, we introduce this method step by step and provide representative results.
Introduction
As adult zebrafish have a strong ability to regenerate axons after optic nerve injury1, a proper method to count the whole RGCs is essential to evaluate RGCs survival and regeneration2. Based on the methods of retrograde labeling RGCs in mammalian and goldfish3-5, we constructed the method to label RGCs from the tectum in adult zebrafish. For adult zebrafish, two critical technical concerns should be noted: the skull of adult zebrafish is very small6; they live in a water environment. Here, we treat the skull with etchant which minimizes the hazards associated with drilling5. Then, we seal the hole with light curing bond which improves animal survival after surgery.
Previously, several other techniques were adopted to count RGCs number in indirect ways. HE staining in the retina sections labels all types of cells in the RGCL7. Antibody labeling in the whole retina, such as islet-1, can also label amacrine cells8. Though retrograde label from the optic nerve stump can label all RGCs in the retina, it cannot be adopted in the crush model because it causes extra injury to the optic nerve. Taking advantage of retrograde label from the tectum, we have researched RGCs survival and regeneration in optic nerve crush. Results show that almost all RGCs survived and over 90% of RGCs regenerated to the tectum at the first week in the crush model9.
In order to successfully label all RGCs, DiI paste was chosen after comparison with several other commercial dyes10. Firstly, it is especially designed for in vivo tissue labeling. Secondly, it is a lipophilic dye which cannot diffuse in water. Additionally, this fluorescence can persist for a long time which makes it an excellent candidate for RGCs survival research.
Protocol
1. Construct the Surgery Apparatus
NOTE: To ensure the fish remains alive during and after the operation, drip anesthesia solution ethyl 3-aminobenzoate methanesulfonate (MS-222, or Tricaine) at a half concentration (0.015%) with a velocity of 1 drop/sec through the fish's mouth using a homebuilt drip system shown in Figure 1.
- Make a box as shown in Figure 1A (length is 28 cm, width is 10 cm, height is 5 cm), put a sponge (length is 6 cm, width is 9 cm, height is 4 cm) in the middle and open a gap on it.
- Make a smooth globular surface on the end of the needle with light curing bond (Figure 1B) which will then be inserted into the fish mouth. Ensure that the surface of the needle is smooth to prevent the fish's mouth from being damaged.
- Add the half concentration of MS-222 (left chamber, length is 6 cm, width is 10 cm, height is 8 cm) and saline (right chamber, length is 4 cm, width is 10 cm, height is 8 cm) to the reservoirs as shown in Figure 1C. Note: Fresh MS-222 is recommended.
- Anesthetize fish in 0.03% MS-222 for 2 min.
- Place the fish in the gap of the sponge and fix the fish with two pairs of pins, inserted behind the pectoral fin and beside the cloacal pore, respectively11.
- Connect the fish’s mouth, MS-222, and rearing water with a T type tube as shown in Figure 1C.
- Switch on the tube connected to the MS-222.
2. Retrograde Label RGCs from the Right Tectum
NOTE: Prior to beginning, disinfect all apparatuses with 70-75% ethanol, ensuring that no ethanol remains.
- Rossing: slightly remove the skin over the whole skull with sclerectome (dorsal view of the skull is shown in Figures 2A and 2B).
- Clearing: wash the skull with saline and then dry it with ear washing bulb.
- Initial corrosion: corrode the skull with the etchant for 10 sec.
- Clearing: completely wipe away the etchant with a cotton swab and then wash the area with saline and dry it.
- Protection: in order to protect the skull in other areas except the right tectum from excess corrosion, cover these areas with light curing bond and then cure it by exposure to blue light with portable illuminant.
- Complete corrosion: put the etchant in the central area of the right tectum again for complete etching for 2 min. Note: Since skulls of older fish are calcified, corrosion will take longer.
- Clearing: completely wipe away the etchant with a cotton swab and then wash the area with saline and dry it again.
- Skull opening: carefully remove the malacic skull (Figure 2C) with forceps to expose the right tectum (Figure 2D).
- Clearing: wipe the mucus and blood effusing from the hole with pieces of gelfoam and wash it with saline until the bleeding has stopped.
- Dye placement: put the dye (tissue-labeling paste) on the whole right tectum and cover it with gelfoam.
- Segregating: cover the hole with a sterile scale from the same animal.
- Sealing: place light curing bond onto the scale and then cure it with blue light again for 40 sec.
- Revival: wash the surface of the bond and revive the animal with saline.
- Nursing and breeding: Keep the fish in 28.5 °C embryo medium (ZFIN) for 5 days and feed 2 times/day. Do not include fish whose dye fails to stay in the tectum for 5 days in the experiment. Note: Embryo medium (EM) solution is vital for fish survival and temperature is the key factor for DiI labeling.
3. Wholemounting Retina and Imaging
- Place the fish in a dark field for 2 hr before retina wholemount.
- Anesthetize the fish with ice water.
- Puncture a hole in the margin of cornea and make an eye cup by removing the cornea with venus scissors.
- Remove the lens and then flush the center of retina with ice-cold 1x PBS.
- Flush the gap between retina and sclera with ice-cold 1x PBS until no pigment is left.
- Cut the optic nerve at the disc.
- Keep the RGC layer upward and hold the retina with a self-made glass spoon (Figures 3A and 3B). A dropper can also be used to transfer the retina.
- Put the retina in a drop of icy 30% glycerin on a slide with the RGC layer upward (Figure 3C).
- Remove the glycerin and then flatten the retina on the glass slide before cutting retina into four quadrants (Figure 3D). Keeping the retina unfolded with RGC layer upward will be helpful for flattening the retina.
- Drip icy 50% glycerin on the retina to wash away the fragments of pigment.
- Remove the glycerin and then drop 20 µl of icy 75% glycerin on the retina.
- Cover the sample with a 1.8 cm x 1.8 cm square coverslip.
- Seal the coverslip with nail polish. Immediate imaging of the retina is recommended.
- Non-interlace image the whole retina under a 20X object lens (NA=0.70) with DP72 CCD (each image is 1360 x 1024 pixels). Each field has three different focuses.
- Extend the depth of each field with the tool of “extended depth of field” in the software.
- Assemble a virtual retina with the function of photomerge.
- Select three fields of 680 x 512 pixels (actual area is 350 µm by 264 µm, 0.092 mm2) in each orientation of the retina to analyze average density of RGCs (Figure 4A).
- Obtain the area with the function of measurements – create a polygon feature in the image software.
- Calculate the total number of RGCs by the equation “RGCs number = destiny x area” 9.
Representative Results
As Figures 4B-D show, the number of DiI+ cells is two thirds of DAPI+ cells in RGCL. In a normal retina, a montage image of a whole retina (Figure 4E) shows that DiI+ RGCs are distributed over the entire retina but in a regenerated retina (Figure 4F), as RGCs in the central area have not regenerated to their target at the first week, they could not be labeled.
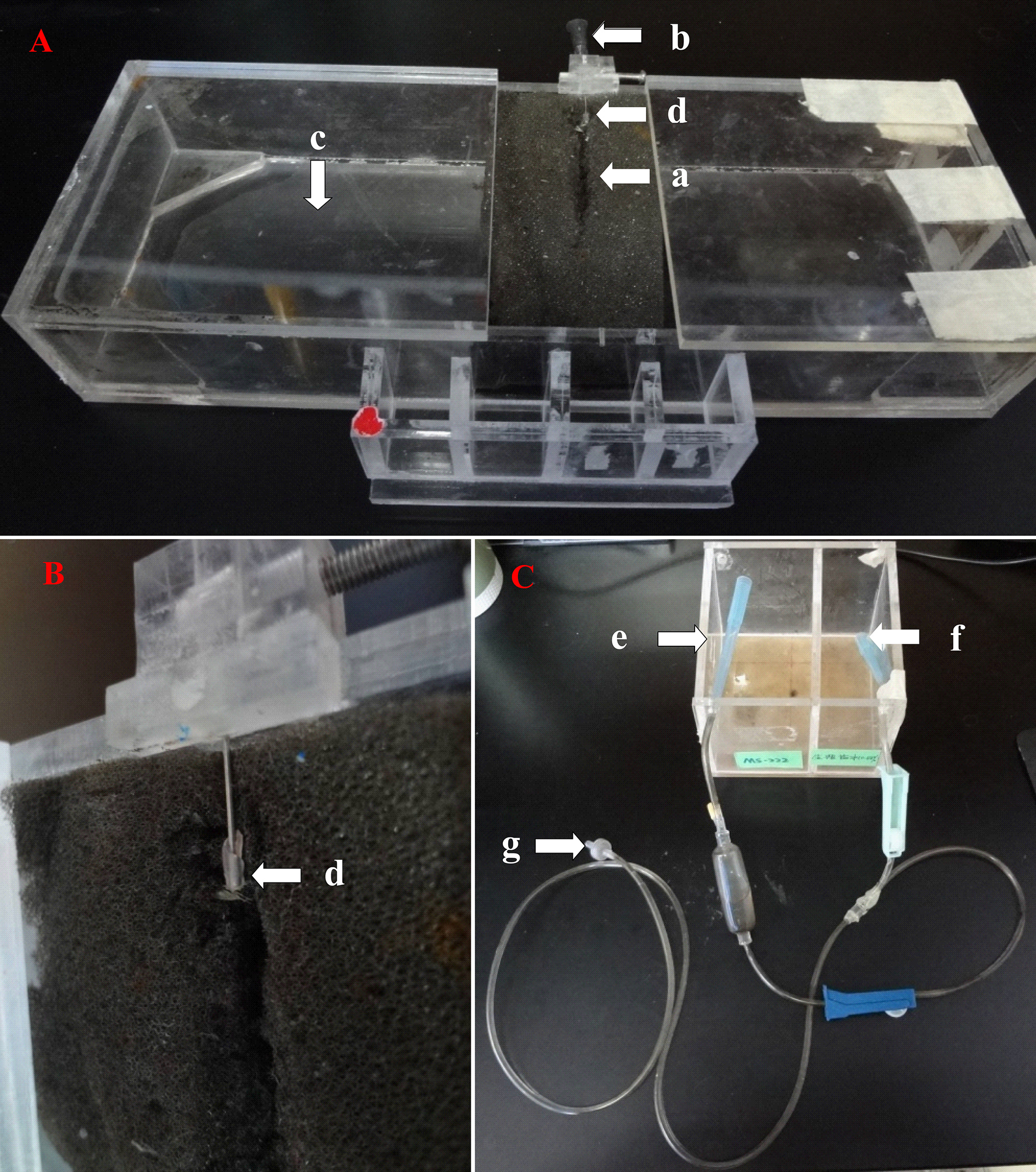
Figure 1. The surgery apparatus used for retrograde labeling of RGC. (A) Use a sponge (a) to fix the fish. The operating platform (length is 28 cm, width is 10 cm, height is 5 cm) and infusion tube are connected via a metal needle (b). Excess water is stored in the cavity (c). (B) A close-up for the metal needle. The needle’s head is wrapped with light curing bond (d). (C) Reservoirs are used for storing MS-222 (e, length is 6 cm, width is 10 cm, height is 8 cm) and saline (f, length is 4 cm, width is 10 cm, height is 8 cm), respectively. The infusion tube (g) connects the reservoir and the metal needle.
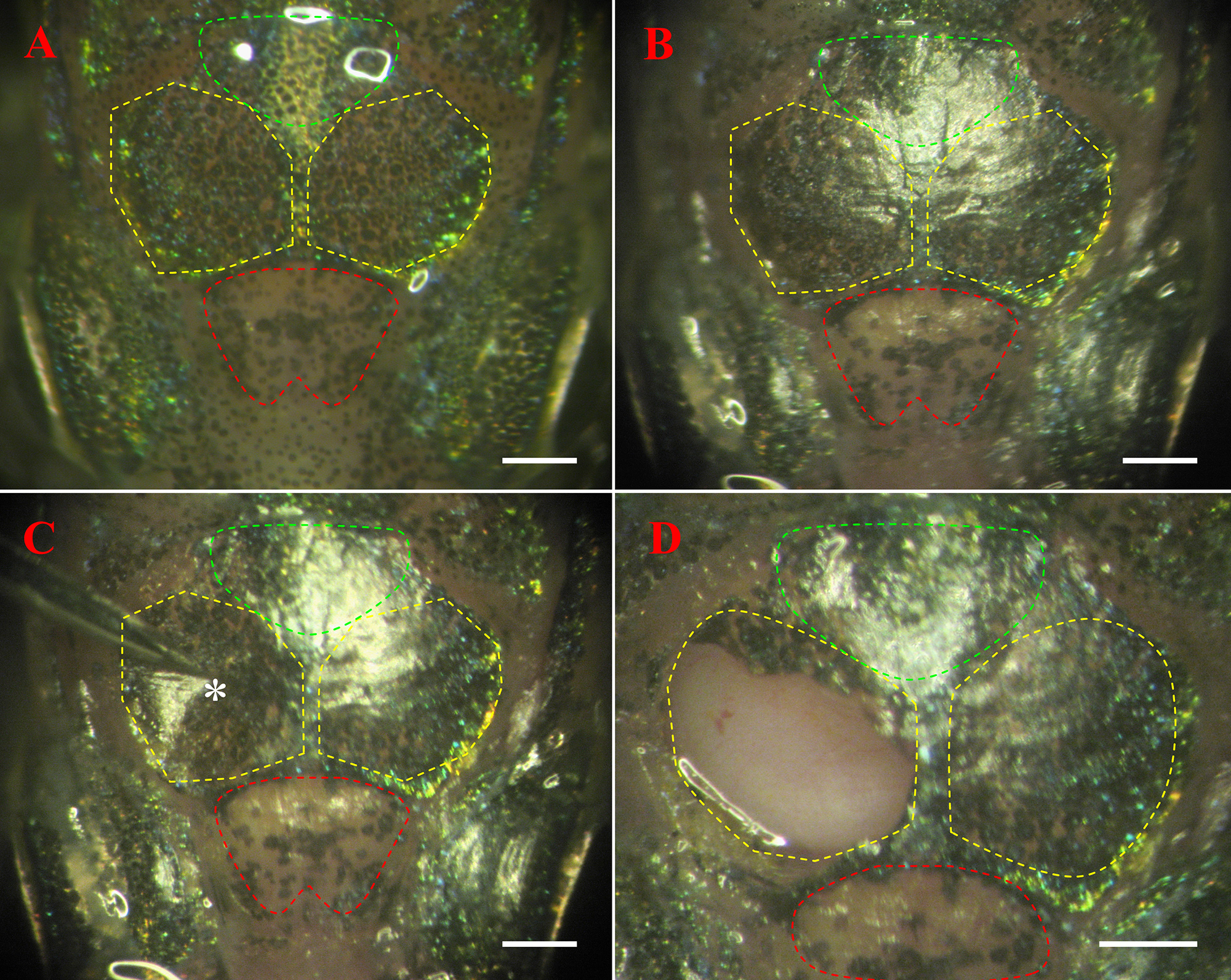
Figure 2. Dorsal view of the skull of adult zebrafish. (A) Intact skull before rossing. The yellow dotted line marks the tectum; the red dotted line marks the telencephalon; and the green dotted line marks the cerebellum. (B) Skull after skin removed. (C) After corrosion with etchant, the skull is malacic and a concave area is pointed out by forceps (white *). (D) Right tectum is exposed after the skull is removed. Scale is 500 μm.
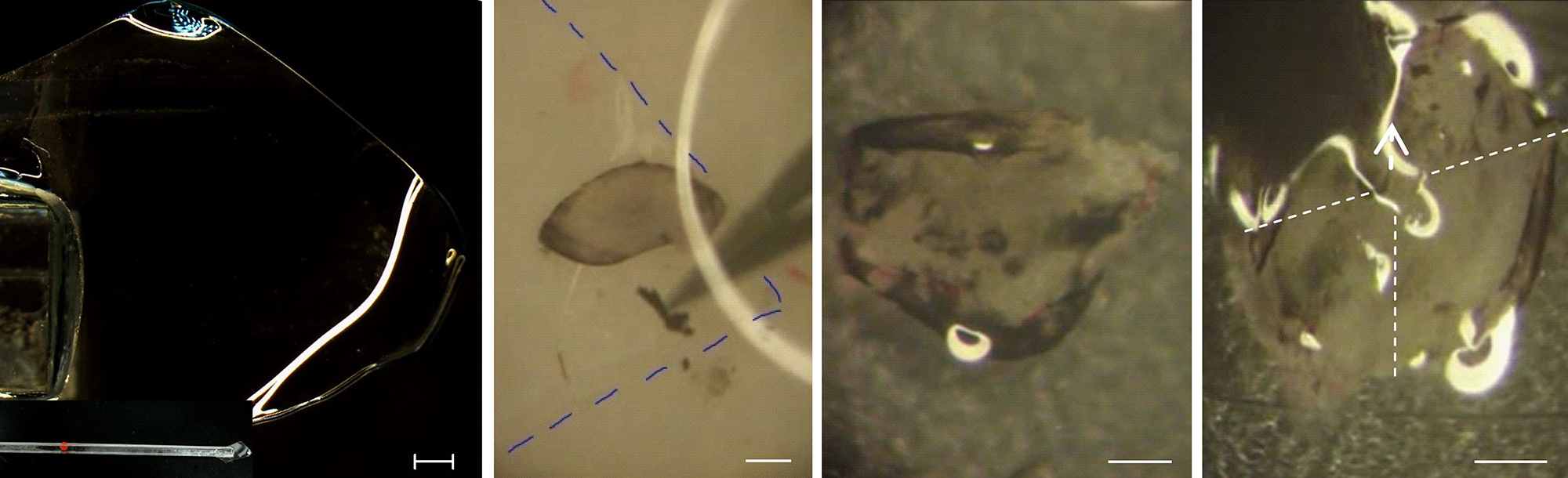
Figure 3. Key steps of wholemounting retina. (A) Homebuilt glass spoon for scooping retina, bottom image shows the whole view. (B) Hold the retina with the “spoon” (marked by dotted line). (C) Transport the retina into ice-cold 30% glycerin. (D) Cut the retina into 4 quarters; arrow indicates the orientation of cutting. Scale bars (A) 1 mm; (B-D) 500 μm.
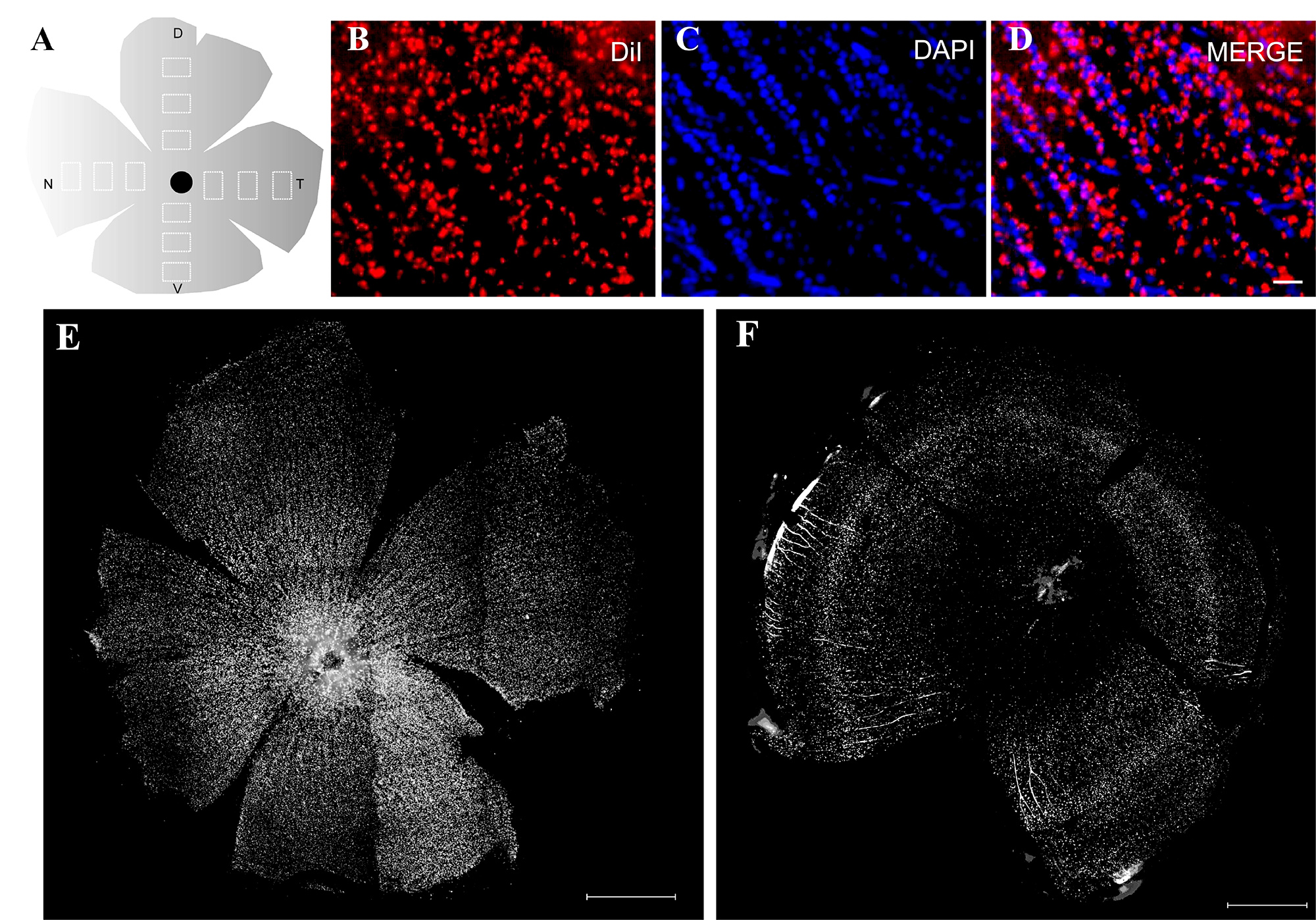
Figure 4. The sampling of RGCs counting in virtual retina. (A) Schematic view of the retina has four fields (N, D, V, T field) and each of them has three fields which are shown in a white box. Notice the optic disc is usually located at the ventral-temporal orientation (black dot). (B) DiI labeled RGCs. (C) DAPI labeled nucleus. (D) Merged picture of RGCs and nucleus. (E) Montage image of whole retina in normal zebrafish. (F) Montage image showing regenerated retina after optic nerve injury. The central area of the retina is not labeled as RGCs have not regenerated to their targets at the first week. Abbreviations: N=nasal, D=dorsal, V=ventral, T=temporal. Scale bars: 30 μm (B-D); 500 μm (E, F).
Discussion
Retrograde labeling of RGCs is important to research RGCs survival in mammalian animals, but it has not been used in zebrafish. The alternative methods, HE staining7 and antibody staining8, are not gold standards for counting RGCs number, and transgenic lines with all RGCs labeled has not yet been constructed12,13. In this video, we introduce a method to retrograde label RGCs in the retina of adult zebrafish. Though about 1% of axons project to olfactory bulb or other areas14, this method can label almost all RGCs in the retina.
As zebrafish are too fragile to bear the physical injury, opening the skull of adult zebrafish with scalpel incision3 or a drill5,15 may cause death. Softening the skull with etchant can minimize the damage to the brain. By sealing the hole with a light curing bond and fixing it under blue light, most fish survive after surgery. It only takes 12-15 min to perform the surgery under proficient operation, which is more convenient and efficient compared with rat and other mammalian animals5.
DiI tissue-labeling paste is a lipophilic dye. Compared to DiI crystals or microinjection of concentrated solutions, this improves the penetration of the dye into the tissue, labeling axons both on and below the surface. Additionally, the fluorescence can persist for a long time period10. On the other hand, this dye is absorbed by passive transport pathway, so it is necessary to leave the dye in the tectum for at least 5 days for complete labeling.
The zebrafish is an excellent model organism of visual regeneration. At 7 days after optic nerve crush, almost all RGCs axon regenerate to the tectum, but only half of RGCs regenerate in the optic nerve cut model9. This method is an ideal way to research RGCs survival and regeneration after optic nerve crush, which could avoid extra injury on the optic nerve.
We have presented a full protocol for successfully retrograde labeling RGCs in adult zebrafish and measuring the RGCs number in a wholemount retina. Additionally, by using a light curing bond, we increase the survival rate of zebrafish, which make this method more effective.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This is supported by 973 MOST grant (Grant No. 2011CB504402, 2012CB947602), National Natural Science Foundation of China (Grant No. 91132724, U1332136) and the ‘Hundred Talents Project’ of Chinese Academy of Science. The protocol was approved by the Committee on the Ethics of Animal Experiments of the USTC (Permit Number: USTCACUC1103013).
Materials
| MS222 | Sigma Aldrich | E10521 | USA |
| DiI | Invitrogen | N22880 | USA |
| lightcuring bond | Heraeus Kulzer | Durafill bond | Germany |
| Gluma Etch | Heraeus Kulzer | Gluma Etch 35 Gel | Germany |
| Blue LED | Shenruo Medical Equipment Co. | Power Blue Light Curing Unit | China |
Referenzen
- Wyatt, C., et al. Analysis of the astray/robo2 zebrafish mutant reveals that degenerating tracts do not provide strong guidance cues for regenerating optic axons. J Neurosci. 30, 13838-13849 (2010).
- Grieshaber, P., Lagreze, W. A., Noack, C., Boehringer, D., Biermann, J. Staining of fluorogold-prelabeled retinal ganglion cells with calcein-AM: A new method for assessing cell vitality. J Neurosci Methods. 192, 233-239 (2010).
- Schwalb, J. M., et al. Two factors secreted by the goldfish optic nerve induce retinal ganglion cells to regenerate axons in culture. J Neurosci. 15, 5514-5525 (1995).
- Watanabe, M., Inukai, N., Fukuda, Y. Survival of retinal ganglion cells after transection of the optic nerve in adult cats: a quantitative study within two weeks. Vis Neurosci. 18, 137-145 (2001).
- Chiu, K., Lau, W. M., Yeung, S. C., Chang, R. C., So, K. F. Retrograde labeling of retinal ganglion cells by application of fluoro-gold on the surface of superior colliculus. J Vis Exp. , (2008).
- Bakken, T. E., Stevens, C. F. Visual system scaling in teleost fish. J Comp Neurol. 520, 142-153 (2012).
- Zhou, L. X., Wang, Z. R. Changes in number and distribution of retinal ganglion cells after optic nerve crush in zebrafish. Shi Yan Sheng Wu Xue Bao. 35, 159-162 (2002).
- Sherpa, T., et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 68, 166-181 (2008).
- Zou, S., Tian, C., Ge, S., Hu, B. Neurogenesis of retinal ganglion cells is not essential to visual functional recovery after optic nerve injury in adult zebrafish. PLoS One. 8, (2013).
- Choi, D., Li, D., Raisman, G. Fluorescent retrograde neuronal tracers that label the rat facial nucleus: a comparison of Fast Blue, Fluoro-ruby, Fluoro-emerald, Fluoro-Gold and DiI. J Neurosci Methods. 117, 167-172 (2002).
- Zou, S. Q., et al. Using the optokinetic response to study visual function of zebrafish. J Vis Exp. , (2010).
- Tokuoka, H., Yoshida, T., Matsuda, N., Mishina, M. Regulation by glycogen synthase kinase-3beta of the arborization field and maturation of retinotectal projection in zebrafish. J Neurosci. 22, 10324-10332 (2002).
- Xiao, T., Roeser, T., Staub, W., Baier, H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 132, 2955-2967 (2005).
- Li, L., Dowling, J. E. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. Journal of Neuroscience. 20, 1883-1892 (2000).
- Kassing, V., Engelmann, J., Kurtz, R. Monitoring of Single-Cell Responses in the Optic Tectum of Adult Zebrafish with Dextran-Coupled Calcium Dyes Delivered via Local Electroporation. PLoS One. 8, (2013).

