Adapting Human Videofluoroscopic Swallow Study Methods to Detect and Characterize Dysphagia in Murine Disease Models
Summary
This study successfully adapted human videofluoroscopic swallowing study (VFSS) methods for use with murine disease models for the purpose of facilitating translational dysphagia research.
Abstract
This study adapted human videofluoroscopic swallowing study (VFSS) methods for use with murine disease models for the purpose of facilitating translational dysphagia research. Successful outcomes are dependent upon three critical components: test chambers that permit self-feeding while standing unrestrained in a confined space, recipes that mask the aversive taste/odor of commercially-available oral contrast agents, and a step-by-step test protocol that permits quantification of swallow physiology. Elimination of one or more of these components will have a detrimental impact on the study results. Moreover, the energy level capability of the fluoroscopy system will determine which swallow parameters can be investigated. Most research centers have high energy fluoroscopes designed for use with people and larger animals, which results in exceptionally poor image quality when testing mice and other small rodents. Despite this limitation, we have identified seven VFSS parameters that are consistently quantifiable in mice when using a high energy fluoroscope in combination with the new murine VFSS protocol. We recently obtained a low energy fluoroscopy system with exceptionally high imaging resolution and magnification capabilities that was designed for use with mice and other small rodents. Preliminary work using this new system, in combination with the new murine VFSS protocol, has identified 13 swallow parameters that are consistently quantifiable in mice, which is nearly double the number obtained using conventional (i.e., high energy) fluoroscopes. Identification of additional swallow parameters is expected as we optimize the capabilities of this new system. Results thus far demonstrate the utility of using a low energy fluoroscopy system to detect and quantify subtle changes in swallow physiology that may otherwise be overlooked when using high energy fluoroscopes to investigate murine disease models.
Introduction
Dysphagia (swallowing impairment) is a common symptom of numerous medical conditions affecting people of all ages. Examples include stroke, Parkinson’s disease, Alzheimer’s disease, cerebral palsy, muscular dystrophy, amyotrophic lateral sclerosis (ALS), Batten disease, head and neck cancer, premature birth, and advanced aging. Dysphagia is highly correlated with mortality, typically as a consequence of severe malnutrition or pneumonia that develops when bacterial-laden food/liquid/saliva is aspirated into the lungs1-4. This debilitating and life-threatening medical condition affects over 15 million people each year in the United States alone3. Despite the high prevalence and associated negative outcomes, current treatment options for dysphagia are limited to palliative (rather than curative) approaches, such as diet modification (e.g., avoiding specific food/liquid consistencies), postural changes (e.g., tucking the chin when swallowing), motor approaches (e.g., exercises targeting muscles in the oral cavity, pharynx, and larynx), sensory approaches (e.g., implementing flavor, temperature, and/or mechanical stimulation), and tube feeding (e.g., nutrition and hydration administered via nasogastric (NG) tube or percutaneous endoscopic gastrostomy (PEG) tube). These treatments merely serve as symptomatic therapy rather than targeting the underlying causes of the problem. Indeed, a major barrier to the discovery of novel, effective treatments for dysphagia is the limited scientific knowledge of the responsible pathological mechanisms, which are likely different for each disease.
Dysphagia diagnosis is predominantly made using a radiographic procedure called a videofluoroscopic swallowing study (VFSS), also known as a modified barium swallow study. Over the past 30-plus years, this diagnostic test has been considered the gold-standard for evaluating swallow function5-7. This test entails having the patient sit or stand within the path of the X-ray beam of a fluoroscopy machine while voluntarily ingesting food and liquid consistencies mixed with an oral contrast agent, typically barium sulfate8,9 or iohexol10. As the patient swallows, food and liquid containing contrast agent can be seen in real time via a computer monitor while traveling from the mouth to the stomach. Soft tissue structures also are visible and can be assessed relative to structure and function. Patients are asked to perform several swallows of each food and liquid consistency, all of which are video recorded for subsequent viewing and frame-by-frame analysis to quantify the presence and degree of dysphagia. Numerous physiological components of swallowing are typically analyzed, such as the anatomical trigger point of the pharyngeal swallow, bolus transit time through the pharynx and esophagus, extent and duration of laryngeal elevation, location and amount of post-swallow residue, and occurrence of and physiological reason for aspiration7,11.
Aspects of the human VFSS protocol were recently adapted for studying freely-behaving rats; however, results were limited because the rats did not remain in the videofluoroscopic field of view during testing12. VFSS has not previously been attempted with mice. Successful adaptation of the human VFSS protocol for use with mice and rats would provide a novel research method to investigate the hundreds of currently existing murine (mouse and rat) models of diseases that are known to cause dysphagia in humans. This new method (henceforth referred to as murine VFSS) would therefore hasten identification and validation of murine models of dysphagia that are suitable for investigating the underlying neurophysiological mechanisms within muscles, nerves, and brain tissue that are pathological and contributing to dysphagia in humans. Moreover, murine VFSS would permit identification of objective measures (biomarkers) of swallow function/dysfunction that could be directly compared with humans. These cross-species videofluoroscopic biomarkers could then serve as novel outcome measures to quantify treatment efficacy in preclinical trials with mice and rats, which would better translate to clinical trials with people.
To this end, the murine VFSS protocol was established using ~100 mice of either sex. All mice were either C57 or hybrid C57/SJL strains. The C57 mice were not genetically altered, whereas C57/SJL was the background strain for a colony of transgenic SOD1-G93A (or SOD1) mice, the most widely used animal model of ALS. The SOD1 colony was an approximate 50-50 mix of transgenic (i.e., ALS-affected) mice and nontransgenic (i.e., unaffected) littermates.
The murine VFSS protocol consists of three components:
- Custom-designed observation chambers that permit voluntary feeding and swallowing while standing unrestrained in a confined space within a fluoroscopy machine,
- Recipes that mask the aversive taste/odor of oral contrast agents and produce sufficient radiodensity to permit adequate visualization of swallowing,
- A step-by-step test protocol that maximizes animal compliance, minimizes total test time and radiation exposure, and permits quantification of several swallow parameters for each stage of swallowing (i.e., oral, pharyngeal, and esophageal).
The combined effect produces a comfortable, low stress, self-feeding examination environment that permits assessment of typical feeding and swallowing behaviors of mice.
Protocol
The murine VFSS protocol follows an approved Institutional Animal Care and Use Committee (IACUC) protocol and NIH Guidelines.
1. Construct Observation Chambers from Polycarbonate Tubing and Sheeting (Figure 1)
- Cut 5 cm wide, square polycarbonate tubing (~2 mm wall thickness) into 16 cm lengths using a manual milling machine. Most mice adequately fit within these dimensions, which results in a narrow test chamber that permits walking and turning around as desired. A wall thickness of ~2 mm provides adequate rigidity without significantly attenuating the X-ray beam.
- Two types of chambers are essential to this protocol: “spout tubes”, designed for delivering liquids via spout, and “ventilation tubes”, designed for delivering liquids via peg-bowl.
- For “spout tubes”, make a small oblong hole (12 x 8 mm) in the top of each tube near one end using a manual milling machine. This hole is used to deliver drinking solutions via a sipper tube spout during behavioral conditioning and VFSS testing.
- For “ventilation tubes”, drill 9 small ventilation holes in the top of each tube near one end. This tube is used during VFSS testing with a peg-bowl instead of sipper tube.
- It is possible to use spout tubes when delivering liquid via peg-bowl; however, the opening in the chamber ceiling must be blocked to prevent distracting exploratory behaviors by mice (see step 6.2.2).
- Two types of chambers are essential to this protocol: “spout tubes”, designed for delivering liquids via spout, and “ventilation tubes”, designed for delivering liquids via peg-bowl.
- Cut polycarbonate sheeting (3/4” thickness) into end-caps (50 x 50 mm, 2 per tube) using a computerized milling machine, also called a computerized numerical control (CNC) machine.
- Mill one oblong groove (19 x 6 mm) near one edge of the interior face of each end-cap. Use this groove to secure a peg-bowl for mice to drink from during VFSS testing.
- Mill 5 round ventilation holes (6 mm diameter) through each end cap.
- Mill one smaller round hole (5 mm diameter) through the end-cap, directly above the oblong groove. Use this hole to deliver liquid into the peg-bowl during VFSS testing.
- On the exterior face of the end-cap, mill a 9/16” diameter counterbore that is 1/4” deep around this smaller hole.
- Mill away 2 mm along the perimeter of the interior face of the end-cap to a depth of 7 mm to make a step that easily inserts into the end of the tube.
- Mill a 1 mm groove into the step of the end-cap to accommodate an O-ring, which is necessary to prevent the end-cap from falling off the end of the tube.
- Round off exposed edges and bevel all corners of the end-caps to prevent chewing by mice.
- Make peg-bowls from polycarbonate sheeting using a CNC machine. Overall dimensions should be 24 x 19 x 6 mm3, with a 10 x 3 mm2 bowl-shape depression at one end. One peg-bowl is needed for each tube. Peg-bowls should insert snugly into the oblong groove in the end-caps (Figure 2).
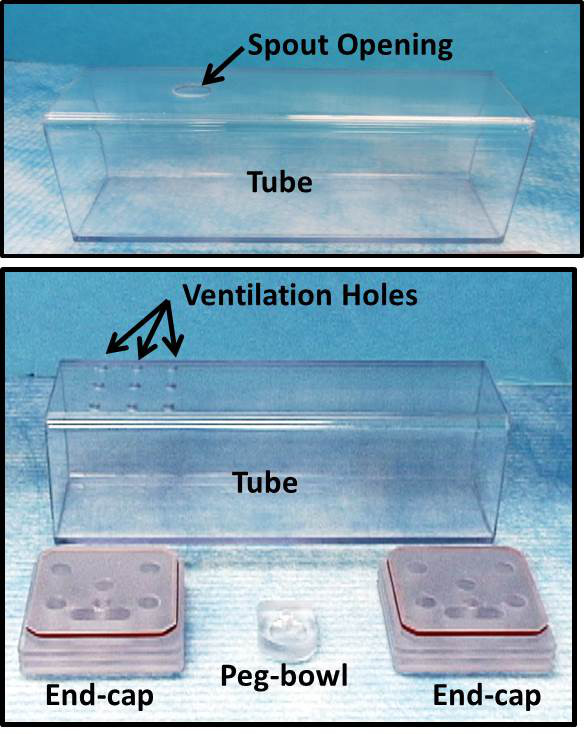
Figure 1: Observation Chambers. Observation chambers were designed to maintain freely behaving animals in the fluoroscopy field of view. These pictures show chamber components essential for conducting VFSS. Top: “spout tube”, designed for delivering liquids via spout. Bottom: “ventilation tube”, designed for delivering liquids via peg-bowl. The two end-caps are interchangeable between spout and ventilation tubes.

Figure 2: Peg-bowls. Each peg-bowl snaps into a groove in the interior face of each end-cap. Left: unassembled components. Middle: assembled components. Right: exterior face of end-cap. Please click here to view a larger version of this figure.
2. Construct Sipper Tube Bottles from Centrifuge Tubes, Silicone Stoppers, and Metal Spouts (Figure 3)
- Use a stopper borer (5/16”) to make a center hole through each silicone stopper.
- Apply a few drops of mineral oil into the bore hole and manually insert a metal spout into the wide end of the stopper. Straight ball-point spouts are preferred because straight open-end spouts result in excessive leaking and splattering of the contrast agent within the observation chamber, which can interfere with visualization during testing.
- Adjust the spout length so that it spans the entire length of the silicone stopper and extends approximately 3 cm beyond the wide end of the stopper.
- Insert the narrow end of each stopper (containing a sipper tube) into a 30 ml centrifuge tube.
- Verify that the spout length is adequate by inserting it through the oblong hole in the top of the observation chamber. The spout tip should rest approximately 1 cm from the chamber ceiling, which is sufficiently long for healthy adult mice to reach.
NOTE: Longer lengths result in mice drinking while turning/tilting the head, which obscures visualization of swallowing during VFSS. - Extend the spout length to accommodate younger mice, smaller sized mouse strains, and mouse disease models that cannot reach the spout due to motor impairment of the limbs.
- Wash the newly made spouts before use to remove mineral oil, silicone debris, and other contaminants during handling.

Figure 3: Sipper Tube Bottles. Left: unassembled components. Middle: assembled components. Right: mouse drinking from sipper tube in observation chamber. Please click here to view a larger version of this figure.
3. Construct a Syringe Delivery System for use with Peg-bowls (Figure 4)
- Use a lathe to make adapters for connecting polyethylene (PE) tubing to the observation chamber end-caps, described as follows.
- Cut 1/2” diameter acetal resin rod material into 1 1/4” length sections, herein referred to as tube adapters (or adapters).
- At one end of each adapter, reduce a 1/2” length section at the tip to 3/16” diameter, referred to herein as the narrow end.
- For the remaining 3/4” length section of each adapter (i.e., the 1/2” diameter end), machine grooves for manual gripping during use. This section is herein referred to as the wide end.
- In the wide end of each adapter, drill a center hole that is 0.098” diameter and 1” deep.
- Drill and ream the remaining portion of the center hole in each adapter to 0.096” to provide a snug fit on the PE tubing.
- Cut PE tubing (PE 240, inner diameter 1.67 mm) to the desired length using a scissor. A 3-4 foot length sufficiently increases the distance between the investigator and fluoroscope during VFSS testing to improve radiation safety.
NOTE: Longer lengths will utilize a larger volume of the contrast agent solution during VFSS testing, perhaps greater than the standard 30 ml recipe. - Insert a blunt-tipped 15 G needle fully into one end of the PE tubing. The fitting should be snug.
- Insert the other (free) end of the PE tubing through the center hole of the adapter tube, starting at the wide end.
- Pull the PE tubing out of the narrow end of the adapter so that it extends ~2 mm.
- Insert the narrow end of the adapter (with ~2 mm PE tubing extending from it) into the end-cap of an observation tube; it should fit snugly into the counterbored hole located directly above the peg-bowl.
- Adjust the PE tubing length at the narrow end of the adapter so that it barely extends above the bowl depression in the peg-bowl.
- Fill a 10 ml syringe (without needle attached) with water from a beaker and remove any air bubbles.
- Attach the filled syringe to the needle end of the PE tubing.
- Slowly push the syringe plunger to deliver water into the peg-bowl in the observation chamber. Stop when the peg-bowl is nearly full. Avoid overfilling, which will cause splattering during drinking.
- If the peg-bowl does not fill properly, adjust the length of the PE tubing extending above the peg-bowl.
- Over-extension of the PE tubing will entice mice to chew on it during testing, rather than drinking from the peg-bowl.
- If the PE tubing is not extended far enough, liquid will run onto the floor of the observation chamber rather than filling the peg-bowl.
- After use, detach the syringe and wash the entire syringe delivery system with soap and water. Use a 10 ml syringe to push air through the PE tubing to remove water. Sterilize by autoclaving as needed.

Figure 4: Syringe Delivery System. Left: unassembled components. Middle: assembled components. Right: mouse drinking from peg-bowl in observation chamber. Please click here to view a larger version of this figure.
4. Construct a Motorized Scissor Lift Table for Remote Positioning of the Observation Chamber (Figure 5)
- Build a scissor lift with a 12 x 12 cm platform which can raise and lower by 5 cm to accommodate viewing mice in different positions within the fluoroscopy field of view. Lift material should be metal or plastic for ease of cleaning with disinfectants.
- Mount stepper motors to adjust the height and longitudinal position of the lift.
- Couple the first stepper motor to the scissor lift mechanism to control the height by translating a crossbar. This coupling can be a lead screw or rack-and-pinion gearing.
- Couple the second stepper motor to the scissor lift frame to control the longitudinal position by translating the entire lift frame relative to the table. This coupling can be a lead screw or rack-and-pinion gearing.
- Wire a remote control system to the stepper motors to allow adjustment of the observation chamber position during imaging while minimizing investigator exposure to radiation.
- Interface handheld remote control buttons with a microcontroller chip to control the activation and direction of each stepper motor.

Figure 5: Remote-controlled Scissor Lift Table. Left: side view of scissor lift table. Right: lift table with observation chamber positioned in fluoroscope. The lift table adjusts the position of the observation chamber to maintain mice in the field of view. Please click here to view a larger version of this figure.
5. Perform Behavioral Conditioning Before VFSS Testing to Ensure Maximal Participation
- 1-2 weeks prior to VFSS testing, subject mice to one overnight (12-16 hr) water regulation period to induce thirst, during which time water is withheld from the home cage. The goal of the water regulation is for animals to be thirsty, not dehydrated. Animals should remain alert and responsive. This duration and time frame is essential to prevent dehydration, which may occur as a result of 2 water regulation episodes within 1 week (i.e., one for behavioral conditioning and another for VFSS testing).
- Place a single "spout tube" (with one end closed by an end-cap) on the floor of a home cage containing fresh bedding material. The closed end should be nearest the spout opening in the chamber ceiling. This step ensures adequate ventilation while multiple mice sleep huddled within the chamber depth overnight. The open end allows mice to freely enter/exit the chamber.
- Remove other enrichment material (e.g., nestlet and hut) to encourage mice to explore and sleep in the chamber overnight (Figure 6). This step ensures that mice are acclimated to being in the chamber for lengthy durations prior to VFSS testing.
- Provide a single standard food pellet per mouse on the floor of the cage for overnight eating; do not provide water or other hydration sources.
- Use a standard filter top to contain the mice in the cage overnight, as the dimensions of the observation chamber prevent a standard wire lid from fitting in the cage. Store the removed wire lid (containing food and water bottle) on top of the filter top to weigh down the lid and prevent mice from escaping.
- Perform palatability testing the following morning, described as follows.
- Make a chocolate-flavored test solution in a 30 ml sipper tube bottle, without adding contrast agent (i.e., substitute water for iohexol). This recipe is described in Table 1. Make one bottle per cage to be tested.
- Remove the observation chamber and replace the standard wire lid. Offer the chocolate-flavored solution (room temperature, ~22 °C) for 2 min per cage, inserted through the wire lid.
- Assess palatability by observing drinking behaviors during the 2 min test period.
- Score palatability according to the following criteria:
- Latency until the first mouse drinks at the spout for at least 5 sec without interruption.
- Percentage of mice per cage that drink the solution.
- Number of mice that simultaneously drink at the spout.
- The solution is deemed palatable if the majority of mice in each cage have multiple long (>5 sec) bouts of drinking and if multiple mice simultaneously drink from the spout (Figure 7).
- If the chocolate-flavored solution is not palatable, repeat palatability testing with other flavor enhancers at various concentrations to identify a single preferred solution.
- Offer up to four different solutions (at various concentrations) one at a time in randomized order to multiple cages of mice in a single test day, without a washout period or washout solution. Suitable flavors enhancers to consider for mice include sugar, cheese, peanut butter, various fruit and nut flavors, and milk.
NOTE: Do not perform palatability testing more than once per week to prevent dehydration from repeated water regulation episodes. - It may take several weeks to successfully identify the preferred solution for each strain of mice. The goal is to identify candidate flavors that result in multiple long (>5 sec) bouts of drinking by mice immediately (<30 sec) after exposure, as these qualifications are deemed essential to obtaining successful VFSS outcomes.
- After a preferred flavor solution is identified, return the observation chamber to each home cage to continue behavioral conditioning, described as follows.
- Attach one end-cap to the observation chamber at the end nearest the oval (spout) hole.
- Offer mice the chocolate-flavored solution for 2-3 hr by inserting the sipper tube bottle through the oval hole in the top of the chamber. This step ensures that all mice have been conditioned to drinking deep within the observation chamber.
- Remove the wire lid to accommodate the observation chamber.
- Place 1 food pellet per mouse on the cage floor for ad libitum consumption during the test period.
- Cover the cage with a standard filter top to prevent mice from escaping for the remainder of the behavioral conditioning period. Store the removed wire lid (containing food and water bottle) on top of the filter top to weigh down the lid.
- Provide water and food ad libitum in the home cage when behavioral conditioning is completed.
- Wash the observation chambers (tubes and end-caps) and sipper tube bottles (spouts and centrifuge tubes) with soap and water; sterilize by autoclaving as needed. Avoid using acetone to clean the tubes as it causes a permanent clouding effect that makes the tube opaque rather than translucent.

Figure 6: Mice Exploring Observation Chambers. Mice are naturally inclined to seek shelter in small spaces. As a result, they freely enter and explore the observation tube when it is placed in the home cage. Most mice are found sleeping in the chamber in the morning. Please click here to view a larger version of this figure.
| INGREDIENTS | Chocolate Solution (for Palatability Testing) | Chocolate-Flavored Iohexol (for VFSS Testing) |
| Chocolate Syrup | 3 ml | 3 ml |
| Iohexol (350 mg iodine/ml) | 0 ml | 15 ml |
| Water (DI or filtered) | Adjust to 30 ml final volume (27 ml) | Adjust to 30 ml final volume (12 ml) |
| Final Volume | 30 ml | 30 ml |
Table 1: Chocolate-flavored Test Solution Preferred by C57 and C57/SJL Mouse Strains.

Figure 7: Palatability Testing. One indicator of taste preference during palatability testing is the number of mice that simultaneously drink from a single spout in the home cage. This image shows four mice simultaneously drinking a chocolate-flavored solution, which was identified as the preferred flavor enhancer by C57 and C57/SJL strains.
6. VFSS Testing Preparation
- Subject mice to an overnight water regulation period (i.e., withhold water for 12-16 hr), as described in Step 5 above.
- Place a single “ventilation tube” (with one end closed by an end-cap) on the floor of a home cage containing fresh bedding material. The closed end should be nearest the ventilation holes in the chamber ceiling. This step ensures adequate ventilation while multiple mice sleep huddled within the chamber depth overnight. The open end allows mice to freely enter/exit the chamber.
- The following morning, remove soiled observation chambers from cages and briefly rinse with tap water and completely dry in preparation for VFSS testing.
- Remove and clean only one chamber at a time to prevent mixing up chambers between cages, which can cause excessive exploratory behaviors that significantly interfere with VFSS testing.
- If “spout tubes” are used instead of “ventilation tubes” for VFSS testing, insert a silicone plug into the spout opening of the observation chamber ceiling to prevent exploratory behaviors (Figure 8).
- Label each chamber (e.g., with the home cage number) to prevent mix up.
NOTE: Use a dry erase marker to label each cleaned tube before placing it back in the home cage. Permanent marker should be avoided because it is absorbed by the tube material and does not wash off, even with alcohol or acetone.
- Prepare the chocolate-flavored iohexol solution (or other palatable solution).
- Make a single recipe (30 ml) of the test solution (Table 1) for several cages.
- PRECAUTIONS FOR IOHEXOL: Store unopened iohexol bottles at room temperature, protected from light. Use opened iohexol bottles within 24 hr, as the viscosity and taste may change within a day or so after exposure to air. Alternatively, freeze aliquots of single-servings (15 ml) in centrifuge tubes for long-term storage. Prepared iohexol test solutions must be used within a few hours to ensure freshness and prevent avoidance by mice. Administer iohexol solutions at room-temperature to avoid confounding the study due to temperature effects on swallow function. Do not freeze any remaining prepared test solution, as the chocolate flavor becomes bitter with freezing and results in avoidance by mice.
- Prepare the fluoroscopy environment.
- Use a spare (empty) observation chamber and peg-bowl (or sipper tube spout) to determine the optimal height and position within the fluoroscope beam that permits visualization of drinking in the lateral (horizontal) plane.
- Set the fluoroscopy frame rate to 30 frames per sec; higher (but not lower) frame rates can be used if available.
- Ensure that a radiopaque calibration marker is appropriately placed on the fluoroscope camera/detector so that it is visible on the display monitor during the entire test. This step is necessary to permit calibration of length measurements used for quantifying swallow parameters.

Figure 8: Silicone Plug When Using Peg-Bowls. Left: silicone plug. Right: A silicone plug is pulled through the sipper tube opening in the top of the observation chamber. This plug prevents mice from becoming distracted by the spout opening when using a peg-bowl rather than sipper tube during VFSS testing. Please click here to view a larger version of this figure.
7. VFSS Testing of Mice
- Observe each cage to identify when a mouse freely enters the observation chamber.
- Lift the chamber out of the cage and gently attach the 2nd end-cap (with peg-bowl attached, if a sipper tube will not be used), being careful not to pinch the mouse (especially the tail).
NOTE: This approach minimizes the mouse’s stress response due to handling, which is especially important for mice that are being tested for the first time. - With repeated testing, mice can be easily coaxed to enter the chamber when it is placed in front of them inside the cage, or when suspended by the tail over the chamber opening.
- Lift the chamber out of the cage and gently attach the 2nd end-cap (with peg-bowl attached, if a sipper tube will not be used), being careful not to pinch the mouse (especially the tail).
- Position the observation chamber (containing the mouse) within the fluoroscopy machine to begin VFSS testing in the lateral plane (i.e., horizontal X-ray beam).
- Provide the chocolate-flavored iohexol solution (Table 1) via peg-bowl or sipper tube bottle.
- If using a peg-bowl, deliver the solution via the syringe delivery system described in Step 3 above. This system permits fast and easy refilling of the peg-bowl as needed.
- If using a sipper tube bottle, insert the sipper tube through the oval opening in the top of the observation chamber. Tilt the bottle so that the spout is directed toward the center of the chamber.
- Start the videofluoroscopy recording when the mouse starts drinking.
- Adjust the position of the observation chamber (using the remote-controlled scissor lift table described in Step 4) so that the swallowing mechanism is visible in the field of view.
- Pause recording each time the mouse turns away from the peg-bowl or spout to minimize the duration of radiation exposure.
- Resume recording when the mouse returns to the spout or peg-bowl.
- Refill the peg-bowl as needed.
- Stop testing if the mouse does not drink within 5 min. The goal is to record several long (>5 sec) bouts of continuous drinking, which is typical for most mice within the first 2 min of testing.
- Return noncompliant mice to the home cage (without water) for re-testing at a later time the same day; do not exceed a 24 hr water regulation period. Mice that remain noncompliant for three trials are removed from the study.
- If needed, reposition the fluoroscope to test mice in the dorsal-ventral plane (i.e., vertical X-ray beam). This plane is used to identify deviations in bolus flow through the pharynx and esophagus during swallowing.
- When testing multiple mice from the same home cage:
- Clean the peg-bowl (and tip of the PE tubing) or sipper tube spout with a dry paper towel between mice.
- Clean the observation chamber as needed between mice to remove any splattered iohexol on the chamber walls. Rinse the chamber with tap water and dry with a paper towel.
- When testing mice from a different home cage:
- Use a new peg-bowl (or change the sipper tube spout). Otherwise, mice may be distracted by the odor of other mice that drank from the same peg-bowl or sipper tube. The peg-bowls and sipper tubes should be labeled to avoid confusion.
- When testing of all mice in a single cage is completed, provide water and food in the home cage.
- Wash the observation chambers (tubes and end-caps), peg-bowls, syringe delivery system, and sipper tube bottles (spouts and centrifuge tubes, if used) with soap and water; sterilize by autoclaving as needed.
- Dispose of any remaining iohexol solution as directed by safety guidelines; drain disposal may be acceptable at most facilities.
8. Video Analysis
- Use a video editing software program that permits frame-by-frame analysis of the videofluoroscopy recordings to quantify the swallow parameters of interest (Table 2).
- Identify at least two trained reviewers to analyze each video in a blinded fashion: A primary reviewer and one or two secondary reviewers.
- Primary reviewer: View each video to identify and analyze 3-5 long (approximately 5 sec) drinking bouts. This criterion is based on published non-radiographic swallow studies with mice13,14 and VFSS with rats12 showing that 3 – 5 measures per swallow parameter are sufficient for statistical analyses.
- Secondary reviewers: Independently analyze the 3-5 measures per swallow parameter for each mouse that were initially identified and analyzed by the primary reviewer.
- Identify reviewer discrepancies for each mouse. Re-analyze all discrepancies as a reviewer group to reach 100% consensus.
- Average the 3-5 consensus (i.e., undisputed) values for each swallow parameter to obtain the mean value for each mouse for use in statistical analyses. When fewer than 3 measures are obtained for a single swallow parameter for a given mouse, enter a missing value (i.e., not zero) in the statistical data base for the corresponding swallow parameter.
| SWALLOW PARAMETERS | DESCRIPTION |
| Inter-Swallow Interval (ISI) | The number of video frames between two successive, uninterrupted swallows. The start frame for calculating ISI is the “rest frame” that immediately precedes visible transfer of the bolus from the valleculae to the esophagus. The end frame is the “rest frame” of the next swallow. The number of frames between the two successive swallows is then divided by 30 frames per sec (fps) to convert to time (sec). |
| Jaw Excursion Rate (Lick Rate Equivalent) | The tongue is not clearly visible during VFSS to permit quantification of lick rate; however, jaw excursion rate is easily quantifiable. During licking, the jaw must open to permit the tongue to protrude from the mouth. Therefore, the number of jaw open/close (excursion) cycles per second (30 frames) while drinking is equivalent to lick rate. Each jaw excursion cycle begins with the jaw maximally opened (which coincides with tongue protrusion) and ends when the jaw returns to maximally opened position. Subsequent cycles of the jaw closing and re-opening are counted as individual jaw excursion episodes. |
| Jaw Excursion Distance | The distance the jaw opens during jaw excursion cycles, measure in mm between the maxillary and mandibular incisors. |
| Lick-Swallow Ratio | The number of jaw excursion cycles that occur during each ISI (i.e., between two successive, uninterrupted swallows). |
| Swallow Rate | The number of swallows occurring during each 2 sec episode of uninterrupted drinking at the spout. |
| Pharyngeal Transit Time (PTT) | The time it takes the bolus to be swallowed through the pharynx. The start frame is identical to the ISI start frame (i.e., the “rest frame” that immediately precedes visible transfer of the bolus from the valleculae). The end frame is when the tail of the bolus has completely passed the 2nd cervical vertebra (C2), which is the most obvious anatomical landmark in the cervical spine of the mouse. The number of frames between the start and end frames is then divided by 30 fps and converted to milliseconds (msec). |
| Bolus Speed through Pharynx | Pharyngeal bolus speed is measured relative to PTT (described above). Using ImageJ software, the distance (mm) from the valleculae to the C2 vertebra is measured, scaled using a calibration marker. This distance (mm) is then divided by PTT (msec) to determine bolus speed (mm/msec). |
| Esophageal Transit Time (ETT) | The ETT start frame is identical to the PTT end frame (described above). The ETT end frame is when the bolus has completely entered the stomach, which is defined as the disappearance of the bolus from the esophagus. The number of frames between the ETT start and end frames is then divided by 30 fps and converted to msec. |
| Bolus Speed through Esophagus | Esophageal bolus speed is measured relative to ETT (described above). Using ImageJ software, the distance (mm) measured is from the C2 vertebra to the gastroesophageal junction, scaled using calibration marker. This distance (mm) is then divided by ETT (msec) to determine bolus speed (mm/msec). |
| Bolus Speed through Pharynx and Esophagus | This parameter is used when C2 is not a readily visible anatomical landmark; hence, it is not possible to distinguish between the pharyngeal and esophageal stages of swallowing. In such cases, bolus speed through the pharynx and larynx is combined into a single swallow parameter. The start frame is identical to the PTT start frame (i.e., the “rest frame” that immediately precedes visible transfer of the bolus from the valleculae). The end frame is identical to the ETT end frame (i.e., when the bolus has completely entered the stomach). The number of frames between these two events is divided by 30 fps and converted to msec. |
| Bolus Area | Using ImageJ software, bolus area is measured at the vallecular “rest frame” before initiation of pharyngeal swallow, scaled using a calibration marker. |
| Pharyngeal Residue Area | Pharyngeal residue area is measured using ImageJ software, scaled using a calibration marker. |
| Volume of Liquid Consumed | The volume of liquid consumed from a sipper tube bottle is difficult to estimate due to leakage from the spout. However, the volume of liquid consumed from a peg-bowl may be more accurately calculated as follows: 1) determine the density (i.e., ratio of weight to volume) of the calibrated volume of liquid that was administered into the peg-bowl, 2) determine the weight of the peg-bowl containing the residual liquid, 3) enter these values into a weight to volume converter (e.g., http://www.thecalculatorsite.com/conversions/weighttovolume.php. |
Table 2: Swallow Parameters Quantifiable During Murine VFSS.
Representative Results
We have successfully designed a novel and replicable murine-specific VFSS protocol that includes test chambers that permit self-feeding, recipes for flavoring oral contrast agents, and a step-by-step test protocol that permits quantification of swallow physiology. The energy level capability of the fluoroscopy system determined which swallow parameters could be investigated in mice. We initially used high energy fluoroscopes designed for use with people and larger animals (e.g., GE Advantx, GE OEC 9600, and Omega Cardiac Cath CS-25, each at 30 frames per second). However, these systems had insufficient magnification capabilities for testing mice, which resulted in the animal filling only a small portion of the field of view (Figure 9). As a result, image quality was exceptionally poor, rendering it impossible to visualize most structures of the swallowing mechanism. Despite this limitation, we identified 7 objective VFSS swallow parameters that were consistently quantifiable for mice when using a conventional (i.e., high energy) fluoroscope in combination with the new murine VFSS protocol (Table 3). In addition, we identified the vallecular space as the anatomical trigger point for swallowing in healthy adult mice (3-17 months of age), as well as mice with conditions of advanced age (>18 months) and end-stage ALS.

Figure 9: High Energy Fluoroscopy Systems. Left: Representative image of a mouse obtained using high energy (i.e., conventional) fluoroscopy systems. Note that the mouse fills only a small portion of the fluoroscopy field of view, thereby demonstrating the insufficient magnification capability of conventional fluoroscopes for imaging rodents. Right: Same image magnified post-capture using a video editing software program. Black arrow: swallow trigger point (valleculae). White arrow: bolus in the distal esophagus, immediately prior to passing through the GE junction (white asterisk). Please click here to view a larger version of this figure.
| SWALLOW PARAMETERS | High Energy System | Low Energy System |
| Inter-Swallow Interval (ISI) | X | X |
| Jaw Excursion Rate (Lick Rate Equivalent) | X | X |
| Jaw Excursion Distance | X | X |
| Lick-Swallow Ratio | X | X |
| Swallow Rate | X | X |
| Pharyngeal Transit Time (PTT) | X | |
| Bolus Speed through Pharynx | X | |
| Esophageal Transit Time (ETT) | X | |
| Bolus Speed through Esophagus | X | |
| Bolus Speed through Pharynx and Esophagus | X | X |
| Bolus Area | X | |
| Pharyngeal Residue Area | X | |
| Volume of Liquid Consumed | X | X |
Table 3: Swallow Parameters Quantifiable Using High Versus Low Energy Fluoroscopy Systems.
We recently obtained a low energy magnification fluoroscopy system called The LabScope (Glenbrook Technologies, Randolph, NJ) that was specifically designed for our lab for use with mice and other small rodents (Figure 10). However, the markedly greater magnification levels of this system rendered it impossible to view the entire swallowing mechanism of a mouse in a single field of view. Instead, two test positions are required, as shown in Figure 11. Position 1 permits visualization of the entire head and proximal thoracic region. This position is necessary for assessing the oral and pharyngeal stages of swallowing. Position 2 permits visualization from the swallow trigger point (i.e., valleculae) to the gastroesophageal (GE) junction. This position is necessary for assessing the esophageal stage of swallowing. Preliminary work using The LabScope in combination with the new murine VFSS protocol has identified 13 objective swallow parameters that are consistently quantifiable in mice, which is nearly double the number obtained using high energy (i.e., conventional) fluoroscopes (Table 3). This outcome is attributed to the advanced magnification capabilities of The LabScope, which allows for visualization of numerous anatomical structures (Figure 12) that were essentially invisible when using conventional systems: e.g., hyoid bone, trachea, and cervical vertebrae. As a result, we also were able to analyze the videos for evidence of laryngeal penetration and aspiration. Neither penetration nor aspiration was observed for any mouse in this study, regardless of health or disease conditions.

Figure 10: The LabScope. Left: The LabScope performs as a desktop fluoroscope for small animals. Right: Close-up view of The LabScope with labeled components. The scissor lift table is positioning an observation chamber within the fluoroscope field of view. Please click here to view a larger version of this figure.

Figure 11: Low Energy Fluoroscopy System. Images of a mouse obtained using a low energy fluoroscopy system. Note that the high magnification capability prevents visualization of the entire swallow mechanism within the fluoroscopy field of view. Left: Position 1 – permits visualization of the entire head and proximal thoracic region. The swallow trigger point (black arrow) is essentially centered within the field of view. Right: Position 2 – permits visualization from the swallow trigger point (black arrow) to the GE junction (white asterisk). Note the bolus passing through the distal esophagus (white arrow). Please click here to view a larger version of this figure.

Figure 12: Anatomical Structures Visible Using a Low Energy Fluoroscopy System. Even at the lowest magnification setting (left), boney structures of the head and neck of a mouse are readily visible using our low energy fluoroscopy system (i.e., The LabScope). Anatomical structures within the black square are shown (and labeled) at higher magnification to the right. Improved visualization of boney structures permits quantification of several additional swallow parameters that were impossible to analyze using high energy fluoroscopes. Please click here to view a larger version of this figure.
Swallow rate and inter-swallow interval are representative VFSS parameters that can be quantified using either low or high energy fluoroscopy systems in combination with the new murine VFSS protocol. These two swallow parameters were quantified for three groups of mice: SOD1-G93A (SOD1) transgenic mice (i.e., a model of ALS) at disease end-stage between 4-5 months of age, aged C57 mice (18-24 months of age), and a control group of healthy young (4-8 months of age) C57 mice and nontransgenic littermates from the SOD1 colony. All data pertain to spout drinking only, using either a low or high energy fluoroscopy system. No significant differences were found between young C57 mice and young nontransgenic (control) mice from the SOD1 colony relative to these two swallow parameters; therefore, data were combined into a general “control” group of young healthy mice for comparison with aged C57 mice and end-stage SOD1 mice. Swallow rate (i.e., the number of swallows during 2 consecutive seconds of uninterrupted drinking) was significantly slower for SOD1 mice compared to aged C57 mice and controls. Inter-swallow interval (i.e., the time between two successive swallows) was not significantly different between groups. These findings support the notion that dysphagia profiles are likely to be distinctly different for each disease condition (Figure 13).

Figure 13: Preliminary Findings. This figure shows representative preliminary findings for two VFSS swallow parameters quantified using the murine VFSS protocol: swallow rate (left) and inter-swallow interval (right). Swallow rate was significantly slower for SOD1 mice compared to aged C57 mice and controls. No significant group differences were identified for inter-swallow interval. Lines at the top of the bars indicate statistically significant differences (p < 0.05) between groups, identified using Bonferroni pairwise comparisons. Error bars represent ± 1 SEM. Please click here to view a larger version of this figure.
Discussion
Hundreds of murine (mouse and rat) models are commercially available to study human diseases. However, only three murine disease models have been specifically investigated relative to dysphagia: a mouse model of ALS13,14 and rat models of Parkinson’s disease12,15-17 and stroke18. Each of these preliminary studies utilized different methodologies to assess dysphagia, rendering it impossible to derive meaningful comparisons between species and diseases. This major limitation may be overcome in future studies by utilizing the newly developed murine VFSS protocol that permits objective quantification of numerous swallow parameters in self-feeding animals.
Successful VFSS outcomes are dependent upon three critical components: 1) test chambers that permit self-feeding while standing unrestrained in a confined space, 2) recipes that mask the aversive taste/odor of commercially-available oral contrast agents, and 3) a step-by-step test protocol that permits quantification of swallow physiology. The combined effect produces a comfortable, low stress, self-feeding examination environment that evokes typical feeding and swallowing behaviors. Elimination of one or more of these components will have a detrimental impact on the study results. Examples of negative outcomes include inability to maintain animals in the fluoroscopy field of view, undesirable behaviors that distract from drinking, aversion to the oral contrast agent, and inability to quantify swallow parameters due to insufficient drinking episodes.
A major challenge in obtaining optimal VFSS outcomes was designing a suitable test chamber. Numerous revisions of our prototype design culminated in an observation chamber that sufficiently maintains mice in the field of view and prevents behaviors that distract from drinking. The chambers were made using milling machines to obtain uniform dimensions of the tubes and end-caps, thereby ensuring interchangeability of components for several observation chambers of the same diameter. The inner dimensions (diameter and length) were matched to be slightly larger than an adult mouse’s body size, which resulted in a narrow test chamber that sufficiently permits walking in a straight line and turning around. The narrow design, in combination with strategic positioning of the spout and peg-bowl at only the end, maintains the head and body of mice aligned along the length of the chamber while drinking. Once engaged in drinking, mice remain remarkably self-stabilized at the spout or bowl for several seconds at a time, resulting in minimal movement artifact to interfere with testing. Thus, it is possible to obtain undistorted, close-up observation/video recording and videofluoroscopic imaging of mice while drinking in the lateral and dorsal-ventral planes.
Mice (and other small rodents) are naturally inclined to seek shelter in small spaces. As a result, they freely enter the test chamber (with one end already closed by an end-cap) when it is placed in the home cage, thereby eliminating stress/anxiety caused by handling (i.e., manually picking up the animal to place it in the chamber). Once the mouse enters the chamber, the other end is closed by attaching a 2nd end-cap. This design prevents escape while creating a low anxiety test chamber for mice to freely explore.
The square shape of the chamber provides built-in motion stability that permits it to be used in a free-standing fashion, thus eliminating the need for testing within a standard rodent cage. The entire apparatus is lightweight, portable, stackable for storage purposes, sturdy, easy to clean, and can be autoclaved. While the chambers were initially designed for use with fluoroscopy, they also are compatible with spot-film radiography, neuroimaging (e.g., MRI, PET, CT), and visual observation/video recording of various behaviors.
A second major challenge to overcome was masking the aversive taste/odor of oral contrast agents (i.e., barium sulfate and iohexol). Given that taste sensitivity varies widely among mouse strains19-21 and perhaps with age22,23, it was necessary to identify a single test solution that was palatable to all mice, regardless of strain and age. This outcome is essential to permit direct comparisons of swallow function/dysfunction across strains and ages, while eliminating confounding results due to differences in rheological (e.g., viscosity, density, etc.) and chemical properties of the test solutions. To this end, we developed a simple, rapid palatability screening approach to identify the preferred flavor enhancer to mask the aversive taste/odor of oral contrast agents during murine VFSS. Methods were modeled after the brief exposure test, which requires a lickometer (i.e., lick sensor) to record lick rates during the first 2 min after a water regulation period (i.e., withholding water overnight) to induce thirst24,25. A lickometer was not available for this study; therefore, preference was assessed by behavioral observations, as well as standard video recording methods for lick rate that have been previously validated in our lab13,14. Using this palatability screening approach, chocolate was identified as the preferred flavor enhancer by C57 and C57/SJL strains. Specifically, 100% of the mice in each cage readily drank chocolate-flavored solutions within 30 sec of exposure, with multiple mice simultaneously drinking at the spout. However, the addition of barium resulted in only brief drinking bouts by most mice, regardless of barium or chocolate concentration.
An alternative to barium is iohexol, an iodine-based contrast agent that has only recently been recognized as a suitable alternative to barium sulfate for human VFSS10; thus, it has not yet been standardized for this purpose. Several different concentrations of chocolate-flavored iohexol were offered to mice. Recipes containing up to a 50% solution of stock iohexol (350 mg iodine per ml) were readily drank by most mice after an overnight water regulation period. Higher concentrations resulted in avoidance behaviors. A 50% iohexol (350 mg iodine per ml) solution produced sufficient radiodensity while being swallowed by mice, whereas lower concentrations were markedly less visible and hindered quantification of swallow physiology. Therefore, the optimal test solution for VFSS with mice was identified as a 50% iohexol solution with chocolate flavor added. Repeat palatability testing did not result in avoidance behaviors or adverse events.
A third challenge to overcome was preventing mice from turning/tilting their head while drinking, which obscures visualization of the swallowing mechanism during VFSS. Drinking from a peg-bowl positioned just above the floor at one end of the chamber resolved this problem. There are several additional advantages of using a peg-bowl instead of a sipper tube bottle. For example, a calibrated volume of liquid can be pipetted into the peg-bowl through a ventilation hole in the end-cap of the observation tube. This approach permits quantification of the minute volume of test solution consumed during the brief VFSS test duration. Moreover, the increased surface area of the test solution in the peg-bowl, compared to a small sipper tube opening, may provide increased olfactory stimulation to further motivate drinking. Peg-bowls may be better suited for studying young or smaller strain mice, as the bowl height is a standardized distance from the floor. In contrast, sipper tube lengths must be adjusted to accommodate different sized mice, which adds another potentially confounding variable to consider. Also, mouse models of neurological diseases may have difficulty reaching a sipper tube bottle due to motor impairment of the limbs, whereas they can easily reach a peg bowl. Mice with tongue and/or jaw dysfunction may be unable to sufficiently press the ball in the spout to access the liquid; using peg-bowls can eliminate this confound. For these reasons, the use of peg-bowls over sipper tube bottles is the preferred method of murine VFSS testing. However, the observation chambers were designed to accommodate spout drinking as needed. An important caveat to consider is that lick rates are known to differ between spout and bowl drinking13,26. Therefore, the choice of either spout or peg-bowl for VFSS must be consistent within and between experiments.
A fourth challenge was to identify quantifiable swallow parameters for mice that are comparable to the VFSS parameters commonly used in human research studies and clinical practice. Our preliminary findings showed the type of fluoroscopy system determines which swallow parameters can be investigated in mice. Most research centers and medical settings have high energy (75-95 kV, 1-5 mA) fluoroscopes designed for use with people and larger animals, which result in exceptionally poor image quality when testing mice and other small animals. As an example, a recent study using a high energy fluoroscope with rats was able to identify only 4 quantifiable swallow parameters12, and we were able to identify only 7 swallow parameters for mice in this present study. To overcome this major limitation, we recently obtained a low energy fluoroscopy system called The LabScope (Glenbrook Technologies). The system is a miniature fluoroscope that generates a continuous cone-beam of X-rays with photon energies between 15 and 40 kV and a peak tube current of 0.2 mA (8 W maximum power). The lower energy levels of this system are better attenuated by the thin bone and soft tissue of mice and thus provide higher contrast resolution than conventional (i.e., high energy) fluoroscopes. The X-ray beam of The LabScope is directed at a 5 cm diameter image intensifier, which is markedly smaller than the 15-57 cm diameter image intensifier of conventional fluoroscopes. The minimum source-to-intensifier distance (SID) of The LabScope is ~6 cm (in contrast to ~30 cm for conventional fluoroscopes), which provides increased magnification capabilities. In addition, The LabScope uses patented technology that digitally magnifies the image up to 40 times in real time, without altering the SID. The result is in essence an X-ray microscope that can zoom in and out in real time to view small regions of interest, such as the swallowing mechanism of a mouse.
A major advantage of this low-energy fluoroscopy system is improved radiation safety. In addition to animals receiving lower radiation doses with The LabScope, researchers using the system are exposed to significantly less radiation scatter. The radiation exposure directly in front of the unit at the control panel is 10.3 mR/hr. At a distance 1 m in front of the unit, exposure drops to 580 µR/hr. Most other locations in the room have very low exposure below 10 µR/hr. Despite this improvement, we have taken extra measures to improve radiation safety. For example, leaded acrylic shielding has been added around The LabScope to block scattered X-ray photons, which enables researchers to conduct murine VFSS testing without wearing personal shielding (e.g., lead aprons, thyroid shields, and glasses). In addition, the clear acrylic permits visualization of the mouse from a distance. Further radiation safety is provided by a motorized scissor lift table, which is controlled remotely by the investigator. From a distance up to 3 m from the fluoroscope, researchers can use the remote-controlled device to adjust the vertical and horizontal position of the observation chamber within the X-ray beam. As a result, the anatomical regions of interest can be maintained within the fluoroscopy field of view while the mouse freely moves within the observation chamber. Although the scissor lift was designed for use with The LabScope, it also is compatible for use with conventional fluoroscopes to improve radiation safety for researchers. A final step to improve radiation safety during murine VFSS entails the use of a syringe delivery system for liquids. This system includes a 3-4 foot (or longer, if needed) length of PE tubing, which permits fast and efficient delivery of liquids into the peg-bowl from a distance. This syringe delivery system for liquids, in combination with the observation chambers, also can be used with conventional fluoroscopes.
Preliminary work using The LabScope, in combination with the new murine VFSS protocol, demonstrates a major advantage of over conventional systems: the number of swallow parameters that can be reliably quantified is nearly doubled. However, soft tissue structures of the swallowing mechanism (e.g., tongue, velum, posterior pharyngeal wall, and epiglottis) of mice are not readily visible when using low or high energy fluoroscopy systems. Therefore, we focused on quantifying bolus flow measures rather than the biomechanics of swallowing. We were predominantly interested in parameters that could be quantified based on units of time, area, distance, volume, etc., rather than using Likert-type scale measures. Numerous bolus flow parameters meeting this requirement have been described in the human VFSS literature, such as oral transit time27-29, pharyngeal transit time27-33, and esophageal transit time34-36, to name but a few. Bolus transport through the oral cavity was not readily visible in mice, likely due to the small bolus size during spontaneous drinking. However, we were able to reliably quantify pharyngeal and esophageal transit times, as well as several other measures pertaining to bolus flow and clearance. Identification of additional translational swallow parameters is expected as we optimize the capabilities of The LabScope.
Results of this study showed that mice take several rhythmic licks per swallow during spontaneous drinking, with each small liquid bolus sequentially filling the vallecular space before triggering the pharyngeal swallow. This behavior, which is typical for mammals that use licking as the primary means of ingesting liquid37-40, resembles the rhythmic suck-swallow pattern of human infant swallowing and all infant mammals in general. Infant swallowing physiology is characterized by several rhythmic sucks followed by a reflexive pharyngeal swallow, commonly described as the suck-swallow cycle37,41-43. Thus, the rhythmic tongue and jaw movements involved in the ingestive licking behaviors of mice may be more comparable to ingestive sucking behaviors of human infants rather than cup drinking by children and adults. We have therefore been quantifying the lick rate and lick-swallow ratio of mice for future comparisons with the suck rate and suck-swallow ratio of human infants. Perhaps murine VFSS research will provide insight into developmental swallowing disorders.
As with any new research method, areas for improvement have been identified. For example, the murine VFSS protocol was developed using only C57 and C57/SJL mouse strains; it has not yet been tested with rats. The observation chambers will need to be scaled up in size (diameter and length) to accommodate the larger body size of rats. Also, it is unknown if chocolate-flavored iohexol is suitable as a universal murine VFSS test solution. Therefore, larger scale testing with multiple strains of mice and rats is warranted for this purpose. Also, the use of barium as a contrast agent for murine VFSS should not be ruled out. Mice clearly preferred the iohexol recipes over barium; however more rigorous and systematic attempts at masking the aversive taste/odor of barium may provide palatable alternatives to iohexol. Future studies comparing the effects of iohexol and barium sulfate (as well as other potential oral contrast agents) on taste preference and swallow physiology in mice and rats would undoubtedly provide important information that is directly relevant and translational to human VFSS.
VFSS with humans includes several consistencies of foods and liquid, and dysphagia is most apparent when swallowing thin liquids and dry, solid foods44,45. The murine VFSS protocol is therefore being expanded to include additional consistencies that may facilitate detection and quantification of dysphagia in disease models. It also will be necessary to conduct viscosity testing of the liquid recipes for murine VFSS in order to adjust the viscosities to match those used during human VFSS. Addressing these limitations will facilitate identification of translational VFSS biomarkers of dysphagia that can be directly compared between mice, rats, and humans.
The utility of murine VFSS may be significantly improved by implanting radiopaque markers into soft tissue structures of the swallowing mechanism that are otherwise not visible, thereby permitting investigations of the biomechanics of swallowing. This approach has been successfully used for many years to study the biomechanics of swallowing in infant pigs, using an assortment of metal clips and wires37,42. We expect the use of similar, but smaller, markers in mice would permit quantification of several additional swallow parameters for comparison with larger mammals, including humans. We are currently developing methodology for implanting radiopaque markers into the tongue, soft palate, pharynx, larynx, and proximal esophagus of mice to test this hypothesis.
The video recording frame rate of The LabScope and conventional fluoroscopes is limited to 30 frames per sec (fps). However, our preliminary results showed that the entire pharyngeal stage of swallowing for healthy mice occurs in less than 66 msec (i.e., 2 frames), which is approximately 10 times faster than humans. Thus, the pharyngeal phase of swallowing in mice occurs so quickly that the details are not appreciable with a 30 fps camera. A higher frame rate (likely >100 fps) will be necessary to sufficiently visualize and quantify the extremely rapid and complex movements of the pharyngeal stage of swallowing in mice and other rodents. In conjunction with a higher frame rate, incorporating biplanar technology for 3D fluoroscopic imaging would certainly expand the utility murine VFSS. Therefore, future design considerations should include a higher frame rate camera and biplanar imaging capabilities.
Lastly, low-dose radiation has been shown to cause sterility in female C57 mice, resulting in altered levels of ovarian-stimulated hormones that may confound life-span studies46. Outcomes pertaining specifically to the effects of repeated low-dose radiation exposure associated with VFSS testing have not yet been investigated in mice, other animals, or humans. However, ovarian dysfunction (not related to radiation exposure) in human females has been linked to gastrointestinal motility disorders, and specifically to dysphagia in some cases47, which provides yet another caveat to consider when designing future VFSS studies that include females (animals and humans). Exclusion of females should be avoided, as significant gender differences in swallow function have been reported for people48,49 and would be important to detect and characterize in animal disease models as well. Therefore, outcomes from longitudinal VFSS studies in mice and rats of both sexes have tremendous translational potential for humans relative to dysphagia, as well as to the risks of low-dose radiation exposure associated with repeat VFSS testing.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We graciously thank additional members of the Lever Lab who contributed to data collection (Andries Ferreira, Danarae Aleman, Alexis Mok, Kaitlin Flynn, Elizabeth Bearce, and Matan Kadosh) and manuscript review (Andries Ferreira, Rebecca Schneider, and Kate Robbins). We also acknowledge Roderic Schlotzhauer and Edwin Honse from the MU Physics Machine Shop for their design input and fabrication of the rodent observation tubes used in this study. We are especially appreciative of Malea Jan Kunkel (Radiology Supervisor in the Veterinary Medicine and Surgery Department at the University of Missouri – College of Veterinary Medicine) and Jan Ivey (Manager of the Research Animal Cath Lab at the University of Missouri – School of Medicine) for demonstrating constant patience and motivation while operating the high energy fluoroscopes as we developed the murine VFSS protocol. Funding sources for this study included NIH/NIDCD (TE Lever), NIH/NINDS (GK Pavlath), Otolaryngology – Head and Neck Surgery start-up funds (TE Lever), MU PRIME Fund (TE Lever), Mizzou Advantage (TE Lever), and the MU Center on Aging (TE Lever).
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Polycarbonate tubing for observation chambers | McMaster-Carr | 3161T41 | Body of observation tubes, 2"X2" diameter, 0.080" thick wall |
| Polycarbonate sheet for observation chambers | McMaster-Carr | 9115K71 | End-caps for observation tubes, 2"x12"x3/4" |
| Polycarbonate sheet for observation chambers | McMaster-Carr | 8574K281 | Peg-bowls for observation tubes |
| Silicone O-rings for end-caps of observation chambers | McMaster-Carr | 9396K108 | S1138 AS568-029, pack of 25 http://www.mcmaster.com/#o-rings/=t0wt5r |
| Silicone stoppers for observation chambers | McMaster-Carr | 2903K22 | Package of 10 stoppers to plug the oval opening in the top of the observation chamber when using a peg-bowl http://www.mcmaster.com/#catalog/120/3803/=t0y5at |
| Centrifuge tubes for sipper tube bottles | Evergreen Scientific | 222-3530-G80 | 30 ml freestanding centrifuge tubes, with caps, sterile https://www.evergreensci.com/labware-catalog/tubes-and-vials/30-and-50-ml-centrifuge-tubes/ |
| Silcone stoppers for sipper tube bottles | Saint-Gobain Performance Plastics | DX263031-10 | Number 31D, size: 26 mm bottom, 32 mm top, 30 mm high; 10 pack; http://www.labpure.com/en/Products.asp?ID=179&PageBrand=STOPPERS |
| Stopper borers for sipper tube bottles | Thomas Scientific | 3276G40 | Cork Borer Set that ranges from 3/16-15/16 inch http://www.thomassci.com/Supplies/Corks/_/CORK-BORER-SET-316-1516-IN?q=Humboldt |
| Drinking tubes for sipper tube bottles | Ancare | TD-100 | 2 1/2” long drinking tubes with 5/16” opening, straight ball-spout http://www.ancare.com/products/watering-equipment/open-drinking-tubes/straight-tubes-ball-point |
| Iohexol for making oral contrast agent solution | GE Healthcare | 350 mg iodine per ml http://www3.gehealthcare.com/en/products/categories/contrast_media/omnipaque |
|
| Chocolate syrup for flavoring oral contrast agent | Herseys | ||
| 10 ml syringe for syringe delivery system | Becton, Dickinson and Company | 309604 | Luer lock tip syringe without needle, 100 per box http://www.bd.com/hypodermic/products/syringeswithoutneedles.asp |
| Catheter tubing for syringe delivery system | Becton, Dickinson and Company | 427451 | Polyethylene Tubing (Non-Sterile) (PE 240) 100' http://www.bd.com/ds/productCenter/427451.asp |
| Needle for syringe delivery system | Becton, Dickinson and Company | 427560 | 15-gauge needle, fits into PE 240 catheter tubing http://www.bd.com/ds/productCenter/427560.asp |
| Delrin acetal resin rod for syringe delivery system | McMaster-Carr | 8576K15 | 1/2 inch diameter, black http://www.mcmaster.com/#catalog/120/3609/=t0wvaf |
| Acrylic sheeting for scissor lift | Ponoko | Laser cut http://www.ponoko.com |
|
| 3D printed ABS frame | Engineering Rapid Prototyping Facility, University of Missouri | ||
| Brass rods for scissor lift | Amazon | TTRB-03-12-03 | made into axles http://www.amazon.com/Brass-Seamless-Round-Tubing-Length/dp/B000FN898M |
| Drawer slide for scissor lift | Richelieu | 10292G116 | Attaches to base of scissor lift http://www.lowes.com/pd_380986-93052-T35072G16_0__?productId=50041754 |
| 28BYJ-48 stepper motor for scissor lift | 2 each | ||
| ULN2003 Darlington transistor array for scissor lift | Toshiba | ULN2003APG | Used as stepper drivers (2 each) |
| ATTINY85 microcontroller for scissor lift | Atmel | ATTINY85-20PU | 2 each http://www.taydaelectronics.com/attiny85-attiny85-20pu-8-bit-20mhz-microcontroller-ic.html |
| Nylon spur gear | McMaster-Carr | 57655K34 | 2 each http://www.mcmaster.com/#57655k34/=t0yaqz |
| Nylon spur gear rack | McMaster-Carr | 57655K62 | 2 each http://www.mcmaster.com/#57655k62/=t0ybh9 |
| 4-40 nylon machine screws | McMaster-Carr | 95133A315 | Lift assembly http://www.mcmaster.com/#95133a315/=t0yd8q |
| 4-40 nylon hex nuts | McMaster-Carr | 94812A200 | Lift assembly http://www.mcmaster.com/#94812a200/=t0ye29 |
| Buna-N O-Ring AS568A Dash No. 104 | McMaster-Carr | 9452K318 | Lift assembly http://www.mcmaster.com/#9452k318/=t0yem7 |
Referenzen
- Shigemitsu, H., Afshar, K. Aspiration pneumonias: under-diagnosed and under-treated. Curr Opin Pulm Med. 13 (2), 192-198 (2007).
- Gresham, S. L. Clinical assessment and management of swallowing difficulties after stroke. Med J Aust. 153 (7), 397-399 (1990).
- Marik, P. E., Kaplan, D. Aspiration pneumonia and dysphagia in the elderly. Chest. 124 (1), 328-336 (2003).
- Marik, P. E. Pulmonary aspiration syndromes. Curr Opin Pulm Med. 17 (3), 148-154 (2011).
- Logemann, J. A., Larsen, K. Oropharyngeal dysphagia: pathophysiology and diagnosis for the anniversary issue of. Diseases of the Esophagus. Dis Esophagus. 25 (4), 299-304 (2012).
- Logemann, J. A. Swallowing disorders. Best practice & research Clinical gastroenterology. 21 (4), 563-573 (2007).
- Martin-Harris, B., Jones, B. The Videofluorographic Swallowing Study. Physical Medicine and Rehabilitation. Clinics of North America. 19 (4), 769-785 (2008).
- Dietsch, A. M., Solomon, N. P., Steele, C. M., Pelletier, C. A. The effect of barium on perceptions of taste intensity and palatability. Dysphagia. 29 (1), 96-108 (2014).
- Stokely, S. L., Molfenter, S. M., Steele, C. M. Effects of barium concentration on oropharyngeal swallow timing measures. Dysphagia. 29 (1), 78-82 (2014).
- Harris, J. A., et al. The Use of Low-Osmolar Water-Soluble Contrast in Videofluoroscopic Swallowing Exams. Dysphagia. , (2013).
- Hillel, A., Miller, R. Bulbar Amyotrophic Lateral Sclerosis: Patterns of Progression and Clinical Management. Head & Neck. 11, 51-59 (1989).
- Russell, J. A., Ciucci, M. R., Hammer, M. J., Connor, N. P. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 28 (1), 95-104 (2013).
- Lever, T. E., et al. An animal model of oral dysphagia in amyotrophic lateral sclerosis. Dysphagia. 24 (2), 180-195 (2009).
- Lever, T. E., et al. A mouse model of pharyngeal dysphagia in amyotrophic lateral sclerosis. Dysphagia. 25 (2), 112-126 (2010).
- Ciucci, M. R., et al. Tongue force and timing deficits in a rat model of Parkinson disease. Behavioural Brain Research. 222 (2), 315-320 (2011).
- Ciucci, M. R., Schaser, A. J., Russell, J. A. Exercise-induced rescue of tongue function without striatal dopamine sparing in a rat neurotoxin model of Parkinson disease. Behavioural Brain Research. 252, 239-245 (2013).
- Plowman, E. K., Kleim, J. A. Behavioral and neurophysiological correlates of striatal dopamine depletion: A rodent model of Parkinson’s disease. Journal of Communication Disorders. 44 (5), 549-556 (2011).
- Sugiyama, N., et al. A novel animal model of dysphagia following stroke. Dysphagia. 29 (1), 61-67 (2014).
- Bachmanov, A. A., Reed, D. R., Li, X., Beauchamp, G. K. Genetics of sweet taste preferences. Pure Appl Chem. 74 (7), 1135-1140 (2002).
- Ishiwatari, Y., Bachmanov, A. A. NaCl taste thresholds in 13 inbred mouse strains. Chem Senses. 37 (6), 497-508 (2012).
- Pinhas, A., et al. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav. 105 (2), 451-459 (2012).
- Midkiff, E. E., Bernstein, I. L. The influence of age and experience on salt preference of the rat. Dev Psychobiol. 16 (5), 385-394 (1983).
- Niimi, K., Takahashi, E. Differences in saccharin preference and genetic alterations of the Tas1r3 gene among senescence-accelerated mouse strains and their parental AKR/J strain. Physiol Behav. , (2014).
- Weijnen, J. A. Licking behavior in the rat: measurement and situational control of licking frequency. Neurosci Biobehav Rev. 22 (6), 751-760 (1998).
- Weijnen, J. A. Lick sensors as tools in behavioral and neuroscience research. Physiol Behav. 46 (6), 923-928 (1989).
- Kobayashi, M., et al. Electrophysiological analysis of rhythmic jaw movements in the freely moving mouse. Physiol Behav. 75 (3), 377-385 (2002).
- Dantas, R., et al. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. 258, G675-681 (1990).
- Johnsson, F., Shaw, D., Gabb, M., Dent, J., Cook, I. Influence of gravity and body position on normal oropharyngeal swallowing. American Journal of Physiology. 35 (5), G653-G658 (1995).
- Han, T. T., Paik, N. -. J., Park, J. W. Quantifying swallowing function after stroke: A functional dysphagia scale based on videofluoroscopic studies. Archives of Physical Medicine and Rehabilitation. 82 (5), 677-682 (2001).
- Molfenter, S. M., Steele, C. M. Kinematic and temporal factors associated with penetration-aspiration in swallowing liquids. Dysphagia. 29 (2), 269-276 (2014).
- Kendall, K. A., McKenzie, S., Leonard, R. J., Goncalves, M. I., Walker, A. Timing of events in normal swallowing: A videofluoroscopic study. Dysphagia. 15, 74-83 (2000).
- Choi, K. H., Ryu, J. S., Kim, M. Y., Kang, J. Y., Yoo, S. D. Kinematic analysis of dysphagia: Significant parameters of aspiration related to bolus viscosity. Dysphagia. 26, 392-398 (2011).
- Molfenter, S. M., Steele, C. M. Variation in temporal measures of swallowing: Sex and volume effects. Dysphagia. 28, 226-233 (2013).
- Alves, L. M. T., Secaf, M., Dantas, R. Effect of a bitter bolus on oral, pharyngeal, and esophageal transit of healthy subjects. Arquivos de gastroenterologia. 50 (1), 31-34 (2013).
- Dalmazo, J., Aprile, L. R. O., Dantas, R. O. Esophageal contractions, bolus transit and perception of transit after swallows of liquid and solid boluses in normal subjects. Arquivos de gastroenterologia. 49 (4), 250-254 (2012).
- Kahrilas, P. J., Dodds, W. J., Hogan, W. J. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 94 (1), 73-80 (1988).
- German, R. Z., Crompton, A. W., Levitch, L. C., Thexton, A. J. The mechanism of suckling in two species of infant mammal: Miniature pigs and long-tailed macaques. Journal of Experimental Zoology. 261 (3), 322-330 (1992).
- Herring, S. W., Scapino, R. P. Physiology of feeding in miniature pigs. Journal of Morphology. 141 (4), 427-460 (1973).
- Gordon, K. R., Herring, S. W. Activity patterns within the genioglossus during suckling in domestic dogs and pigs: Interspecific and intraspecific. Brain, Behavior, and Evolution. 30 (5-6), (1987).
- Hiiemae, K. M., Palmer, J. B. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia. 14 (1), 31-42 (1999).
- Thexton, A. J., Crompton, A. W., German, R. Z. EMG activity in the hyoid muscles during pig suckling. Journal of Applied Physiology. 112, 1512-1519 (2012).
- Thexton, A. J., Crompton, A. W., German, R. Z. Transition from suckling to drinking at weaning: A kinematic and electromyographic study in miniature pigs. Journal of Experimental Zoology. 280 (5), 327-343 (1998).
- Goldfield, E. C., Richardson, M. J., Lee, K. G., Margetts, S. Coordination of sucking, swallowing, and breathing and oxygen saturation during early infant breast-feeding and bottle-feeding. Pediatric Research. 60 (4), 450-455 (2006).
- Ottaviano, F. G., Linhares Filho, T. A., Andrade, H. M., Alves, P. C., Rocha, M. S. Fiberoptic endoscopy evaluation of swallowing in patients with amyotrophic lateral sclerosis. Braz J Otorhinolaryngol. 79 (3), 349-353 (2013).
- Inamoto, Y., et al. The effect of bolus viscosity on laryngeal closure in swallowing: kinematic analysis using 320-row area detector CT. Dysphagia. 28 (1), 33-42 (2013).
- Spalding, J. F., Thomas, R. G., Tietjen, G. L., Rein, S. e. r. e. n. e. . Los Alamos National Laboratory. , (1982).
- Palomba, S., Di Cello, A., Riccio, E., Manguso, F., La Sala, G. B. Ovarian function and gastrointestinal motor activity. Minerva Endocrinol. 36 (4), 295-310 (2011).
- Alves, L. M., Cassiani Rde, ., Santos, A., M, C., Dantas, R. O. Gender effect on the clinical measurement of swallowing. Arq Gastroenterol. 44 (3), 227-229 (2007).
- Logemann, J. A., Pauloski, B. R., Rademaker, A. W., Kahrilas, P. J. Oropharyngeal swallow in younger and older women: videofluoroscopic analysis. J Speech Lang Hear Res. 45 (3), 434-445 (2002).

