Accurate and Phenol Free DNA Sexing of Day 30 Porcine Embryos by PCR
Summary
This protocol describes an accurate, inexpensive, rapid and non-toxic method to determine the sex of Day 30 porcine embryo using PCR method after grinding an embryo into powder without phenol chloroform extraction and DNA column purification.
Abstract
Research into prenatal programming in the pig has shown that the sex of the developing embryo or fetus can influence the developmental outcome. Therefore, the ability to determine an embryo's sex is necessary in many experiments particularly regarding early development. The present protocol demonstrates an inexpensive, rapid and non-toxic preparation of pig genomic DNA for use with PCR. Day 30 embryos must be humanely collected according to the guidelines established by Institutional Animal Policy and Welfare Committees for the present protocol. The preparation of the whole embryo for this PCR based sexing technique simply involves grinding the frozen embryo to a fine powder using a pre-chilled mortar and pestle. PCR-quality DNA is released from a small amount of embryo powder by applying a hot incubation in an alkaline lysis reagent. Next, the DNA solution is mixed with neutralization buffer and used directly for PCR. Two primer pairs are generated to detect specific sex determining region of the Y- chromosome (SRY) and ZFX region of the X- chromosome with high accuracy and specificity. The same protocol can be applied to other elongated embryos (Day 10 to Day 14) earlier than Day 30. Also, this protocol can be carried with 96-welled plates when screening a large number of embryos, making it feasible for automation and high-throughput sex typing.
Introduction
The domestic pig has become a fundamental research subject in development, genetics and nutrition in both human and livestock sectors. The potential of pigs as biomedical models for human research can be attributed to their physiological similarities. In livestock, the manipulation of sex ratio can enhance the effectiveness of selection and genetic improvement programs1. Sexing individual embryos is a fundamental tool used in many experimental investigations including but not limited to genotype, epigenetics and X inactivation of sexual dimorphism during early embryo development2.
Studies in mice suggest that maternal diet and other factors may result in gender imbalance3. In pigs, causes of sex ratio imbalance include paternal breed4, uterine capacity5, and the sow's metabolic condition6. Since the differences observed in embryos and litters can be influenced by sexual dimorphism, researchers should be aware of embryo sex and sex ratios before drawing conclusions regarding their research. The development of efficient tools and protocols for sexing pig embryos at Day 30 of development will be discussed here.
Various methods of sex typing have been developed for genetic studies in model organisms and livestock. Particularly in livestock, identifying male and female early embryos is a very common practice to enhance genetic selection for breeding programs. Early embryos karyotyping in pig using Giemsa7 or the intense fluorescence8,9 techniques have been used for sex typing. However, these methods are time consuming and not suitable for screening large numbers of embryos quickly and accurately.
The most effective sex typing method is DNA amplification using a heat stable DNA polymerase and a pair of primers. DNA sexing by PCR method is more specific, rapid and sensitive, only requiring a minute amount of cellular materials. The first PCR-based embryo sexing was performed on humans10, and later in mice11, cattle12, buffalo13 and sheep14 pre-implantation embryos. In the pig, the earliest DNA sex typing method was established for pre-implantation embryos via a single pair of Y-chromosome specific DNA primers15. However, the most common PCR primers for sex determination were selected from the Y-chromosome of male specific SRY gene16 and the non-sex discriminative region of a zinc-finger gene located at both X and Y chromosome17. Subsequently, these primers have been applied to determine the sex of Day 30 embryos in this study with improved specificity of the primers to detect only the X- chromosome of a zinc-finger gene.
Genomic DNA from porcine pre-implantation embryos can be extracted by exposing an intact blastocyst to buffer with proteinase K16 or by taking a biopsy of a few cells from the individual early cleavage embryos15 and using them for direct PCR. However, release of DNA from porcine blood, hair, tissues or a large conceptus over a few centimeters in size is not effectively done using the proteinase K method. DNA extraction methods for these materials have been established using either time consuming phenol/chloroform protocols6 or expensive column based kits18. In order to avoid the use of potentially toxic chemicals, there is a trend to develop inexpensive, easy and phenol-free DNA extraction methods. This type of protocol for the isolation of PCR-quality genomic DNA from mouse19 and zebrafish20 tissues has been established using hot sodium hydroxide and Tris (HotSHOT). This study provides a protocol to obtain DNA with modified HotSHOT and redesigned duplex primer pairs for PCR sex typing directly from cell lysates of Day 30 porcine embryos with high accuracy.
Protocol
In accordance with the Canadian Council on Animal Care guidelines and with approval of the Faculty Animal Policy and Welfare Committee – Livestock of the University of Alberta, pregnant sows were euthanized by trained staffs at approximately Day 30 of pregnancy and embryos were collected. Use examination gloves at all times during the procedures.
1. Sample Collection and Sample Storage
- Euthanize sow using captive bolt followed by exsanguination. Wear examination gloves to collect the reproductive tracts and embryos from each euthanized sow.
Note: Rapid collection of samples and transfer embryos to dry ice or liquid nitrogen will result in higher quality tissues for other molecular works such as microarray and qPCR. - Dissect the uterus and gently separate all the embryos within their extraembryonic placental membranes from the underlying uterine wall21. Make sure there is no maternal tissue contamination.
- Record length and weight of all viable embryos prior to freezing.
- Collect each individual embryo and immediately wrap it up in an aluminum foil before snap freezing with liquid nitrogen.
- Transfer the frozen embryos from liquid nitrogen directly to a -80 °C freezer.
2. Grinding Embryos
- Label all sample tubes (15 ml) with the sample ID needed for the number of samples to be analyzed.
- Fill thermal container with dry ice to half full and insert the pre-labelled sample tube in the dry ice.
- Wear winter gloves with another pair of examination gloves on top for transferring the pre-chilled mortars and pestles from -80 °C freezer to dry ice container.
- Place the frozen embryo inside the mortar on the dry ice.
- Pour adequate liquid nitrogen to cover the embryo and grind it with the pestle into a fine powder.
- Transfer the embryo powder to a pre-labeled sample tube with a microspatula (Figure 1) and place the tube in a -80 °C freezer.
- Use a clean pre-chilled mortar and pestle for each embryo and change the examination gloves after grinding each embryo to avoid cross contamination between samples.
3. Genomic DNA Preparation using Modified Sodium Hydroxide Method
- Label all the microcentrifuge tubes (1.5 ml) needed for the number of samples to be analyzed.
- Transfer the sample tubes containing embryo powder from the -80 °C freezer to a container with dry ice prior to starting.
- Pipette 180 µl of 50 mM NaOH (sodium hydroxide) into each pre-labelled microcentrifuge tube.
- Turn on an incubator and pre-heat to 95 °C.
- Use a toothpick to transfer the correct amount of embryo powder (about 5-10 mg) (Figure 2) from the sample tube into a pre-labelled microcentrifuge tube containing the 50 mM NaOH solution.
- Pull up the same toothpick slowly to visualize the DNA lysate as a sticky, gooey, white transparent-like substance (Figure 3).
- Transfer the microcentrifuge tubes with DNA lysate to the pre-heated incubator at 95 °C for five minutes. Immediately transfer the tube to a styrofoam box filled with ice.
- Add 20 µl of 1 M Tris-HCl directly into the microcentrifuge tube and mix it by gently tapping the tube. Use a pH paper to ensure the pH is approximately 8.0 (Figure 4) prior to centrifugation.
Note: When dealing with a large number of sample preparations, it is acceptable to randomly pick few samples to check the pH. - Centrifuge the tube with the DNA lysate at 2,000 x g in a microcentrifuge for two min at room temperature to remove undissolved tissue debris.
- Transfer 150 µl of the top clear supernatant into a new tube or 96 well plates.
Note: The clear DNA lysate is ready to use as a template in PCR reaction and it is stable at 4 °C for two weeks, or it can be kept at -20 °C for a year.
4. Design Sex-specific PCR Primers
- Obtain accession numbers for Porcine sex determining region Y (SRY) (NM_214452.3) and and zinc finger protein X-linked (ZFX) genes (XM_005673501.1) from NCBI website http://www.ncbi.nlm.nih.gov/.
- Copy and paste these NCBI accession numbers to an online primer design tool.
Note: The best primers information including length, Tm and GC% as well as PCR product size (bp) will be generated on the computer screen (Figure 5). - Validate the specificity of these primers using nucleotide Blast program (Blastn) against the current porcine genomic database 104 to ensure these sequences are only located on X and Y chromosome for SRY and ZFX respectively (Figure 6).
5. Genomic DNA PCR Directly from DNA Lysates
- Use only 1 µl of the DNA lysate as a template for a 15 µl PCR reaction.
Note: A high throughput screening method for 96-well plates PCR can be performed in similar way using a multichannel pipette. - Do the following just for the first PCR: prepare one PCR tube for the no template negative control and two additional tubes for positive controls by adding 1 µl (0.5 ng) of commercially obtained known sex porcine genomic DNA. Later, include a sample from the last successful sexing analysis as a positive control and run together with the new PCR reaction for sex determination.
- Use any HotStart ready mix PCR enzyme and prepare a master mix by adding primers and nuclease free water depending on the total number of PCR reactions. Add 1 µl of the DNA lysate into a pre-existing PCR tube with 14 µl of the PCR master mix. Add primers such that the final concentration of the primers from two sex-specific genes is 0.3 µM in total of 15 µl PCR reaction.
- Set up the following PCR program in a thermal cycler: 95 °C for 3 min, 35 cycles each with a 20 sec melting step 98 °C, followed by a 15 sec annealing step at 65 °C, followed by a 15 sec elongation step at 72 °C. In a final step, incubate the reactions for 1 min at 72 °C and continue to incubate at 4 °C until removal of the PCR tube for gel electrophoresis verification to determine the sex.
Note: PCR conditions and optimization are according to the manual of the PCR kit mentioned in the Materials Table. - Add the appropriate amount of non-toxic green- fluorescent SYBR DNA gel stain when preparing a 2% TBE agarose gel.
Note: Ethidium bromide can be substituted for green- fluorescent stain if it is not available. - Add 1.5 µl of the loading dye (10x) into the PCR tube and mix well by pipetting the PCR reaction buffer up-and-down.
- Load 10 µl of the sample into the well and run the agarose gel with appropriate voltage settings (small gel apparatus at 100 V, 96-well gel apparatus at 150 V until the dye band runs half way through the gel).
- Observe and adjust the bands intensities under the fluorescent light setting using a laser scanner and capture an image of the gel. Note: Common gel imager can be substituted for the ethidium bromide gel.
- Determine the sex of the porcine embryos by identifying embryos with one band as a female and two bands as a male (Figure 7).
Representative Results
A representative result of sex determination from 345 DNA lysates screening by PCR is shown in Figure 7 and summarized in Table 1.
As can be seen in Figure 7, the primers annealing temperature at 65 °C is the optimal condition in this PCR protocol generating similar intensity and predicated amplicon sizes (Figure 5) among different samples.
Two amplified products of SRY (400 bp) and ZFX (506 bp) are shown in the gel picture (Figure 7). Male embryos showed two DNA fragments with the correct size of the upper (400 bp) and lower (506 bp) bands. The intensity of these two bands was shown to be equal in most male individuals. All the female embryos only showed a single DNA fragment corresponding to the ZFX gene located in the X-chromosome.
DNA lysates obtained from the modified 50 mM NaOH can be used directly for PCR with a high success rate and accuracy. DNA lysates did not show any PCR reaction inhibition after screening 345 embryos. Only three individuals were not sexed and the percentage of female embryos was slightly higher than males (Table 1).
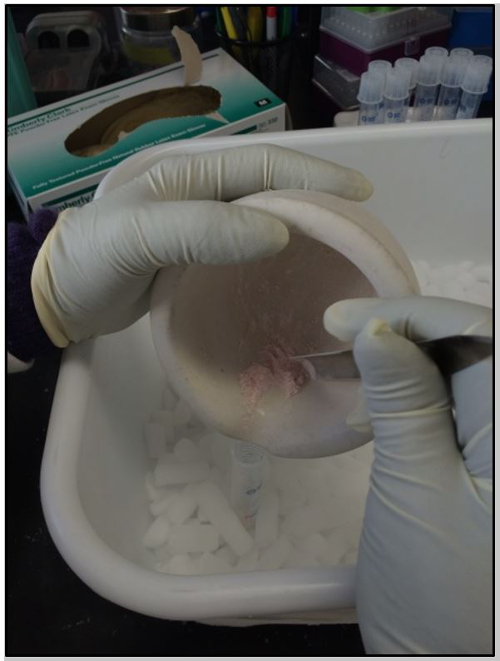
Figure 1: Embryo Powder Transfer. Transferring embryo powder to a 15 ml tube.
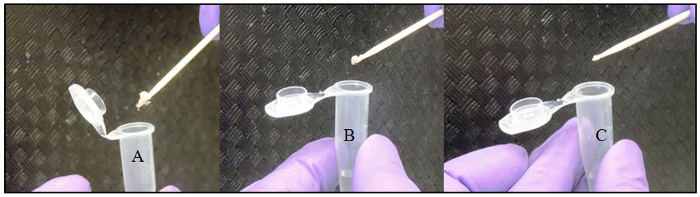
Figure 2: Collecting and Transferring Embryo Powder with a Toothpick. (A) An excessive amount of embryo powder adhering to the toothpick. (B) Correct amount of embryo powder adhering to the toothpick to be transferred to the alkaline lysis reagent solution. (C) An insufficient amount of embryo powder adhering to the toothpick.
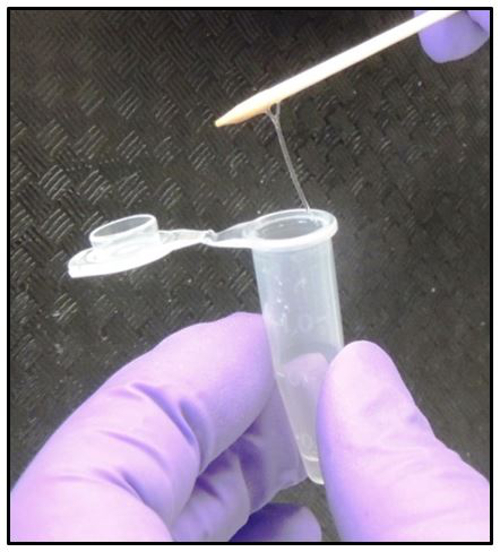
Figure 3: DNA Visualization Technique. Slowly pull up the toothpick, after adding the embryo powder, to determine if the embryo DNA has dissolved as a sticky gooey white transparent-like substance.
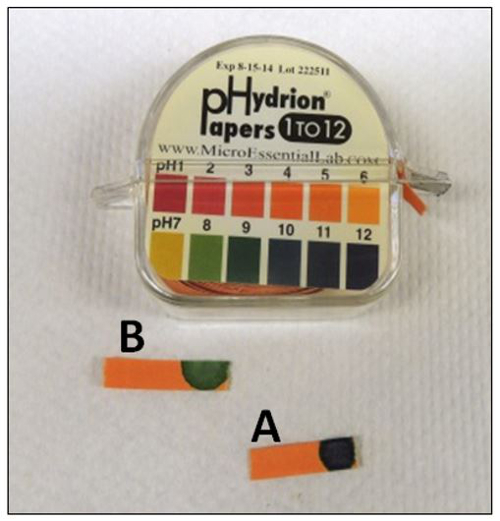
Figure 4: pH Confirmation. (A) pH of the sodium hydroxide (alkaline lysis reagent) solution is 12.0. (B) After addition neutralization buffer to the lysis buffer, color indication of pH paper is green and the pH should be around 8.0.
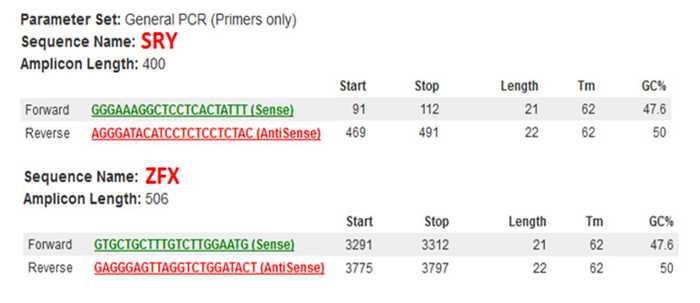
Figure 5: Sex-specific Primer Information. Sequence names, Sequences and the amplicon length obtained from the primer design tool. Please click here to view a larger version of this figure.
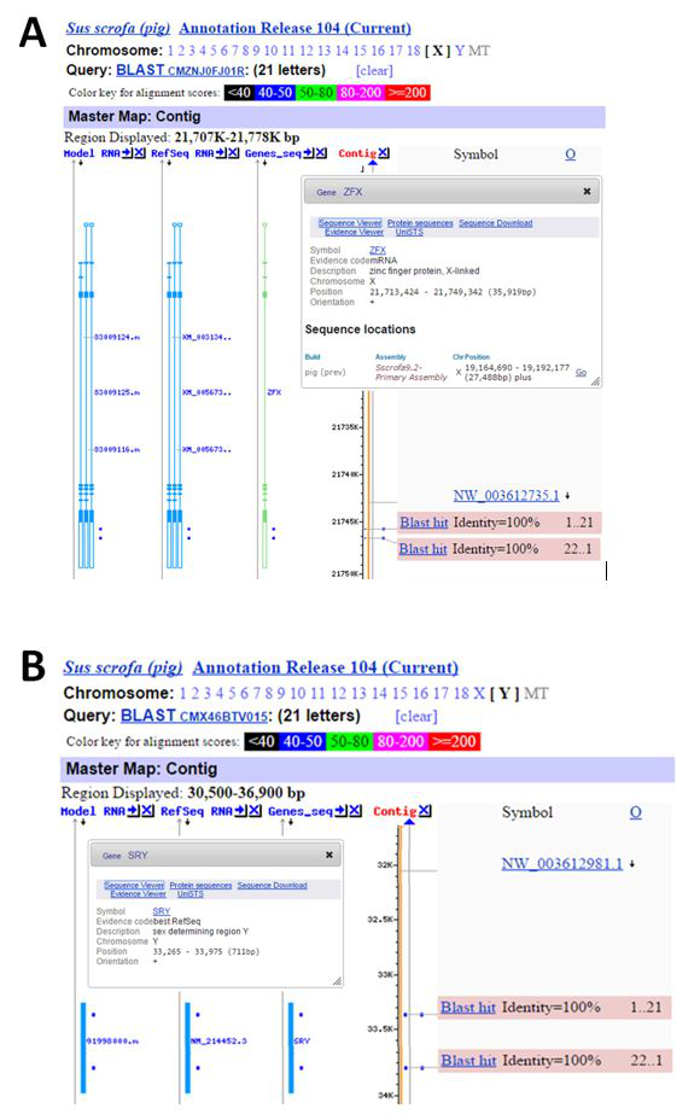
Figure 6: Mapping of Porcine Sex-specific Primers on Chromosome X and Y. (A) Location of the forward and reversed primers specified on the ZFX gene. (B) Location of the forward and reversed primers specified on the SRY gene. Please click here to view a larger version of this figure.
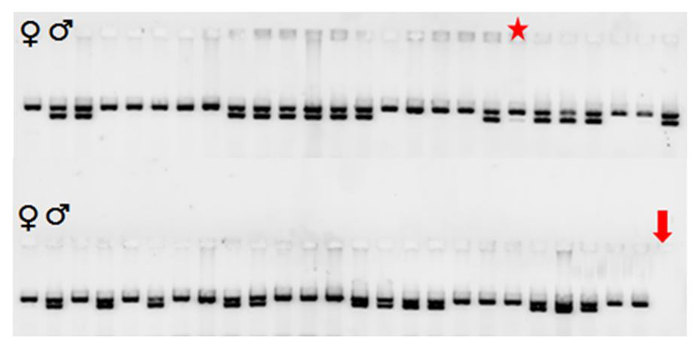
Figure 7: PCR Amplification of Porcine Sex-specific Genes from Day 30 Embryos. 2% TBE agarose gel stained with SYBR DNA gel stain with the known positive controls are indicated with male and female symbols. Two bands indicated as males and a single band as females. Red star indicated the possible sample contamination in the well with the appearance of a very faint lower band (400 bp) compared to upper band (506 bp) PCR product caused by SRY Y-specific primers. Red arrow indicates the no template PCR negative control. Please click here to view a larger version of this figure.
| Sexing PCR | Embryo Count | % |
| ♀ | 185 | 53.60% |
| ♂ | 157 | 45.50% |
| Unknown | 3 | 0.90% |
| Total | 345 | 100% |
Table 1: Percentage of Female, Male and Unknown after PCR Sexing among 345 Day 30 Embryos.
Discussion
Most of the existing protocols related to porcine embryos DNA sex typing are only suitable for early stage pre-implantation stage15,16. We have successfully developed a protocol suitable for porcine embryo screening during late gestation. Based on studies with similar developmental stages of embryos from previous studies6,18, the present protocol is considered to be safer and low cost.
This protocol is also suitable for a large number of samples for sex typing using PCR by transferring of the DNA lysates into a 96-well plate. The plate format allows high-throughput screening by allowing the transfer 1 µl of DNA lysate for the PCR reaction using a multichannel pipette.
However, several steps involved in the procedure should be done carefully. In order to perform the modified 50 mM NaOH lysis method more efficiently, the individually frozen embryo has to be grounded into fine powder using a pre-chilled (-80 °C) mortar and pestle. Avoid spilling embryo powder on the dry ice, by gently and slowly scraping the powder into the 15 ml tube, as shown in Figure 1. Changing examination gloves after each sample is highly recommended to avoid powder on the glove transferring to another embryo sample.
The appropriate amount of embryo powder to be added to the modified 50 mM NaOH lysis solution is shown in Figure 2B. An excessive amount of embryo powder is presented in Figure 2A and should be avoided. Likewise, in Figure 2C, the amount of embryo powder could be insufficient for downstream procedures. Although weighing the embryo powder for each sample is possible, it is not practical when screening embryo sex in a large scale study involving over a hundred embryos.
The amount of DNA may be visibly verified using a toothpick to check for the sticky gooey-like substance as shown in Figure 3, if it is doubtful whether the appropriate amount of powder was added in the previous step.
Adding the right amount of neutralization solution is important after lysing the embryo powder. Figure 4 shows the pH change after and before adding neutralization solution. This step is important to make sure that the pH is slightly alkaline (around 8.0) and therefore similar to most PCR reaction buffers.
Specificity of the oligo primers is the most important element for the accuracy of DNA sexing during PCR. The specificity of the oligo primers can be confirmed through the NCBI Blastn program. A single location will be detected on the X chromosome at the 3' non-coding region of the ZFX gene for each primer sequence (Figure 6A) for females. A similar situation is detected only on Y chromosome at the coding region of SRY gene (Figure 6B) for males.
Error rate due to no sex identification could be very low (<1%) as shown in Table 1, when all steps proceed carefully and correctly. However, in some rare case, cross contamination between DNA lysates of two different samples is possible and can be detected by PCR as indicated with a red star in the gel (Figure 7). This type of banding pattern in female can be interpreted as cross contamination with a male sample.
It is unlikely that the appearance of a very faint lower band is due to the primer competition for PCR reagents. In this case the smaller amplicon (400 bp) related to the SRY gene will generate more PCR product faster than the higher band corresponding to the ZFX gene. In this protocol, one disadvantage is the determination of cross contamination of a male sample with a female. Because our main goal is not quantification of copy number for each target gene, a tiny amount of powder from the female sample would not be easy to visualize on the gel.
The DNA sexing protocol presented here is the most cost effective method to identify embryo sex during late gestation and can be applied to others large livestock animals such as cattle, goat and sheep. Also, similar DNA sexing method has been applied in pigs related to sows metabolic condition18. Another research investigation using DNA sexing could be applied to study the genomic imprinting mechanism during prenatal programming of postnatal development in the pig22. A wide range of applications in livestock using DNA PCR sexing is possible such as pre-sex selection breeding programs, and epigenetic effects of nutrition and infectious diseases.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the cooperation and financial contributions of the following research funding agency: Alberta Livestock and Meat Agency Ltd., Pork CRC, Alberta Pork, Hypor A Hendrix Genetics Company and NSERC CRSNG.
Materials
| KAPA HiFi HotStart Ready Mix PCR kit | KAPABiosystems | KR0370 | other Hot Start Taq polymerase can be used after optimization |
| SYBR Safe DNA Gel Stain | Life Technologies | S33102 | Ethidium bromide can be substituted for SYBR Safe |
| Pig female and male genomic DNA | Zyagen | GP-160-F1 & GP-160-M5 | Postive controls from the tissues of known sex DNA can be used. |
| Typhoon FLA 9500 laser scanner | GE Healthcare Life Sciences | 28-9969-43 | other imaging system can be used |
| Free Soft Nitrile Examination Gloves | WWR | 89038-270 | any other examination glove can be used |
| Sodium hydroxide, solid | Fisher | BP 359 -212 | Molecular Biology Grade |
| Eppendorff DNA LoBind Tubes, 1.5 ml, PCR clean | Eppendorff | 0030 108.051 | heat resistant |
| ThermoStat plus | Eppendorff | 22670204 | Use as a incubator for 95C, don't need to use the heater |
| Toothpick | Bunzl Plc | 75200815 | Any round wooden toothpicks can be used – quality wood |
| Microcentrifuge 5417R/5417C | Eppendorff | 22621807 | This model was discontinued. But another newer model can be used |
| Microspatula | Fisher | SDI28540115 | Autoclaved before use each time. |
Referenzen
- Seidel, G. E. Economics of selecting for sex: the most important genetic trait. Theriogenology. 59, 585-598 (2003).
- Gutiérrez-Adán, A., et al. Development consequences of sexual dimorphism during pre-implantation embryonic development. Reprod. Domest. Anim. 41, 54-62 (2006).
- Rosenfeld, C. S., Roberts, R. M. Maternal diet and other factors affecting offspring sex ratio: a review. Biol Reprod. 71, 1063-1070 (2004).
- Gorecki, M. T. Sex ratio in litters of domestic pigs (Sus scrofa f. domestica Linnaeus, 1758). Biol. Lett. 40, 111-118 (2003).
- Chen, Z. Y., Dziuk, P. J. Influence of initial length of uterus per embryo and gestation stage on prenatal survival, development, and sex ratio in the pig. J. Anim. Sci. 71, 1895-1901 (1993).
- Vinsky, M. D., Novak, S., Dixon, W. T., Dyck, M. K., Foxcroft, G. R. Nutritional restriction in lactating primiparous sows selectively affects female embryo survival and overall litter. Reprod. Fertil. Dev. 18, 347-355 (2006).
- Cassar, G., King, W. A., King, G. J. Influence of sex on early growth of pig conceptuses. J. Reprod. Fertil. 101, 317-320 (1994).
- Zudova, D., Rezacova, O., Kubickova, S., Rubes, J. Aneuploidy detection in porcine embryos using fluorescence in situ hybridization. Cytogenet Genome Res. 102, 179-183 (2003).
- Hornak, M., Oracova, E., Hulinska, P., Urbankova, L., Rubes, J. Aneuploidy detection in pigs using comparative genomic hybridization: from the oocytes to blastocysts. PLoS One. 7, (2012).
- Handyside, A. H., Pattinson, J. K., Penketh, R. J., Delhanty, J. D., Winston, R. M., Tuddenham, E. G. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1, 347-349 (1989).
- Wilton, L. J., Shaw, J. M., Trounson, A. O. Successful single-cell biopsy and cryopreservation of preimplantation mouse embryos. Feri. Steril. 51, 513-517 (1989).
- Peura, J. M., Turunen, M., Janne, J. A reliable sex determination assay for bovine preimplantation embryos using the polymerase chain reaction. Theriogenology. 35, 547-555 (1991).
- Rao, K. B., Pawshe, C. H., Totey, S. M. Sex determination of in vitro developed buffalo (Buhalus buhalis.) embryos by DNA amplification. Mol. Reprod. Dev. 36, 291-296 (1993).
- Herr, C. M., Matthaei, K. I., Petrzak, U., Reed, K. C. A rapid Y-chromosome-detecting ovine embryo sexing assay. Theriogenology. 33, 245 (1990).
- Fajfar-Whetstone, C. J., LaneRayburn, A., Schook, L. B., Wheeler, M. B. Sex determination of porcine during preimplantation embryos via y-chromosome specific DNA sequences. Animal Biotechnology. 4, 183-193 (1993).
- Pomp, D., Good, B. A., Geisert, R. D., Corbin, C. J., Conley, A. J. Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day-10 or -11 pig embryos. J. Anim. Sci. 73, 1408-1415 (1995).
- Aasen, E., Medrano, J. F. Amplification of the ZFY and ZFX genes for sex identification in humans, cattle, sheep and goats. Biotechnology (N Y). 8, 1279-1281 (1990).
- Oliver, G., et al. Restricted feed intake in lactating primiparous sows. II. Effects on subsequent litter sex ratio and embryonic gene expression. Reprod Fertil. Dev. 23, 899-911 (2011).
- Truett, G. E., Heeger, P., Mynatt, R. L., Truett, A. A., Walker, J. A., Warman, M. L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques. 29, 52-54 (2000).
- Meeker, N. D., Hutchinson, S. A., Ho, L., Trede, N. S. Method for isolation of PCR-ready genomic DNA from zebrafish embryos. Biotechniques. 43, 610-614 (2007).
- Almeida, F. C. R. L., Machado, G. S., Borges, A. L. C. C., Rosa, B. O., Fontes, D. O. Consequences of different dietary energy sources during folliculardevelopment on subsequent fertility of cyclic gilts. Animal. 8, 293-299 (2014).
- Foxcroft, G. R., Dixon, W. T., Dyck, M. K., Novak, S., Harding, J. C. S., Almeida, F. C. R. L., Rodriguez-Martinez, Prenatal programming of postnatal development in the pig. Control of Pig Reproduction VIII. , 212-213 (2009).

