Intrarenal Injection of Escherichia coli in a Rat Model of Pyelonephritis
Summary
This manuscript describes a rat surgical model of pyelonephritis using direct intra-renal infection by Escherichia coli into the renal pelvis. The experimental procedure can be utilized to study the pathogenesis of pyelonephritis as well as the associated inflammation and fibrosis.
Abstract
Pyelonephritis is a bacterial infection of the kidney and is most commonly caused by Escherichia coli. Recurrent infections can cause significant renal inflammation and fibrosis ultimately resulting in declining kidney function. Before improved clinical management and prevention of pyelonephritis can be instituted, a reliable animal model must be established in order to study the mechanisms of progression, recurrence, and therapeutic efficacy. The transurethral infection model closely mimics human pyelonephritis but exhibits considerable variation due to its reliance on urethral reflux to transport the bacteria to the kidney. Herein, a detailed surgical protocol for performing bacterial injections into the rat renal pelvis is provided and confirmed by non-invasive Magnetic Resonance Imaging (MRI). Using this protocol, animals receive direct exposure to a desired concentration of E. coli bacteria and can fully recover from the surgical procedure with adequate post-operative care. This facilitates subsequent longitudinal MRI assessments of the experimental animal models for comparison with saline (sham) controls. Using this direct delivery approach, the severity of infection is controllable and applicable for mechanistic studies of progression as well as development of novel treatment strategies.
Introduction
Rodent models have been used to study numerous human disease manifestations, including pyelonephritis and urinary tract infections (UTI). UTIs are a global health problem, and can impact children, men, and women of all ages.1,2,3 The initial manifestation of UTIs includes cystitis, and if the infection ascends along the ureter, a kidney infection (pyelonephritis) may follow. At the same time, the prevalence of diabetes is approaching 400 million people worldwide.4,5 Importantly, UTI incidence may be up to 4 times higher in patients who are obese or have type 2 diabetes mellitus, resulting in increased risk of recurrent UTI infection (rUTI), sepsis, renal fibrosis from pyelonephritis, and bladder dysfunction.6,7,8 Rodent models are important in studying UTIs, because current antibiotic therapies produce a sustained, preventative response only in a subset of UTI patients. To improve clinical UTI care, the key steps are to understand the mechanism of rUTI and its pathophysiological processes from acute infection to inflammation to fibrosis, as well as the impact of type 2 diabetes mellitus.
The goal of improving animal models is to develop techniques that allow for a more accurate evaluation of disease progression and therapeutic interventions. Several different approaches have been employed to induce pyelonephritis in rats and/or mice to study the pathophysiology of kidney damage, the effect of antibiotic treatment, and other aspects of the natural course of UTIs. A common approach to establish retrograde UTI is transurethral catheterization.10,11,12,13 This method introduces bacteria via the urethra into the urinary bladder of anesthetized animals. While this technique closely simulates human pyelonephritis, the actual incidence and magnitude of pyelonephritis infection can be highly variable because of multiple factors including a lack of spontaneous ureteric reflux or urinary voiding during or immediately following inoculation.11 As a result, the experimental variability in inducing an ascending pyelonephritis infection can limit the utility of this model to study kidney infections as well as therapeutic strategies.
This report describes a surgical pyelonephritis rat model where E. coli is directly injected into the rat kidney. Despite this rat model being invasive, the amount of E. coli delivered to the kidney can be effectively controlled enabling a robust kidney infection and inflammation.14 Within this procedure, we also describe how these induced renal infections can be monitored longitudinally with in vivo Magnetic Resonance Imaging (MRI).
Protocol
All animal studies were performed according to approved Institutional Animal Care and Use Committee (IACUC) protocols at Case Western Reserve University. The duration of the surgical procedure described below is approximately 45-60 min. The MRI procedure itself is approximately 15 min for each time point.
1. Anesthesia
- Anesthetize rat in isoflurane chamber set at 2% isoflurane mixed with oxygen to facilitate animal handling and restraint prior to administering injectable anesthesia intra-peritoneally.
- After 3-5 min of exposure to isoflurane, check that the animal is anesthetized and exhibits no response to toe pinch.
- Further sedate rat with an intra-peritoneal injection of a mix of xylazine and ketamine: 75 mg/kg ketamine/10 mg/kg xylazine. When performing intra-peritoneal injections, draw back the needle to ensure that portions of the intestine or other vital organs have not been punctured.
- Inject 2 mg/kg of bupivacaine subcutaneously at the site of incision to provide topical pain relief.
2. Preparation of Surgical Area
- Sterilize surgical instruments and supplies before they are used for surgery and lay out on the surgical pad for sterility. Most instruments and supplies can be autoclaved and re-used.
- Use sterile gloves for all surgical procedures.
- Use an electric razor to shave the fur off the animal's right side. Shave the animal from the bottom of the rib cage to the top of the hind leg providing a large hair-free area for the incision.
- Place the animal on a sterile surgical pad to isolate the disinfected area from surrounding areas.
- Scrub the skin with a disinfectant such as povidone iodine or betadine. Start the scrubbing at the center of the surgical site and move to the outside in a circular manner. Repeat at least three times with a new wipe of povidone iodine or betadine.
- Scrub the surgical site with 70% alcohol swabs until skin is clear, as iodine may be toxic if absorbed.
3. Surgical Procedure
- Maintain this procedure under aseptic conditions.
- Position the anesthetized animal on a warm heating bed in left lateral decubitus position with right flank facing upwards.
NOTE: Care must be taken to maintain the animal's core body temperature at 35-37 °C to prevent hypothermia. This warming bed should also be sterilized as needed to maintain aseptic conditions. - Feel for the rib cage, and make a small 2-3 cm right dorsal retroperitoneal incision using a sterile size 10 scalpel blade starting at the bottom of the rib cage.
- Place sterile gauze longitudinally along either side of the incision.
- Dissect away the subcutaneous tissue, fat, and muscles in order to visualize and access the abdominal cavity. Use curved-bladed Mayo scissors to allow deeper penetration into wound and cut thick tissues.
- Once the liver is clearly visible and accessible, use blunt forceps to retract the liver upwards.
- Using another pair of blunt forceps in the other hand, expose the right kidney so it sits just outside of the abdominal cavity.
- Use the pointer finger and thumb of the left hand to hold the kidney in position. With the right hand, slowly and steadily inject 0.1 ml UTI89 E. coli solution (concentration between 1 x 108-1 x 109) from a sterile syringe into the renal pelvis (which appears as a white bubble)15.
NOTE: Prepare the bacterial titer as described in reference15. - Place a strip of absorbable hemostat over the needle to prevent outflow of inoculum into the peritoneum. Slowly pull the needle out of the renal pelvis.
NOTE: Care should be taken not to pierce or infect surrounding tissues resulting in off-target infections and/or complications. - Use a larger syringe to thoroughly rinse the kidney with normal saline before placing it back into the abdominal cavity.
4. Sutures
Note: Suture that will be buried in tissues should be 4-0 non-absorbable braided sutures. Absorbable or monofilament sutures may be used for body surfaces.
- Place the sutures evenly and as close to the tissue edge as possible to prevent obstruction of blood flow; typically no more than 0.3 cm from the edge is necessary.
- Grasp the skin and evert it slightly using a pair of fine toothed forceps, and rotate the needle holder into a pronated position in preparation for piercing the skin.
- Drive the needle through the full thickness of skin by supinating the wrist to rotate the needle and pass it through the skin.
- Repeat this process for the skin edge closest to the individual performing the procedure.
- Tighten the sutures enough to oppose the tissue edges. Any tighter will obstruct blood supply, slow wound healing and may result in dehiscence.
- Tie off the suture using square knots, as if it were a simple interrupted stitch, except that only the short strand is cut, leaving about a 3-4 mm tail.
- After the first stitch has been tied off, prepare to place a second stitch about 3 mm away from the first, and continue the running suture.
- Once the end of the incision is reached, do not pull the last stitch completely through. Instead, use the loop which is being held with the needle holder here as the short strand in order to tie off the distal end of the suture closure.
- Using instrument ties, tie off the suture using square knots. This results in 3 strands sticking up from the completed knot at the distal end.
5. Animal Recovery
- Inject 2.1 mg/kg Yohimbine intra-peritoneally after surgery to reverse anesthesia. Animals are expected to fully recover from the surgical procedure after 3-5 hr.
- Keep the animal on a heating pad (to avoid hypothermia) and absorbent soft bedding after surgery.
- Provide rehydration with oral or parenteral fluids until it can return to normal feeding (within 24 hr). Inject approximately 0.6 ml of normal saline solution peritoneally immediately following surgery to limit dehydration effects.
- Provide analgesia as described in the IACUC approved Animal Care and Use Protocol. Inject 5 mg/kg carprofen subcutaneously for pain management.
- Monitor incisions regularly for swelling, exudate, pain, or dehiscence.
6. Validation via Magnetic Resonance Imaging
- Conduct in vivo MRI experiments on high field small animal MRI scanners.
- Induce anesthesia with 3% isoflurane in oxygen and position the animal with the right kidney at isocenter in the MRI scanner and appropriate radiofrequency coil. Provide animals with 1-2% isoflurane anesthesia continuously throughout the imaging procedure via a nosecone.
NOTE: For the in vivo MRI images shown in Figure 1, a rat-sized volume coil (inner diameter = 72 mm) was utilized. - Use an animal monitoring and control system to maintain each animal's respiration rate (40-60 breaths/min) and core body temperature (35 ± 1 °C).
- Use a multi-slice, multi-echo spin echo MRI acquisition to obtain high resolution, axial T2-weighted images of both the infected and control kidneys. Typical MRI acquisition parameters are repetition time = 5,000 msec, echo time = 40 msec, slice thickness = 2.0 mm, in-plane spatial resolution = 200 µm, 3 signal averages, and an acquisition time of 8 min.
Representative Results
Medical imaging techniques offer the opportunity to non-invasively assess UTI and therapeutic efficacy. Therefore, MRI was utilized to validate induction of acute infection after injection of 1-2 x 107 UTI89 E. coli, and to visualize the changes in the kidney before and after surgery. Figure 1a-b shows a progressively increasing region of kidney infection (yellow arrows). MRI images obtained for each animal at days 1 and 4 post-infection help characterize the growth of the acute pyelonephritis infection.
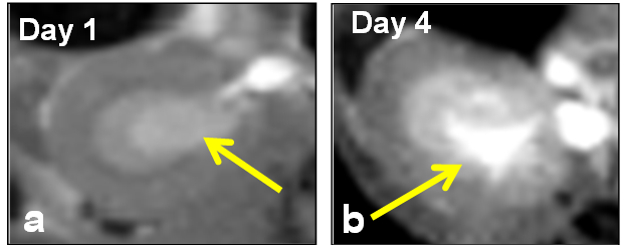
Figure 1: Axial T1-weighted MRI Images from an E. coli – induced Rodent Model of Pyelonephritis at (a) Day 1 and (b) Day 4 post-infection (yellow arrows).
Discussion
Ascending acute pyelonephritis in rodents (i.e. mice and rats) can be produced by transurethral catheterization.16,17,18 This transurethral infection method is advantageous in that it is non-invasive and mimics the human pathophysiology of ascending infection.17,18,19,20 However, this method can also suffer from irregular infection rates and variable E. coli dosing to the kidney due to physiologic limitations such as voiding and anatomic variation.11 Fierer et al. (1971) reported 6 of 40 (15%) rat kidneys showing evidence of pyelonephritis after infusion of E. coli into the bladder.15 Further, repeated infusions of E. coli were required several times a week to reliably produce pyelonephritis.15,19
In this report, we describe an alternative rodent pyelonephritis model that surgically introduces E.coli directly into the renal pelvis. The primary benefit of the surgical model of pyelonephritis technique include administration of a consistent dose of E. coli concentration between 1 x 108 – 1 x 109 as well as injection of the bacteria at the same anatomic site (right renal pelvis) for each animal.15 This direct injection avoids the requirement for reflux providing a more reliable infection as well as a more accurate determination of injected dose. Overall, this surgical pyelonephritis model provides an alternative option for studies requiring consistent, reproducible infection with minimal impact of urethral reflux.
Optimizing the success of this surgical technique include: 1) achieving a deep anesthesia to limit respiratory motion and to allow sufficient working time; 2) shaving the area of surgical incision to prevent contamination from non-prepped areas; 3) sterilization to prevent introduction of contamination; 4) careful injection of a known dose of E. coli into the rat renal pelvis; 5) thorough saline rinse of the kidney post-injection; 6) close approximation of wound edges via sutures; and 7) monitoring of intra-operative and post-operative complications such as hypothermia and systemic side effects. Although a heating pad was used to enhance animal comfort, this protocol may be further optimized by using a rectal temperature sensor to maintain core body temperature. A key step in achieving a successful survival surgery is proper anesthesia and monitoring the animal's respiration. Failure to do so results in extended surgical times to stabilize the animal's anesthesia level and limit undesired injury due to excessive motion. Compromised sterility of the work region and/or unintended infection of the peritoneum with E. coli bacteria are also common complications associated with the surgical model. Therefore, adequate preparation to autoclave surgical tools, to sterilize the entire workspace, and procedural efforts to limit additional E. coli infections all assist in minimizing animal mortality as well as erroneous infection results.
Despite the benefits of the direct injection of the E. coli into the kidney, this surgical model of pyelonephritis also has multiple limitations in comparison to the transurethral delivery method. The greatest drawback to using the direct surgical injection approach is the inherent invasiveness of the technique. However, mortality is considerably low with implementation of a proper sterile technique and use of adequate pain management, temperature control via heating pads, and soft, absorbent bedding post-surgery to ensure speedy recovery. Additionally, autoclaving surgical tools for sterilization is an extremely reliable and cost-effective method for sterilization. The overall surgical time commitment is typically less than 1 h. Moreover, the administration of anesthesia, as well as local and systemic delivery of pain medications calibrated to body weight ensures accurate dosage delivery and subject safety. Another potential limitation in the surgical model is that the surgical techniques are more difficult in mice due to the smaller anatomic sizes. In contrast to the transurethral method that can be implemented in both rats and mice, the requirement to using rat models for the surgical model increases the overall cost of the study and can place constraints on the study of various genetic alterations that may be more readily available and cost-effective in mouse models. As mentioned above, another significant limitation of the surgical method is that it does not represent human pyelonephritis as closely as the transurethral method.
Herein, we used MRI techniques to track the renal infections. As shown in Figure 1, MRI scanning provides the capability to non-invasively monitor the renal infections over time. An alternative approach to tracking E. coli infections is through fluorescent labeling of the bacteria.11,18 However, fluorescent imaging is less effective in this rat model (as opposed to mouse models) due to light absorption by the tissue resulting in greatly reduced detection sensitivity. Therefore, MRI provides a more sensitive means to track E. coli infections at their earliest stages and to provide regional information on the extent of the infection in three dimensions.
The mechanisms of UTI/acute pyelonephritis pathophysiology, and the progression to renal fibrosis are poorly understood. The infecting agents, the underlying immunological host response, and inflammatory reactions play an integral role, but the significance of each is unknown. The improvement in targeted delivery of bacteria to the kidney using this direct injection procedure has the potential to increase the reproducibility of acute pyelonephritis in rodent models, and perhaps to more accurately assess early-stage therapeutic interventions for treatment of UTI. This method was initially developed as an approach to optimize the delivery of bacteria to the kidney, but also has application for delivery of other reagents and pathogens. This method can similarly offer benefits to studies of cystitis, pyelonephritis, UTI, and diabetic kidney disease.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the support of NIH/NIDDK K12 DK100014 (Lan Lu), the Case Comprehensive Cancer Center (NIH/NCI P30 CA43703), and the Clinical and Translation Science Collaborative of Cleveland (NIH/NCATS UL1 TR000439).
Materials
| Absorbing Pad | Fisher | 14-127-47 | |
| Sterile Cotton Gauze Pad | Fisher | 22-415-469 | |
| Latex Surgical Gloves | Henry Schein Animal Health | 21540 | |
| Curved Mayo Scissors | Fisher | S17341 | |
| Straight Blunt Foreceps | Fisher | 08-895 | |
| Scalpel Handle | Fisher | 08-913-5 | |
| Sterile Scalpel Blades | Fisher | 53220 | |
| 1mL Luer-Lok Syringe | BD Biosciences | 309628 | For bacterial injections |
| 20mL Luer-Lok Syringe | BD Biosciences | 301031 | For saline wash |
| Hemostat | Seneca Medical | 240267 | |
| 23 G 3/4 in. Needle | BD Biosciences | 305143 | |
| 30 G 1 in. Needle | BD Biosciences | 305128 | |
| U-100 Insulin Syringe | Exel International | 25846 | For medication injections |
| Isoflurane | Henry Schein Animal Health | 050033 | |
| Xylazine | Henry Schein Animal Health | 33197 | Inject IP |
| Ketamine | Patterson Vetrinary | 07-881-9413 | Inject IP |
| Yohimbine (Atipamezole) | Patterson Vetrinary | 07-867-7097 | Inject IP after surgery |
| Bupivacaine (Marcaine) | Patterson Vetrinary | 07-890-4584 | Inject SQ at site of incision |
| Carprofen (Rimadyl) | Patterson Vetrinary | 07-844-7425 | Should be kept at 4 ᵒC |
| 4-0 Chromic Gut Suture | Ethicon Inc. | U203H | |
| 4-0 Braided Vicryl Suture | Ethicon Inc. | J304H | |
| 1mL SubQ Syringe | BD Biosciences | 309597 | |
| E. coli UTI89 or CFT073 | ATCC | 700928 | |
| Surgicel Absorbable Hemostat | Ethicon Inc. | ETH1951CS | |
| Biospec 9.4T MRI | Bruker | 94/20 USR |
Referenzen
- Saliba, W., Barnett-Griness, O., Rennert, G. The association between obesity and urinary tract infection. Eur J Intern Med. 24 (2), 27-31 (2012).
- Semins, M., Shore, A., Makary, M., Weiner, J., Matlaga, B. The impact of obesity on urinary tract infection risk. Urology. 79 (2), 266-269 (2011).
- Zilberberg, M., Shorr, A. Secular trends in gram-negative resistance among urinary tract infection hospitalizations in the United States, 2000-2009. Infect Control Hosp Epidemiol. 34 (9), 940-946 (2013).
- Whiting, D., Guariguata, L., Weil, C., Shaw, J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94 (3), 311-321 (2011).
- Wild, S., Roglic, G., Green, A., Sicree, R., King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 27 (5), 1047-1053 (2004).
- Ma, D., Gulani, V., Seiberlich, N., Liu, K., Sunshine, J., Duerk, J., et al. Magnetic resonance fingerprinting. Nature. 495 (7440), 187-192 (2013).
- Lu, L., Sedor, J., Gulani, V., Schelling, J., O’Brien, A., Flask, C. A., et al. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol. 34 (5), 476-482 (2011).
- Rosen, D., Hooton, T., Stamm, W., Humphrey, P., Hultgren, S. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4 (12), e329 (2007).
- Torine, L. A. Urinary tract infection: diabetic women’s strategies for prevention. Br J Nurs. 20 (13), 791-792 (2011).
- Rosen, D., Hung, C., Kline, K., Hultgren, S. Streptozocin-induced diabetic mouse model of urinary tract infection. Infect Immun. 76 (9), 4290-4298 (2008).
- Larsson, P., Kaijser, B., Mattsby-Baltzer, I., Olling, S. An experimental model for ascending acute pyelonephritis caused by Escherichia coli or proteus in rats. J Clin Pathol. 33 (4), 408-412 (1980).
- Gupta, R., Ganguly, N., Ahuja, V., Joshi, K., Sharma, S. An ascending non-obstructive model for chronic pyelonephritis in BALB/c mice. J. Med. Microbiol. 43 (1), 33-36 (1995).
- Fernandes, P., Shipkowitz, N., Bower, R. Murine models for studying the pathogenesis and treatment of pyelonephritis. Adv. Exp. Med. Biol. 224, 35-51 (1987).
- Kaye, D. The effect of water diuresis on spread of bacteria through the urinary tract. J. Infect. Dis. 124 (3), 297-305 (1971).
- Fierer, J., Tainer, L., Braude, A. Bacteremia in the pathogenesis of retrograde E. coli pyelonephritis in the rat. Am. J. Pathol. 64 (2), 443-456 (1971).
- Nickel, J., Olson, M., Costerton, J. Rat model of experimental bacterial prostatitis. Infection. 19 (3), S126-S130 (1991).
- Hagberg, L., Engberg, I., Freter, R., Olling, S., Eden, C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Am Soc Microbiol. 40 (1), 273-283 (1983).
- Kurosaka, Y., Ishida, Y., Yamamura, E., Takase, H., Otani, T., Kumon, H. A non-surgical rat model of foreign body-associated urinary tract infection with Pseudomonas aeruginosa. Microbiol. Immunol. 45 (1), 9-15 (2001).
- Anderson, B., Jackson, G. Pyelitis, an important factor in the pathogenesis of retrograde pyelonephritis. J Exp Med. 114 (3), 375-384 (1961).
- Anderson, J. Vesico-ureteric reflux. J R Soc Med. 55 (6), 419-426 (1962).

