Anionic Polymerization of an Amphiphilic Copolymer for Preparation of Block Copolymer Micelles Stabilized by π-π Stacking Interactions
Summary
The key steps of living anionic polymerization of phenyl glycidyl ether (PheGE) on methoxy-polyethylene glycol (mPEG-b-PPheGE) are described. The resulting block copolymer micelles (BCMs) were loaded with doxorubicin 14% (wt%) and sustained release of drug over 4 days under physiologically relevant conditions was obtained.
Abstract
In this study, an amphiphilic copolymer that includes a core-forming block with phenyl groups was synthesized by living anionic polymerization of phenyl glycidyl ether (PheGE) on methoxy-polyethylene glycol (mPEG-b-PPheGE). Characterization of the copolymer revealed a narrow molecular distribution (PDI < 1.03) and confirmed the degree of polymerization of mPEG122–b-(PheGE)15. The critical micelle concentration of the copolymer was evaluated using an established fluorescence method with the aggregation behavior evaluated by dynamic light scattering and transmission electronic microscopy. The potential of the copolymer for use in drug delivery applications was evaluated in a preliminary manner including in vitro biocompatibility, loading and release of the hydrophobic anti-cancer drug doxorubicin (DOX). A stable micelle formulation of DOX was prepared with drug loading levels up to 14% (wt%), drug loading efficiencies > 60% (w/w) and sustained release of drug over 4 days under physiologically relevant conditions (acidic and neutral pH, presence of albumin). The high drug loading level and sustained release is attributed to stabilizing π-π interactions between DOX and the core-forming block of the micelles.
Introduction
In aqueous media, amphiphilic block copolymers assemble to form nano-sized block copolymer micelles (BCMs) that consist of a hydrophobic core surrounded by a hydrophilic shell or corona. The micelle core can serve as a reservoir for the incorporation of hydrophobic drugs; while, the hydrophilic corona provides an interface between the core and the external medium. Poly(ethylene glycol) (PEG) and its derivatives are one of the most important classes of polymers and one of the most widely used in drug formulation.1-3 BCMs have proven to be a worthy drug delivery platform with several formulations relying on this technology now in late stage clinical development.4 Most commonly, the hydrophobic block of the copolymer is comprised of polycaprolactone, poly(D,L-lactide), poly(propylene oxide) or poly(β-benzyl-L-aspartate).5-9
Kataoka's group investigated spherical micelles formed from PEO-b-PBLA and poly(ethylene oxide)-b-(polyaspartic acid-conjugated doxorubicin) for delivery of doxorubicin (DOX).10,11 In their reports, they put forward that π-π interactions between the polymer-conjugated drug or PBLA and free DOX act to stabilize the micelle core resulting in increases in drug loading and retention. It is established that compatibility or interactions between a drug and the core-forming block are determinants of key performance related parameters.12 In addition to DOX, a number of cancer therapeutics include aromatic rings within their core structure (e.g., methotrexate, olaparib, SN-38).
As a result there is significant interest in synthesis of copolymers that include benzyl rings in their core-forming blocks. Anionic ring-opening polymerization of PEG and its derivatives enable control over molecular weight and result in materials of low polydispersity in good yield.13,14 Ethylene oxide with phenyl glycidyl ether (PheGE) or styrene oxide (SO) can be (co)polymerized to form block copolymers that form micelles for solubilization of hydrophobic drugs.15-18 The current report describes the necessary steps for living anionic polymerization of phenyl glycidyl ether monomer on mPEG-OH as macroinitiator (Figure 1). The resulting block copolymer and its aggregates are then characterized in terms of properties of relevance to use in drug delivery.
Protocol
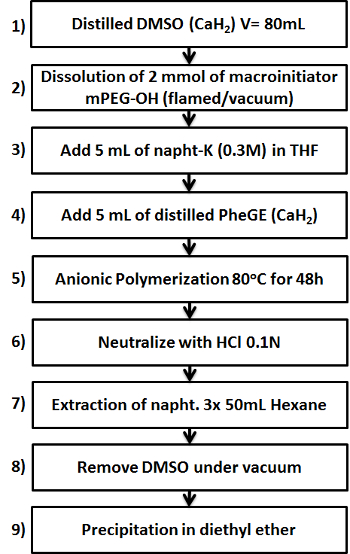
Figure 1. Schematic showing the nine key steps in the preparation of the mPEG-b-PPheGE copolymer. Please click here to view a larger version of this figure.
1. Preparation of the Reagents under Dry Conditions
- Preparation of the reagents.
- Weigh 15 g of mPEG-5K (Mn=5,400 g/mol, PDI 1.03) and place at 50 °C in an oven under vacuum for 48 hr prior to use.
- Dry 200 ml of dimethylsulfoxide (DMSO) over calcium hydride (CaH2) (~1 g), place under vacuum for 30 min, purge under argon and stir for 48 hr prior to use.
- Place 50 ml of the PheGE monomer in a dry and clean flask (100 ml), add 1 g of CaH2, seal under vacuum for 15 min on ice, purge under argon and leave to stir for 24 hr under argon prior to use.
2. Preparation of the Potassium Naphthalene
- Carefully, cut small chunks of sodium (~ 1.5 g) dried with hexane to remove excess mineral oil and add to the round flask containing the tetrahydrofuran (THF) (v = 500 ml).
NOTE: The chunks of sodium must not be exposed to air for long due to risk of fire. - Add benzophenone (~5 g), purge with argon and seal the round flask (2 necks) with glass stoppers.
- Following stirring under argon for 24 hr, connect the round-bottom flask to a distillation apparatus (Figure 2), distill the dark solution under argon while refluxing (i.e., reflux for about a 2 hr period after the solution turns blue). Begin to collect the desired volume ~ 150 ml of THF by closing the left valve (found in the middle of the distillation apparatus).
NOTE: If this solution does not turn blue, stop the distillation, cool down at room temperature (RT) and add more benzophenone or sodium and restart the distillation. This is an indication that THF still contains water. - In a dry Erlenmeyer, add distilled THF (v=100 ml) and dissolve 3.9 g of naphthalene.
NOTE: Stop the distillation, cool down at RT and open the right valve to transfer the volume of THF. - As described in point 2.1, cut small chunks of potassium (1.1 g) and add to the solution containing the naphthalene (final concentration ~0.3 mol/L). Seal the Erlenmeyer with a flushing adapter (T) (on/off) with a septum at the top and purge with argon.
- Following stirring under argon for 24 hr, observe the resulting solution of the potassium naphthalene base as a homogeneous dark green color.
- Under inert conditions, remove a 5 ml aliquot of the basic solution from the flask with a syringe and add to 10 ml of distilled water. Subsequently add 1-2 drops of phenolphthalein indicator to this solution, which turns the solution a fuchsia color.
- Use a burette to titrate the potassium naphthalene solution with a standard hydrochloric acid solution (0.1 N) until the solution turns colorless.
3. Materials and Necessary Precautions for Effective Living Anionic Polymerization
- System argon/vacuum manifold.
NOTE: As described in Figure 2, a double glass manifold with hollow glass stopcocks is used to switch between argon delivery and vacuum conditions in the glassware.- Connect the tank of argon (with manometer) to a dry desiccant column and to the manifold line using inert rubber tubing. At the other extremity of the argon line, connect a bubbler (containing mineral oil).
- To the glass stopcocks, connect flexible inert tubes and needles. To the other line of the manifold, connect a glass trap immersed in a cold Dewar flask (filled with ice/water or liquid nitrogen) to a high vacuum pump.
- Apparatus for distillation of monomer and DMSO.
NOTE: A convenient (i.e., all in one) apparatus for high vacuum distillation is employed (Figure 2). The dry glassware is made with high vacuum valves, and built-in condensers with an inner refrigerated head.- Connect water flow through the inlet (A) and outlet (B) of the cooling unit (letter). Connect the other inlet/outlet (C) to the dual manifold for argon/vacuum. Add and seal a septum (metal wires) at the delivery/extraction port and connect a stainless steel cannula for the transfer of air sensitive liquids (D) (at the top/loop).
- Prior to polymerization, distill PheGE and DMSO on hemispherical heating mantles at 100 °C and 70 °C, respectively, for 2 hr under vacuum with stirring. The PheGE monomer's boiling point is 254 °C whereas the boiling point for DMSO is 189 °C at (1 atm).
- Glassware for anionic polymerization.
- In addition to the distillation system, use only high vacuum resistant glassware including round bottom flasks (certified by the manufacturer), graduated cylinders (for transfer of volumes of solvent, base and monomers), cannulas, septa and metal wires to seal the septa.
NOTE: For living polymerization, heat carefully (under vacuum) and cool down all glassware under argon flow prior to use. Keep the heat gun at a distance ~10 cm from the glassware.
- In addition to the distillation system, use only high vacuum resistant glassware including round bottom flasks (certified by the manufacturer), graduated cylinders (for transfer of volumes of solvent, base and monomers), cannulas, septa and metal wires to seal the septa.
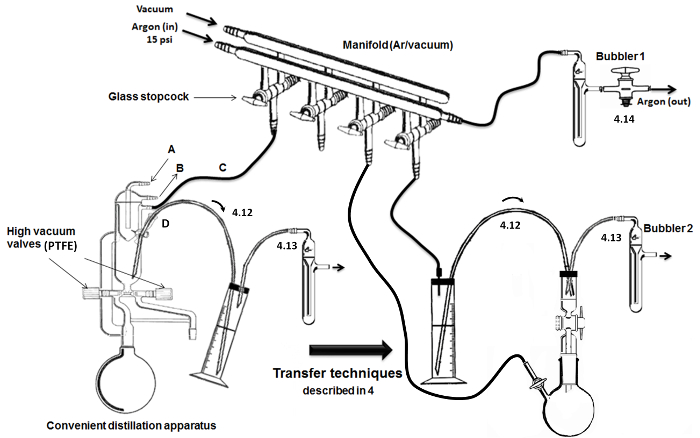
Figure 2. Assembly and key distillation/transfer steps. Please click here to view a larger version of this figure.
4. Description of the Key Steps of Living Anionic Polymerization: Distillation and Transfer
- Weigh mPEG-5K (2 mmol, 10 g) in a dry flask/Schlenk (oven) containing a stir bar and seal the flushing adapter (T) (on/off) with a septum at the top.
- Connect the flask to the manifold and purge the flask for 2-3 min with argon flushes. Turn the valve to the vacuum position to purge the flask.
- Rotate the flask manually and dry the reaction vessel homogeneously with a blow dryer (heat gun) until mPEG-5K melts.
NOTE: Keep the heat gun at a distance ~10 cm from the flask. - After 1 min, break the vacuum by turning the valve on the manifold towards the argon position with several quick snaps.
NOTE: A continuous argon flow has to be observed in the bubbler. When the flow is continuous, the valve stays on the argon position. Repeat heating and cooling steps twice to remove all traces of moisture. - Keep the polymeric macroinitiator under vacuum for ~ 2 hr and under argon before the reaction begins.
- Mount two high vacuum distillation apparatuses under the hood (Figure 2); one for the distillation of DMSO and one for the distillation of the monomer (PheGE).
- Connect the separate flasks containing the DMSO and monomer to the two apparatuses and install each on a hemispherical heating mantle (or in an oil bath). Connect cold water to the top of the apparatus (in/out) and to the manifold (argon/vacuum).
- Ensure that each apparatus is secure and well-sealed. Engage the vacuum via the valve.
NOTE: As described in step 3.3., repeat heating and cooling steps twice to remove all traces of moisture. - Set the heating via a temperature controller and start stirring the solutions. After 2 hr of circulation/distillation of DMSO, close the high vacuum valve (found in the middle of the distillation apparatus) to collect approximately 20 ml of solution (to wash the inside of the apparatus). Then, release the fraction into the flask and repeat the operation once more to ensure the purity of the desired fraction that is collected later.
- Heat the flask containing the mPEG-5K (under vacuum) with the heat gun until the polymer (mPEG-5K) melts. Purge again with argon.
NOTE: The procedure will help to the dissolution after the transfer of the DMSO. - After 2 hr, close the high vacuum valve and collect the volume of solvent (VDMSO = ~ 100 ml). Stop heating and break the vacuum from the manifold. Release argon (by snaps) into the chamber as described above.
- Under a positive pressure of argon, connect one side of the cannula (hold at the stopcock of the apparatus) to a graduated cylinder or directly to the flask containing the mPEG-5K (if the distillation apparatus has graduation) and, immerse the other end carefully into the freshly distilled fraction.
- Using argon pressure, drive the DMSO through the cannula into the reaction flask. Connect an extra bubbler to the flask (or cylinder if needed for measurement) and, close the glass stopcock connected to the bubbler on the opposite side of the manifold.
NOTE: When one side of a cannula is removed for the transfer, make sure that positive argon pressure is applied. - To avoid any accidents caused by argon pressure, open the glass stopcock for 1-2 sec and reclose to continue the flow of DMSO (repeated once per 0.5 min) until the full transfer is completed. Reopen the stopcock when finished.
NOTE: The same procedure must now be followed for distillation and collection of the monomer. The solvent and monomer cannot be collected at the same time. - Transfer 5 ml of 0.3 M naphthalene potassium via cannulation in a graduated cylinder sealed by a septum with cannula inserted (loop).
NOTE: Same precaution as described in NOTE 4.13. Positive argon pressure must be maintained first from the naphthalene potassium flask to the cylinder and then from the cylinder to the reaction flask to avoid air/water contamination. - Insert another needle from the manifold into the cylinder (argon). Remove the cannula connected to the distillation system carefully and insert rapidly into the reaction flask.
NOTE: Use this technique for the transfer of base and monomer. - Add the base drop by drop until the solution becomes dark. Following the slow disappearance of color, add another portion until the dark color appears again, and repeat until the full transfer.
- Transfer the desired volume of monomer (VPheGE = 5 ml) to reach a degree of polymerization of PPheGE ~ n=18-20.
- Leave the reaction for 48 hr at 80 °C under argon atmosphere with constant stirring to ensure complete polymerization.
- Quench the reaction by the addition of drops of HCl 1 N in methanol (measured using litmus paper (neutral pH)) and observed by a color disappearance.
- Extract the naphthalene from the DMSO solution with hexane (3×50 ml). Remove the DMSO by distillation under vacuum ~ 70 ml (same apparatus). Cool down the slurry solution and add 50 ml of THF.
- Remove the salt from the slurry solution by centrifugation at 5,000 x g for 10 min. Transfer the supernatant, and add drop wise to 500 ml of cold diethyl ether.
- Collect the precipitate by filtration or centrifugation (repeat twice) and dry under vacuum at 30 °C for 24-48 hr (yield 85%).
NOTE: The copolymer is now ready for characterization.
5. Characterization of the Copolymers
- Weigh 5-10 mg of copolymer (record the actual mass) in an aluminum sample pan and seal hermetically with the aluminum lid. Load sample pan and reference pan (empty) into the differential scanning calorimeter.
- Program a method ("heat/cool/heat") cycle: 1) heat from 40 °C to 100 °C at 10 °C/min, 2) cool to -70 °C at 10 °C/min, 3) heat to 100 °C at 10 °C/min. Repeat 2) and 3) twice. Determine melting point (Tm), crystallization (Tc) and glass transition temperatures (Tg), and heat of fusion (ΔHf) from the thermal traces from the third cycle (if applicable).
- Dissolve the polymers in THF (2 mg/ml) and filter through a 0.2-µm PTFE filter. Inject the sample into a gel permeation chromatography system (50 µl) and use the retention time for the sample and a calibration curve produced using a range of polystyrene standards to determine the molecular weight of the polymer.19
- Dissolve the (co)polymers (15 mg/ml) in d6 DMSO for 1H NMR spectroscopy analysis.19
- Determine the critical micelle concentration (CMC) of the copolymer using 1,6-diphenyl-1,3,5-hexatriene (DPH) as a fluorescence probe.9
- Prepare a DPH stock solution in THF (2.32 mg/L) in the dark and add 100 µl of this stock solution to each of a series of vials.
- Prepare a copolymer stock solution in THF and add aliquots of equal volume (2 ml) to the series of vials (each containing an aliquot of the DPH stock solution) resulting in final copolymer concentrations that range from 0.01 to 1,000 µg copolymer/ml.
- Subsequently, vortex the copolymer-DPH solutions and add dropwise to 10 ml of double-distilled water with stirring bar. The solutions must then be stirred vigorously in the dark for 48 hr under a stream of nitrogen to allow slow evaporation of THF. The final concentration of DPH in each solution is 0.232 mg/L.
- Measure the fluorescence emission of the samples at 430 nm (λex = 350 nm) using a dual-scanning microplate spectrofluorometer and plot fluorescence versus log[polymer]. The intercept between the two linear slopes provides the CMC value for the copolymer.
6. Procedure for Loading Doxorubicin into BCMs
- Dissolve 12 mg of DOX in 1 ml of acetonitrile, add 10 µl of triethylamine and let the solution stir in the dark for 2 hr.
- Dissolve the copolymer (45 mg) in 1 ml of THF and stir for the same period of time. Add the copolymer solution to the DOX solution and rinse the vial containing residual copolymer with an extra volume of THF (0.5 ml).
- Add the copolymer-drug mixture (2.5 ml) dropwise to a vial (20 ml) containing 15 ml of saline 0.9% (NaCl) with stirring.
- Transfer the solution to a dialysis bag (3.5 kDa cut off) and dialyze against saline 0.9% (500 ml).
NOTE: Change the external saline after 6 hr and let the dialysis continue for 24 hr with stirring in the dark at RT. - Transfer the dialysate to a 50 ml tube and centrifuge at 5,000 x g for 15 min.
- Transfer the supernatant to an ultrafiltration system (with a 10 ml capacity) that contains a dialysis membrane (cut off 10 kDa). Put the stirring adapter into the ultrafiltration system, close the lid and open to a stream of nitrogen.
- Concentrate the BCM solution to a volume of 4 ml and add 6 ml of fresh saline and repeat the procedure twice.
- Concentrate the BCM solution to 4 ml, rinse the chamber with 0.5 ml of saline and add to the solution. Store in brown vials at RT in the dark prior to further use.
7. Evaluation of Doxorubicin Loading in DOX-BCMs
- Dissolve DOX-BCM in dimethylformamide (100 µl in 400 µl) to disrupt the micelles and dilute in HCl aqueous solution (0.1 N) prior to evaluation (100 µl in 900 µl HCl 0.1 N).
- Measure drug loading at 490 nm using a benchtop microplate spectrophotometric system. Use the following equations to determine the drug loading capacity (DLC) and drug loading efficiency (DLE):
DLC (wt%) = (weight of drug loaded / total weight of BCMs) x 100%
DLE (%) = (weight of drug loaded / weight of drug in feed) x 100%
8. Evaluation of In Vitro Release of DOX from DOX-BCMs
- Investigate the release of DOX from BCMs at 37 °C in 0.1 M phosphate-buffered saline (PBS, pH 7.4) against PBS pH 7.4 containing 0.1% (w/v) Tween 80, BCMs + BSA (50 mg/ml) against PBS pH 7.4 and 0.1 M acetate-buffer at pH = 5.5.20,21
- Dilute the BCM-DOX formulation (700 µl) in the selected buffer (2.3 ml) to result in a total amount of ≈ 0.6-0.7 mg of DOX in the dialysis bag.
- Place the solution in the dialysis bag, seal with clips and immerse the bag into 200 ml of the respective external media.
- Remove 2 ml of the solution outside of the dialysis bag at predetermined time points and replace with the same volume of fresh buffer.
- Store the aliquots removed at -20 °C prior to analysis by UV-Vis spectrophotometry (Abs490 nm). The cumulative percentage of drug released (Er) can be calculated using the following equation:
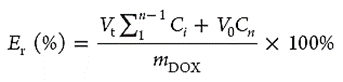
NOTE: Where mDOX represents the amount of DOX in the BCMs, V0 is the total volume of the release media (200 ml), Vt is the volume of the replaced media (Vt = 2 ml), Ci is the concentration before correction, and Cn represents the concentration of DOX in the sample.
Representative Results
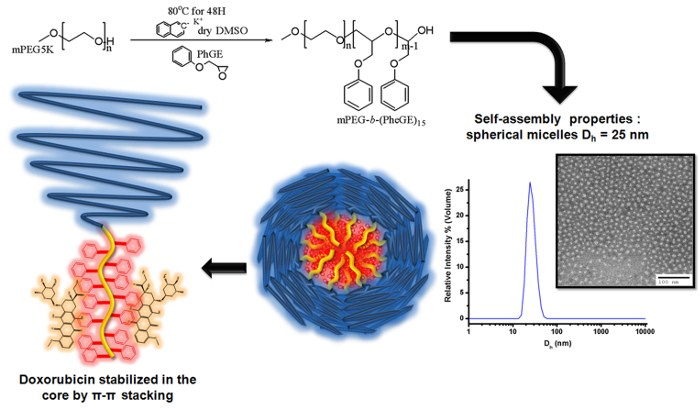
Figure 3. Illustration of the anionic polymerization of phenyl glycidyl ether on mPEG macroinitiator to produce mPEG-b-(PheGE)15 for preparation of block copolymer micelles for loading of doxorubicin. The schematic illustrates the deprotonation of the hydroxyl group of mPEG using naphthalene potassium as a radical-anion, followed by the polymerization of the phenyl glycidyl ether (PheGE) monomer. Representative transmission electron microscopy image (TEM) of the BCMs stained with uranyl acetate (1% w/v) and size distribution of the micelles as determined by dynamic light scattering (DLS). Please click here to view a larger version of this figure.
As shown in Figure 3, anionic polymerization of phenyl glycidyl ether on mPEG macroinitiator was used to prepare block copolymer micelles (DOX-mPEG-b-(PhGE)15 for entrapment of doxorubicin. A narrow molecular weight distribution for the mPEG-b-(PhGE)15 copolymer was confirmed by GPC (PDI=1.03) and the degree of polymerization was determined by 1H NMR analysis (Figure 4) [σ = 7.2 ppm (m, 2H meta, phenyl 2(=CH-)), σ = 6.8 ppm (d, 3H, 2 ortho and 1 para (-CH-), σ = 3.95 ppm (m, 2H, O-CH2-CH-)] with the methyl end group of the mPEG used as a reference peak (σ = 3.22 ppm (s, 3H).
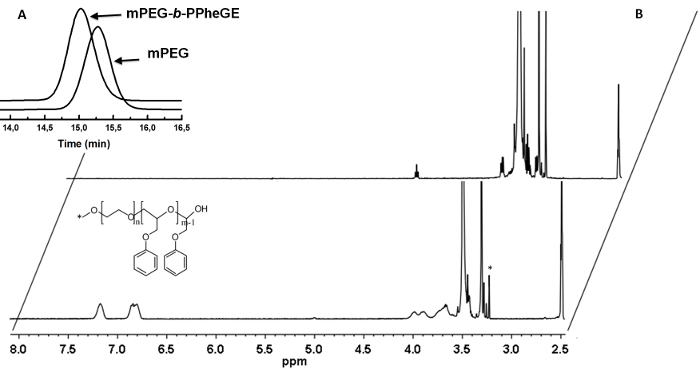
Figure 4. Characterization and analysis. A) GPC analysis of mPEG and the copolymer in THF. B) 1H NMR spectra of mPEG5K (upper spectrum) and mPEG-b-(PheGE)15 (lower spectrum) in d6-DMSO. Please click here to view a larger version of this figure.
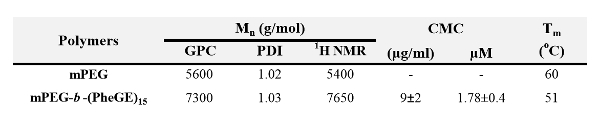
Table 1. Characteristics of the copolymer.
In aqueous media, amphiphilic block copolymers such as mPEG-b-(PheGE)15 assemble to form micelles that consist of a hydrophobic core surrounded by a hydrophilic shell. The CMC of the copolymer was measured using an established fluorescence method. The CMC of mPEG-b-(PheGE)15 was determined to be ~9 µg/ml (Figure 5A inset). Transmission electronic microscopy confirmed a spherical morphology for the copolymer aggregates and thus dynamic light scattering (Figure 3 and Table 2) was employed to assess the hydrodynamic diameter (Dh ~ 25 nm). As shown in the Figure 6-a, L929 mouse fibroblast cells were exposed to mPEG-b-(PhGE)15 BCMs and no cytotoxicity was observed following the 24 hr incubation period.
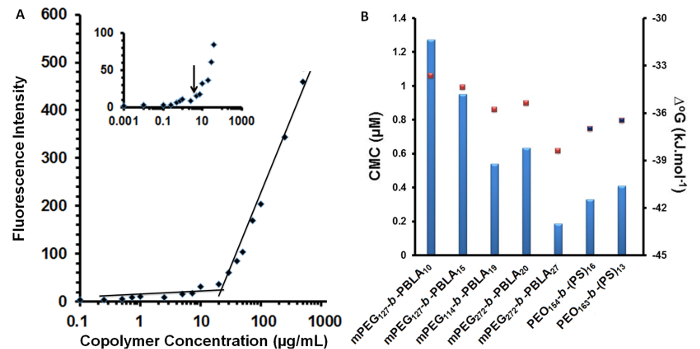
Figure 5. Fluorescence intensity and CMC characterization. A) Plot of the fluorescence intensity of DPH as a function of concentration of mPEG-b-(Phe)15 block copolymer. Inset shows the early stage of aggregation of the block copolymer at 0.1-10 µg/ml. B) Plot of CMC values obtained from the literature for a range of copolymers with pendant phenyl groups on the core-forming block. Red squares represent the calculated values for the Gibbs energy of micellization of the corresponding copolymers (± 0.5 kJ/mol).22,25-28 Please click here to view a larger version of this figure.

Table 2. Characterization of the BCMs prepared by the dialysis method.
Solubilization of drug in BCMs is influenced by the aqueous solubility of the drug as well as the propensity for interaction between the drug and itself and/or the core-forming block of the micelles. In its salt form, DOX is relatively soluble (~10 mg/ml) in water. Thus for loading into the BCMs, DOX was dissolved in acetonitrile and neutralized with TEA to obtain the free base (3 eq.). With a pKa of 8.5, DOX becomes relatively insoluble under basic conditions driving encapsulation in the BCMs with stabilization by π-π stacking interactions (mPEG-b-(PhGE)15). As described in the literature, similar loading capacities for DOX in DOX-mPEG-b-(PhGE)15 have been reported with an average value of 14% (w/w).21-24 After ultrafiltration, it was found that copolymer concentrations as low as 10 mg/ml successfully solubilized up to 1.6 mg DOX/ml. The drug loading efficiency was up to 52% (w/w) for the mPEG-b-(PhGE)15 BCMs (Table 2). The release profiles of DOX from the BCMs in different media were investigated (Figure 6c).
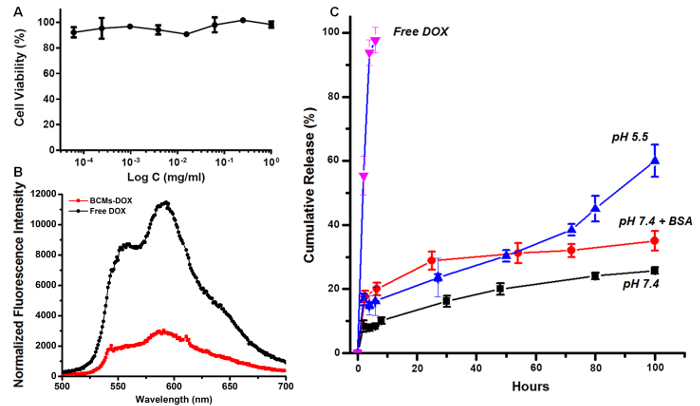
Figure 6. Cytotoxicity and drug release kinetics. A) Evaluation of the cytotoxicity in L929 mouse fibroblast cells of mPEG-b-(PheGE)15 copolymer micelles as determined using the MTS assay following a 24 hr incubation period (n = 3 individual experiments, S.D. < 10%). B) Normalized emission spectra of free DOX and DOX-loaded micelles in PBS, pH 7.4 at 10 µg/ml DOX concentration. The excitation wavelength is 480 nm and the emission spectrum is collected from 500-700 nm. C) Release profiles of DOX from the block mPEG-b-(PhGE)15 copolymer micelles (squares) in PBS 0.1 M pH 7.4, (circles) in PBS 0.1 M pH 7.4 containing BSA 50 mg/ml (in the bag) and (triangles) in acetate Na+ buffer 0.1 M pH 5.5 and (down triangles) free DOX (n=2) in PBS pH 7.4. (for each condition, n=3 individual experiments, S.D. < 10%). Please click here to view a larger version of this figure.
Discussion
Due to the good control that anionic polymerization provides over molecular weight it is one of the most applied processes in the industry for the preparation of polymers based on oxirane monomers (PEG and PPG). Optimal and stringent conditions must be used for successful polymerization to be achieved. Rigorous purification of all reagents and appropriate apparatus are essential for the living character of the synthesis. Limitations of the current setup are mostly associated with the transfer technique that relies on cannulation. Using appropriate pressure, cannulation is a safe laboratory scale technique for the academic setting. Applying these precautions will provide better reproducibility and control during the polymerization process (low PDI). Also, these transfer and purification procedures can be used for the preparation of copolymers such as mPEG-b-PCL, mPEG-b-PLLA, and mPEG-b-PAGE.19,29 However, this convenient procedure may not be adequate for polymerization of some monomers that require more stringent conditions (e.g., styrene). Alternatively, the break-seal technique is usually preferred for anionic polymerization.30 To control these steps in industry, similar systems (stainless/glass) are connected to each other via hermetic valves.
For oxirane monomers, the general mechanism is a nucleophilic attack of the oxyanion (free ion or ion pair) on the oxirane ring, which leads to ring opening polymerization. However, dependent on the nature of substituted oxirane monomers, some monomers may not polymerize or they may not be polymerized to high molecular weight. This type of polymerization does not tolerate acidic or basic components, including the monomer itself, the solvent or other species that lead to termination reactions and / or chain transfer in the medium (loss of control of the reaction). To produce a PEGylated block copolymer bearing phenyl groups by anionic polymerization, alternatives to phenyl glycidyl ether monomer can be found: styrene, styrene oxide or allyl glycidyl ether followed by a radical Michael reaction of benzyl mercaptan are options. mPEG can be prepared by condensation of the ethylene oxide monomer and then polymerized under the same conditions as described in this paper, using a hydroxylated initiator (e.g., methanol). However, mPEG of varying molecular weights with low PDI is available commercially.
To avoid water residue, the macroinitiator (e.g., mPEG-OH) needs to be well dried by pre-drying in an oven, followed by the heat gun-drying procedure. The reactions can be performed in polar aprotic solvents, coordinating solvents, or in bulk. When the polymerization requires specific conditions, such as high temperature, solvents with a stronger polarity than THF must be used, such as DMSO, diglyme or HMPA. As described in the protocol (section 4), efficient distillation of DMSO and the monomer (over CaH2) is required. DMSO is hygroscopic and if the distillation is not well conducted, traces of water may inactivate the active species. Other solvents may be used but DMSO has a high solvating ability for cations, low solvating ability for anions and allows high temperature polymerization.31,32 DMSO is an excellent solvent for base catalyzed polymerization of epoxides and olefins with strong electron withdrawing substituents. Initiation of the polymerization can be achieved by in situ generation of potassium alkoxide initiators through titration of mPEG-OH with a dilute solution of potassium naphthalene.33 It is important to carefully prepare the solution of potassium naphthalene and to titrate the solution with acid prior to its use. Indeed, if the concentration of potassium naphthalene is under or overestimated, the macroinitiator may form aggregates or fail to completely activate the initiator and in turn the polymerization may be compromised. When the potassium naphthalene is added dropwise, the slow disappearance of color provides visual control over consumption of the base by the initiator. Under these conditions, the rapid proton exchange between the hydroxyl groups (dormant) and alkoxides (active) ensures a controlled polymerization of the monomer.13
The aggregation behavior of block copolymers of similar composition to mPEG122–b-(PhGE)15 have been investigated by several groups with reported CMC values ranging from 1 to 10 µg/ml. 25,27,28,34-36 CMC values for a specific copolymer can vary depending on the specific method employed for determination. In this study, a fluorescence-based method was employed with DPH chosen as the probe given that it only results in a fluorescence signal once incorporated in the BCMs (Figure 5A inset). Figure 5B includes the CMC values obtained for various block copolymers with pendant phenyl groups. As shown, the CMC values of the copolymers vary depending on the nature of the polymer backbone bearing the phenyl groups and degree of polymerization.25,27,28,34-36 The exchange of copolymer chains between the micelles and the external medium depends on the state of the micelle core as well as the Flory-Huggins interaction parameter between the two blocks and the solvent. The glass transition temperature (Tg) of bulk PPheGE homopolymer is known to be lower than that of bulk PS.37 Due to the glassy nature of PS, copolymers with a high degree of polymerization of PS (n>35) possess a glassy core at RT (Tg~80 °C).38
Thermodynamically, two main approaches have been put forward to describe the micellization process, namely, the phase separation model (phase separation at the CMC), and the mass-action model (association-dissociation equilibrium micelle/unimers).39 According to both approaches, the standard Gibbs energy change (ΔG◦) for the transfer of 1 mol of amphiphile from solution to the micellar phase (ΔG◦ free energy of micellization), in the absence of electrostatic interactions, is given by ΔG◦ = R T ln(CMC).39 As shown in Figure 5B, the values for CMC and ΔG◦ are in agreement with the values obtained for copolymers that are similar in composition (in terms of total copolymer MW and ratio of hydrophobic to hydrophilic block length) to mPEG-b-(PhGE)15. As shown in Table 1, DSC analysis of the mPEG-b-(PhGE)15 copolymer bulk material confirmed a single Tm at 51 °C which is attributed to the hydrophilic block and is depressed relative to mPEG alone (60 °C). In solution, it is presumed that the cores of mPEG-b-PS copolymer micelles, with polystyrene blocks of similar length to that of PhGE in mPEG-b-(PhGE)15, begin to become mobile at physiological temperature (i.e., 37 °C). 38,40 Therefore, the mPEG-b-(PhGE)15 BCMs likely possess a relatively mobile core which enables local movement at room and physiological temperatures.
In this study dialysis and ultrafiltration were used as a convenient means to remove free drug and to increase the concentration of the drug/copolymer for subsequent in vivo applications. Alternately, freeze drying may be employed to concentrate the formulation; however, this requires optimization including possible addition of stabilizers (e.g., PEG, dextrose) to improve wettability for reconstitution. The resulting DOX-mPEG-b-(PhGE)15 BCMs showed similar sustained release profiles (PBS 7.4) to BCM systems developed by Kataoka and coworkers.21 In PBS at pH 7.4, less than 10% of the total drug was released within six hours whereas more than 95% of the free DOX is released from the dialysis bag within that same period of time. Sustained release at neutral pH indicates good stability of the formulation over the four-day period.
Human blood serum is comprised of approximately 7% protein, two-thirds of which is albumin.41 Therefore, in order to simulate the in vivo conditions drug release is commonly evaluated in buffer solutions containing physiologically relevant concentrations of this protein. In the present study, BSA was included in the dialysis bag at a concentration of 50 mg/ml. In the presence of albumin, the release of DOX from the BCMs increased to about 30% following 4 days of incubation at 37 °C. Release of DOX from the BCMs in buffer at pH 5.5 confirmed that protonation of DOX under these conditions results in an increase in drug release after 72 hours and this increases up to 60% after 4 days. Overall, the resulting DOX-BCMs have shown promising results in vitro, similar or equivalent to other BCM formulations of DOX presented in the literature, and thus encourage additional evaluation in vivo.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
CA acknowledges a Discovery grant from the Natural Sciences and Engineering Research Council of Canada. CA acknowledges a Chair in Pharmaceutics and Drug Delivery from GSK. The authors declare no competing financial interest.
Materials
| DMEM/HAMF12 | Gibco, Life Technologies | 12500 | Supplemented with 10%FBS. Warm in 37 °C water bath |
|||||
| Trypsin-EDTA(0.25%) | Sigma-Aldrich | T4049 | Warm in 37 °C water bath | |||||
| Fetal bovine serum (FBS) | Sigma-Aldrich | F1051 | Canada origin | |||||
| MDA-MB-468 cell line | ATCC | HTB-132 | ||||||
| MTS tetrazolium reagent | PROMEGA | G111B | ||||||
| Phenazine ethosulfate (PES) | Sigma-Aldrich | P4544 | >95% | |||||
| mPEG5K (Mn 5400 g/mol) | Sigma-Aldrich | 81323 | PDI=1.02 | |||||
| Dimethylsolfoxide (DMSO) | Sigma-Aldrich | D4540 | >99.5% | |||||
| Naphthalene | Sigma-Aldrich | 147141 | >99% | |||||
| Phenyl glycidyl ether | Sigma-Aldrich | A32608 | >85% | |||||
| Benzophenone | Sigma-Aldrich | 427551 | >99% | |||||
| Potassium | Sigma-Aldrich | 451096 | >98% | |||||
| Tetrahydrofuran | Caledon Laboratory Chemicals | 8900 1 | ACS | |||||
| Hexane | Caledon Laboratory Chemicals | 5500 1 | ACS | |||||
| Calcium hydride (CaH2) | ACP | C-0460 | >99.5% | |||||
| Diethyl Ether | Caledon Laboratory Chemicals | 1/10/4800 | ACS | |||||
| Microplate reader | BioTek Instruments | |||||||
| Differential scanning calorimetry (DSC) | TA Instruments Inc | DSC Q100 | ||||||
| Gel permeation chromatography (GPC) | Waters | 2695 separation moldule / 2414 detector | 2 Columns: Agilent Plgel 5µm Mixed-D | |||||
| NMR spectroscopy | Varian Mercury 400MHz | |||||||
| Chloroform-d | Sigma-Aldrich | 151858 | 99.96% | |||||
| DMSO-d | Sigma-Aldrich | 156914 | 99.96% | |||||
| Vaccum pump | Gardner Denver Welch Vacuum Tech, Inc. | Ultimate pressure 1.10-4 torr | ||||||
| Drierit with indicator, 8 mesh | Sigma-Aldrich | 238988 | Regenerated at 230°C for 2 hrs | |||||
Referenzen
- Dickerson, T. J., Reed, N. N., Janda, K. D. Soluble Polymers as Scaffolds for Recoverable Catalysts and Reagents. Chemical Reviews. 102, 3325-3344 (2002).
- van Heerbeek, R., Kamer, P. C. J., van Leeuwen, P. W. N. M., Reek, J. N. H. Dendrimers as Support for Recoverable Catalysts and Reagents. Chemical Reviews. 102 (10), 3717-3756 (2002).
- Knop, K., Hoogenboom, R., Fischer, D., Schubert, U. S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angewandte Chemie International Edition. 49 (36), 6288-6308 (2010).
- Eetezadi, S., Ekdawi, S. N., Allen, C. The challenges facing block copolymer micelles for cancer therapy: In vivo barriers and clinical translation. Advanced Drug Delivery Reviews. 91, 7-22 (2015).
- Attwood, D., Booth, C., Yeates, S. G., Chaibundit, C., Ricardo, N. Block copolymers for drug solubilisation: Relative hydrophobicities of polyether and polyester micelle-core-forming blocks. International Journal of Pharmaceutics. 345 (1-2), 35-41 (2007).
- Matsumura, Y., Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Science. 100 (4), 572-579 (2009).
- Chan, A. S., Chen, C. H., Huang, C. M., Hsieh, M. F. Regulation of particle morphology of pH-dependent poly(epsilon-caprolactone)-poly(gamma-glutamic acid) micellar nanoparticles to combat breast cancer cells. Journal of Nanoscience and Nanotechnology. 10 (10), 6283-6297 (2010).
- Diao, Y. Y., et al. Doxorubicin-loaded PEG-PCL copolymer micelles enhance cytotoxicity and intracellular accumulation of doxorubicin in adriamycin-resistant tumor cells. International Journal of Nanomedicine. 6, 1955-1962 (2011).
- Mikhail, A. S., Allen, C. Poly(ethylene glycol)-b-poly(ε-caprolactone) Micelles Containing Chemically Conjugated and Physically Entrapped Docetaxel: Synthesis, Characterization, and the Influence of the Drug on Micelle Morphology. Biomacromolecules. 11 (5), 1273-1280 (2010).
- Kataoka, K., Harada, A., Nagasaki, Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Advanced Drug Delivery Reviews. 47 (1), 113-131 (2001).
- Nakanishi, T., et al. Development of the polymer micelle carrier system for doxorubicin. Journal of Controlled Release. 74 (1-3), 295-302 (2001).
- Liu, J., Xiao, Y., Allen, C. Polymer-drug compatibility: A guide to the development of delivery systems for the anticancer agent, ellipticine. Journal of Pharmaceutical Sciences. 93 (1), 132-143 (2004).
- Flory, P. J. Molecular Size Distribution in Ethylene Oxide Polymers. Journal of the American Chemical Society. 62 (6), 1561-1565 (1940).
- Kazanskii, K. S., Solovyanov, A. A., Entelis, S. G. Polymerization of ethylene oxide by alkali metal-naphthalene complexes in tetrahydrofuran. European Polymer Journal. 7 (10), 1421-1433 (1971).
- Crothers, M., et al. Micellization and Gelation of Diblock Copolymers of Ethylene Oxide and Styrene Oxide in Aqueous Solution. Langmuir. 18 (22), 8685-8691 (2002).
- Taboada, P., et al. Block Copolymers of Ethylene Oxide and Phenyl Glycidyl Ether: Micellization, Gelation, and Drug Solubilization. Langmuir. 21 (12), 5263-5271 (2005).
- Taboada, P., et al. Micellization and Drug Solubilization in Aqueous Solutions of a Diblock Copolymer of Ethylene Oxide and Phenyl Glycidyl Ether. Langmuir. 22 (18), 7465-7470 (2006).
- Attwood, D., Booth, C. . Colloid Stability. , 61-78 (2010).
- Le Devedec, F., et al. Postalkylation of a Common mPEG-b-PAGE Precursor to Produce Tunable Morphologies of Spheres, Filomicelles, Disks, and Polymersomes. ACS Macro Letters. 5 (1), 128-133 (2016).
- Chtryt, V., Ulbrich, K. Conjugate of Doxorubicin with a Thermosensitive Polymer Drug Carrier. Journal of Bioactive and Compatible Polymers. 16 (6), 427-440 (2001).
- Kataoka, K., et al. Doxorubicin-loaded poly(ethylene glycol)-poly(β-benzyl-l-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. Journal of Controlled Release. 64 (1-3), 143-153 (2000).
- Cammas, S., Matsumoto, T., Okano, T., Sakurai, Y., Kataoka, K. Design of functional polymeric micelles as site-specific drug vehicles based on poly (α-hydroxy ethylene oxide-co-β-benzyl l-aspartate) block copolymers. Materials Science and Engineering: C. 4 (4), 241-247 (1997).
- Lv, S., et al. Doxorubicin-loaded amphiphilic polypeptide-based nanoparticles as an efficient drug delivery system for cancer therapy. Acta Biomaterialia. 9 (12), 9330-9342 (2013).
- Kim, J. O., Oberoi, H. S., Desale, S., Kabanov, A. V., Bronich, T. K. Polypeptide nanogels with hydrophobic moieties in the cross-linked ionic cores: synthesis, characterization and implications for anticancer drug delivery. Journal of Drug Targeting. 21 (10), 981-993 (2013).
- Zhao, C. L., Winnik, M. A., Riess, G., Croucher, M. D. Fluorescence probe techniques used to study micelle formation in water-soluble block copolymers. Langmuir. 6 (2), 514-516 (1990).
- Wilhelm, M., et al. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: a fluorescence probe study. Macromolecules. 24 (5), 1033-1040 (1991).
- Cammas, S., Kataoka, K. Functional poly[(ethylene oxide)-co-(β-benzyl-L-aspartate)] polymeric micelles: block copolymer synthesis and micelles formation. Macromolecular Chemistry and Physics. 196 (6), 1899-1905 (1995).
- Kwon, G., et al. Micelles based on AB block copolymers of poly(ethylene oxide) and poly(.beta.-benzyl L-aspartate). Langmuir. 9 (4), 945-949 (1993).
- Ahmed, F., Discher, D. E. Self-porating polymersomes of PEG-PLA and PEG-PCL: hydrolysis-triggered controlled release vesicles. Journal of Controlled Release. 96 (1), 37-53 (2004).
- Uhrig, D., Mays, J. W. Experimental techniques in high-vacuum anionic polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 43 (24), 6179-6222 (2005).
- Parker, A. J. The effects of solvation on the properties of anions in dipolar aprotic solvents. Quarterly Reviews, Chemical Society. 16 (2), 163-187 (1962).
- Cram, D. J. . Fundamentals o] Carbanion Chemistry. , (1965).
- Szwarc, M. . ACS Symposium Series. 166, 1-15 (1981).
- Cho, Y. W., Lee, J., Lee, S. C., Huh, K. M., Park, K. Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles. Journal of Controlled Release. 97, 249-257 (2004).
- Dewhurst, P. F., Lovell, M. R., Jones, J. L., Richards, R. W., Webster, J. R. P. Organization of Dispersions of a Linear Diblock Copolymer of Polystyrene and Poly(ethylene oxide) at the Air−Water Interface. Macromolecules. 31 (22), 7851-7864 (1998).
- Opanasopit, P., et al. Block Copolymer Design for Camptothecin Incorporation into Polymeric Micelles for Passive Tumor Targeting. Pharmaceutical Research. 21 (11), 2001-2008 (2004).
- Allen, G., Booth, C., Price, C. VI-The physical properties of poly(epoxides). Polymer. 8, 414-418 (1967).
- Jada, A., Hurtrez, G., Siffert, B., Riess, G. Structure of polystyrene-block-poly(ethylene oxide) diblock copolymer micelles in water. Macromolecular Chemistry and Physics. 197 (11), 3697-3710 (1996).
- Attwood, D., Florence, A. T. . Surfactant systems : their chemistry, pharmacy, and biology. , (1983).
- Rekatas, C. J., et al. The effect of hydrophobe chemical structure and chain length on the solubilization of griseofulvin in aqueous micellar solutions of block copoly(oxyalkylene)s. Physical Chemistry Chemical Physics. 3 (21), 4769-4773 (2001).

