Preparation of Primary Mixed Glial Cultures from Adult Mouse Spinal Cord Tissue
Summary
The development of neuropathic pain involves pathological changes of spinal cord glial cells. A reliable glial culture system derived from adult spinal cord tissue and designed for studying these cells in vitro is lacking. Therefore, we show here how to establish primary mixed glial cultures from adult mouse spinal cord tissue.
Abstract
It has been well-accepted that spinal cord glial responses contribute significantly to the development of neuropathic pain. Tremendous information regarding glial activities at the cellular and molecular levels has been obtained through in vitro cell culture systems. The in vitro systems utilized, mainly include primary glia derived from neonatal brain cortical tissue and immortalized cell lines. However, these systems may not reflect the characteristics of spinal cord glial cells in vivo. In order to further investigate the roles of spinal cord glial cells in the development of peripheral nerve injury-induced neuropathic pain using a culture system that better reflects the in vivo condition, our laboratory has developed a method to establish primary spinal cord mixed glial cultures from adult mice. Briefly, spinal cords are collected from adult mice and processed through papain digestion followed by myelin removal with a density-gradient medium. Single cell suspensions are cultured in complete Dulbecco's modified Eagle media (cDMEM) supplemented with 2-mercaptoethanol (2-ME) at 35.9 oC. These culture conditions were optimized specifically for the growth of mixed glial cells. Under these conditions, cells are ready to be used for experimentation between 12 – 14 d (cells are usually in log phase during this time) after the establishment of the culture (D 0) and can be kept in culture conditions up to D 21. This culture system can be used to investigate the responses of spinal cord glial cells upon stimulation with various substances and agents. Besides neuropathic pain, this system can be used to study glial responses in other diseases that involve pathological changes of spinal cord glial cells.
Introduction
Chronic pain is a serious health problem that affects approximately 100 million adults in the United States, with an estimated annual cost of up to $635 billion1. Mounting evidence has indicated a significant contribution of spinal cord glial cells in the development of neuropathic pain, one of the most devastating kinds of chronic pain2. A proper in vitro culture system would help significantly in further investigating the roles of glial cells at the cellular and molecular levels.
Currently, the in vitro glial culture systems utilized by researchers include mainly glial cells derived from cortical tissues of neonatal mouse or rat brains and immortalized glial cell lines derived from neonatal mouse or rat primary glial cells. Neonatal cells contain significant numbers of undifferentiated cells that can be further differentiated into glial cells (mainly astrocytes and microglia), thus providing abundant cells for experimental usage3. However, our previous study has shown that neonatal brain glial cells responded significantly differently from adult spinal cord glial cells upon lipopolysaccharide (LPS) stimulation. For example, interleukin (IL)-4 displayed enhanced inhibitory effects on LPS-induced nitric oxide (NO) production in adult rat spinal cord glia compared to neonatal brain glia4. Furthermore, the profiles of chemokine production upon stimulation by a neuropeptide, calcitonin gene-related peptide (CGRP), are unarguably different between neonatal mouse brain glia (methods described in Ref. 5) and adult mouse spinal cord glia (Figure 1). Established cell lines are easy to use and maintain and can provide a large number of cells in a short time period. In general, immortalized cell lines are generated either using a virus-mediated immortalization system (such as the widely-used microglial cell line BV2)6, 7 or following the identification of spontaneous transformation (such as the astrocyte cell line C8-D1A and microglial cell line C8-B4)8, 9. Cell lines are excellent in studying the molecular characteristics of astrocytes and microglia individually; however, the results obtained from cell lines always require further validation in primary cells or in in vivo conditions. It should also be noted that there has not been a report of a glial cell line that is derived from a rodent spinal cord glia.
To help investigating the role of spinal cord glial cells in the development of neuropathic pain, a culture system that is derived from the adult mouse spinal cord has been developed by adapting a previously-reported method used to generate adult rat mixed glial cultures4, 5. The mouse spinal cord glial culture was further refined recently10 and is described in more detail in this article. Adult spinal cord mixed glial cultures can be established with a mouse from selected strains that fit the needs of the particular study, and cells can be maintained in culture for up to 21 d post initiation of the culture. This method can be used in neuropathic pain studies, as well as in investigations of various neurological diseases that involve pathological changes within the spinal cord, such as amyotrophic lateral sclerosis (ALS), human immunodeficiency virus (HIV)-associated sensory neuropathy, and multiple sclerosis (MS).
Protocol
The following protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of New England. The following protocol is for preparing glial cultures from 4 adult mouse spinal cords.
1. Preparation of Solutions in a Culture Hood under Aseptic Conditions
- Prepare culture media: complete Dulbecco's modification of Eagle's media (cDMEM) containing DMEM (with 4.5 g/L glucose), 10% Fetal Bovine Serum (FBS), 2 mM L-glutamine, 100 IU/mL penicillin, 100 mg/mL streptomycin, 250 ng/mL Amphotericin B, and 50 µM 2-mercaptoethanol (2-ME; see 1.1.1). Mix and filter sterilize all other components and then add FBS. Store the culture media at 4 oC.
- Prepare 50 mM 2-ME/phosphate-buffered saline (PBS) stock solution: add 100 µL of concentrated 2-ME (14.3 M) into 28.6 mL of 1x PBS, and filter sterilize (the solution can be stored at 4 oC for several months). Use 1 mL of 2-ME stock solution for every 1,000 mL of culture media.
- Preparation of the density gradient media
- Prepare a stock isotonic density gradient media solution (e.g., 100% Percoll) from 1 part 10x regular sterile PBS and 9 parts sterile stock density gradient media (e.g., Percoll).
- Dilute the 100% density gradient media (made in 1.2.1) with regular 1x sterile PBS to make a 20% density gradient media (e.g., 10 mL of 100% density gradient media with 40 mL of 1x PBS) and store it at 4 oC. Bring the 20% density gradient media to room temperature (RT) before preparing the cell culture.
- Preparation of solutions from the papain dissociation system
NOTE: The papain dissociation system can be identified in the Materials Table. Other similar dissociation kits (including those assembled in-house) can also be used. All components must be sterile.- Add 32 mL of Earle's Balanced Salt Solution (EBSS) to the ovomucoid inhibitor mixture (powder).
- Add 5 mL of EBSS to one papain vial (powder). Place the papain vial in a 37 oC water bath for 10 min or until the papain is dissolved.
- Add 500 µL of EBSS to one DNase vial (powder). Mix gently.
- Add 250 µL of the DNase solution (above) to the papain vial.
- Calculate the total amount of the above papain/DNase mixture needed (approximately 800 µL of papain/DNase mixture per mouse spinal cord) and transfer the needed mixture into a 50 mL tube for tissue digestion (step 3.2). Store the leftover in the original vial at 4 °C for at least two weeks.
- Keep all components from the kits on ice until needed.
- Prepare spinal cord collection tubes: one sterile 15 mL tube with 5 mL of Hank's balanced Salt Solution (HBSS; from the papain dissociation system, for collecting spinal cords) and one sterile 15 mL tube with 1x PBS (for filling up the 5 mL syringe, see step 2.4). In addition, prepare one sterile petri dish (35 mm or 60 mm) with 5 mL of HBSS and keep it in the culture hood.
2. Spinal Cord Collection
NOTE: All equipment must be sterile. Perform the collection in a designated area approved by the IACUC.
- Obtain the following tools for harvesting mouse spinal cords: straight sharp/blunt 18-cm surgical scissors, a 12-cm standard scalpel (#3 solid), carbon steel sterile scalpel blades #10, a small animal decapitator, 12-cm standard curved forceps, and a sterile 20G needle attached to a 5-mL syringe.
- Euthanize the animal with CO2 and disinfect the mouse head and the back region with 70% ethanol (EtOH).
- Decapitate animal with the decapitator. Make a middle longitudinal incision on the lower back to expose the muscle layer. Make a clean transection through the vertebrate column at the hip level (also cutting through the muscle layer that covers the vertebrate column) with the sterile straight surgical scissors (18 cm).
- Place the animal on a sheet of fresh disposable wipe. Carefully insert the 20 G needle (attached to a 5 mL syringe filled with sterile 1x PBS (step 1.4)) into the spinal column towards the rostral side. Hold the animal down tightly and push the syringe quickly. The whole spinal cord should come out from the cervical end. Collect the spinal cord into the 15 mL tube containing HBSS.
- Repeat step 2.4 for the rest of the mice. Between each animal, disinfect all used instruments with 70% EtOH.
NOTE: Typically, a single 12-well plate can be established for every 4 mouse spinal cords (see 4.3).
3. Preparation of the Single Cell Suspension
Note: Perform steps 3 and 4 in a culture hood to keep everything sterile.
- Transfer all spinal cords into the HBSS-containing petri dish (step 1.4). Cut each of the spinal cords into many fine, small pieces with sterile scissors and forceps. Transfer the spinal cord tissue to the 50-mL conical tube containing the prepared papain/DNase enzyme mixture (step 1.3.5) using sterile forceps or a 10 mL pipette (avoid adding HBSS to the enzyme mixture, as this will further dilute the prepared enzyme solution and may result in decreased performance of the enzyme).
- Vortex the tube gently to mix. Incubate the tube at 37 oC for 1 h in an incubator/shaker with orbital shaking at 150 rpm.
- Vortex the tube again and vigorously triturate the enzyme solution with the tissue using a 5 mL pipette to promote further dissociation.
- Transfer the cell suspension into a 15-mL tube and centrifuge at 300 x g for 5 min at RT.
- During centrifugation, mix 2.7 mL EBSS with 300 µL of reconstituted albumin-ovomucoid inhibitor solution (1.3.1) in a sterile tube. Add 150 µL of the DNase solution (step 1.3.3).
- Following centrifugation, remove the supernatant and resuspend the cell pellet with the solution prepared above (step 3.5). Vortex well to break the cell pellet.
- Add 3 mL of reconstituted albumin-ovomucoid inhibitor solution (step 1.4.1) to the cell suspension. Centrifuge cells at 70 x g for 6 min at RT. Remove the supernatant (which contains membrane fragments).
4. Further Removal of Myelin from the Single Cell Suspension
- Add 8 mL of 20% density gradient media (prepared in step 1.2.2) into the tube containing the cell pellet, vortex gently to disrupt the pellet, and centrifuge the cells at 800 x g for 30 min at RT without braking. Carefully remove the top layer of debris (mostly myelin) and the supernatant, but keep the pellet.
- To remove remnants of the density gradient, wash the cells by resuspending the cell pellet with 8 mL of a diluted cDMEM (1 part cDMEM and 2 parts HBSS). Centrifuge the cells at 400 x g for 10 min at 4 oC. Remove the supernatant and wash the cells again with the diluted cDMEM (above) in the same manner. Keep the cells on ice until seeding them.
- Remove the supernatant and resuspend the cell pellet in culture media (cDMEM supplied with 2-ME (prepared in step 1.1)). For one 12-well plate, use 3 mL x 4 (number of mice used) + 2 mL = 14 mL media. This will ensure that there is sufficient cell suspension for the entire plate (12 wells) and will provide for extra wells that can be used to determine the average cell number per well and the microglial content of the culture. If other types of culture vessels will be used, calculate the total volume of needed culture media proportionally.
- Add 1 mL of the cell suspension into each well of a 12-well plate.
- Incubate the cells at 35.9 oC with 5% CO2.
- Change the media (remove old media via aspiration) on D 1 and then every 3 – 4 d thereafter (typically change the media on D: 1, 4, 8, and 11, and then use the cells on day 12).
NOTE: On day 1, the culture may contain significant amounts of debris due to the residue myelin from the spinal cord tissue; thus, changing the media on day 1 is recommended (please see the Discussion for more information). Cultures are ready for treatment between D 12 – 14. Cells usually are 80% confluent at D 12 and can be near 100% confluent by D 14. Typically, on D 12, there are about 100,000 cells per well in a 12-well plate.
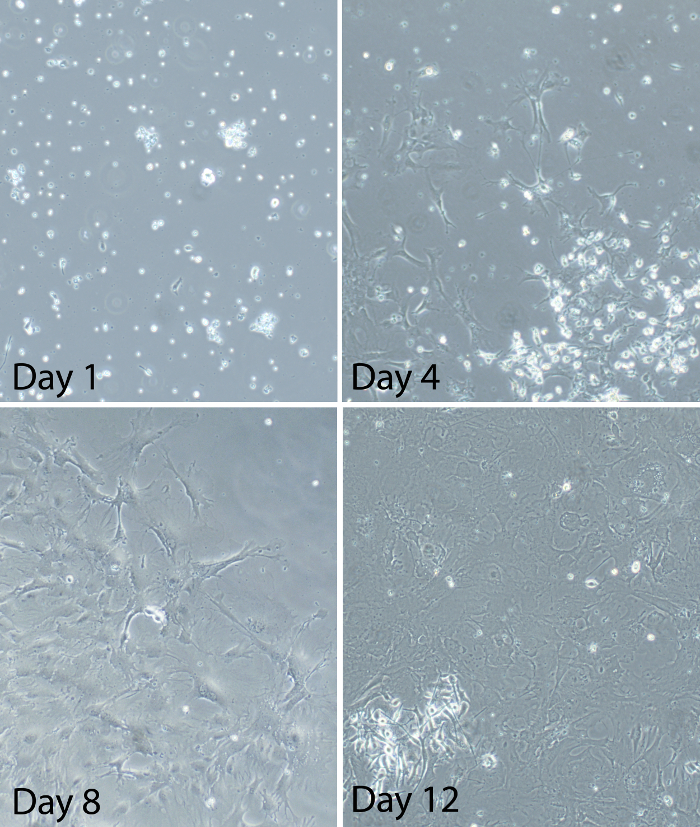
Figure 2: Representative images of mixed glial cells at different times after the establishment of the mixed glia culture. Primary spinal cord mixed glia were prepared from adult C57Bl/6 mice. Representative images show the progress of the glial culture after plating. At day 1, some cells are attached to the culture plate, but they are still mostly round. There are also many floating cells and significant debris. At day 4, the majority of the cells are attached to the culture plate. Cells appear ramified with visible processes. Cells are sporadically distributed and cultures are about 20-30% confluent at this time. At day 8, cultures are between 50-60% confluent. Some areas of the cultures have large patches of cells. Other areas of the cultures have sparse growth of cells. Some cells within the patches take on a “square-like” appearance. At day 12, cultures are at or above 80% confluent (about 90% confluent in the image shown here). Cell are in the log phase of growth at this time and between days 12-14 is the optimal time for using the glia cells for experiments. Please click here to view a larger version of this figure.
- Examine the microglial content using cells from the extra wells (step 4.3) via standard fluorescence-activated cell sorting (FACS) staining protocol11 using the combination of anti-mouse-CD45 and anti-mouse CD11b monoclonal antibodies. CD45+CD11b+ cells are identified as microglia.
Representative Results
This method can be used to prepare mixed glial cells from both mice and rats. The average total cell number per well in a 12-well plate on D 12 post-initiation of the culture should be relatively stable, with around 100,000 cells per well when cells are derived from mouse spinal cords. Glial cells obtained from this method can be used in experiments that are designed to examine adult spinal cord glial responses upon administration of substances and agents of interest. Figure 3 provides an example of cytokine and chemokine responses from one typical experiment in which adult spinal cord microglia were obtained from adult BALB/c mice and treated with LPS (Salmonella Minnesota Re595) on D 13 post-initiation of the culture. Supernatants were collected 24 h post-LPS treatment for the determination of cytokine and chemokine levels via an enzyme-linked immunosorbent assay (ELISA) using commercially-available kits.
When this adult glial culture system was first established, cells were incubated in a standard culture environment at 37 oC. Under this condition, the average microglial content ranged from 5 – 10%. However, it was observed that despite the use of consistent culture techniques, cultures would in some instances have either very low microglial content (<2%) or relatively high microglial content (15 - 20%)10. It can be frustrating to obtain cultures that have very few microglia when the experimental results rely on the microglial responses within the mixed culture. Following the suggestion of Dr. Alejandro M. S. Mayer (Department of Pharmacology, Chicago College of Osteopathic Medicine)12, the method was modified by growing the mixed glial cells at 35.9 oC. This resulted in a more consistent and higher microglial yield (ranging between 10 – 40% and remaining mostly around 20%) within the majority of cultures. This improvement is illustrated in Figure 4. As reported previously, microglia are estimated to make up 5 – 21% of the CNS glial population in adult mice13-15. Although both culture conditions provide cultures that contain similar amounts of microglia as estimated in vivo, the modified culture condition (35.9 oC) is more appropriate for experiments in which it is critical to examine the responses from both astrocytes and microglia.
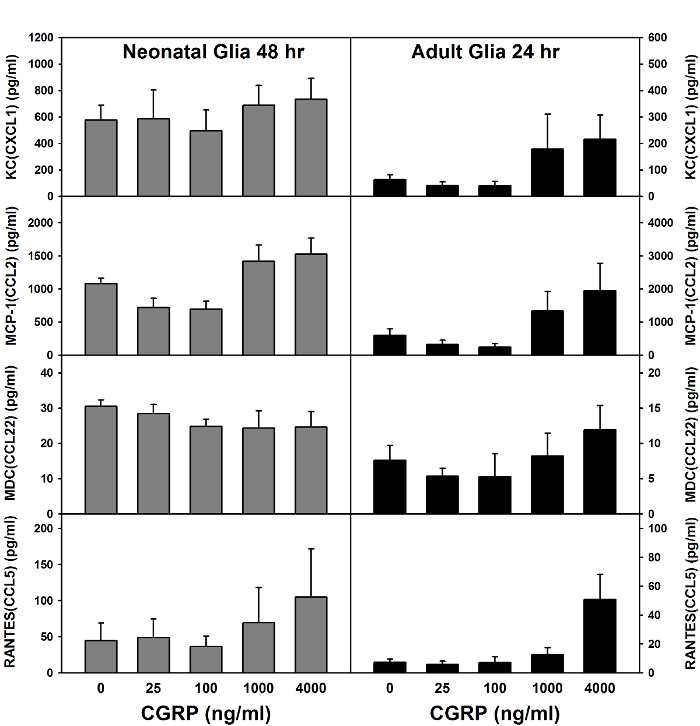
Figure 1: Calcitonin Gene-related Peptide (CGRP)-induced Chemokine Production by Mixed Glial Cells. Mixed glial cells prepared from neonatal BALB/c mouse brains (left) or adult BALB/c mouse spinal cords (right) were treated with various doses of CGRP. Levels of several chemokines in culture supernatants were determined via multiplex assay (performed by the manufacturer) at optimal times (mean ± SEM, n = 2 – 6). Please click here to view a larger version of this figure.
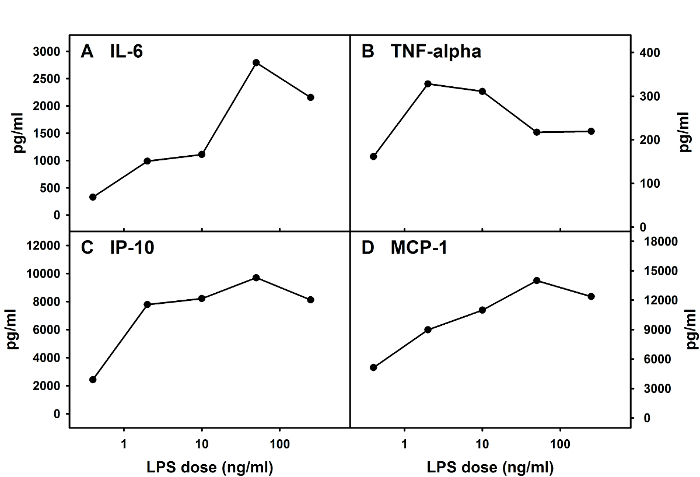
Figure 3: Cytokine and Chemokine Responses of Mouse Adult Spinal Cord Mixed Glia upon LPS Stimulation. Adult spinal cord mixed glial cells were prepared from BALB/c mice and stimulated with various doses of LPS. Levels of IL-6 (A), tumor necrosis factor (TNF)-alpha (B), interferon-gamma-inducible protein 10 (IP-10, also known as CXCL10) (C), and monocyte chemoattractant protein 1 (MCP-1, also known as CCL2) (D) in culture supernatants were determined via ELISAs at 24 h post-LPS treatment. Please click here to view a larger version of this figure.
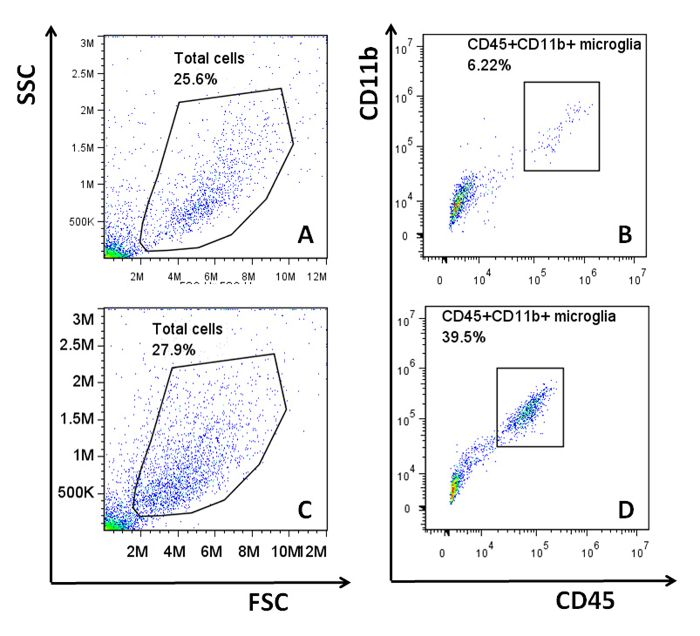
Figure 4: Microglial Content in Adult Mixed Glial Cultures. Mixed glial cells were generated from adult C57B6/L mice and cultured at either 37 oC or 35.9 oC. Microglial content from each culture was analyzed via flow cytometry using APC-anti-mouse-CD45 (clone 30-F11) and FITC-anti-mouse-CD11b (clone M1/70). Representative plots from the flow cytometric analyses are shown. The total cell populations from the cultures were identified in plots A (37 oC) and C (35.9 oC), and the microglial populations (CD45+CD11b+ cells) were further isolated from the respective total cell populations in B (37 oC) and D (35.9 oC). Flow cytometric analysis was performed, and all data were analyzed as previously described5. Please click here to view a larger version of this figure.
Discussion
It is critical to perform all steps-including the media changing schedule-in a consistent manner in order to obtain reproducible results between experiments. The following steps are particularly critical in obtaining excellent quality adult mixed glia.
The enzymatic digestion is an important aspect of this protocol. It is crucial that the tissue pieces are well-digested in order to obtain a single cell suspension. However, over-digestion will result in significantly fewer cells. Each lab needs to perform pilot tests to determine the exact digestion time based on the types of vials used for incubation, the average tissue amount used each time, and the type of shaker (rotating versus orbital shaker). Furthermore, regardless of the papain dissociation system chosen, it is essential to consistently use the same components from the same sources, as the occasional substitution of components could lead to significant variations in the yield of total number of cells.
In this protocol, the myelin is removed using a density gradient medium. It is critical to make and use density gradient solutions at RT since the densities of these solutions are temperature-sensitive. Density gradient solutions are usually stored at 4 oC. If necessary, the density gradient solutions can be kept at RT overnight before the day of setting up the culture. In addition, following the 30 min centrifugation of cells in the 20% density gradient, cells should be removed immediately from the centrifuge to prevent large pieces of debris from shifting (i.e., moving away from the top of the solution; see step 4.1).
It is important to remove debris on D 1 post initiation of the culture. Although cells will survive and proliferate if the culture media is changed every 3 to 4 d, changing media on D 1 will provide a more "quiescent" and "cleaner" culture later on. This allows cells to grow in a less disturbed environment, and cells can be kept up to 21 d post-initiation of the culture.
Microglial content for each experiment can be examined prior to using the mixed glia. Even with the most consistent technique, microglial content may still vary significantly between experiments. Some cellular effects are dependent on microglial content4, 10; thus, it is crucial to monitor the microglial content of each set of cultures established. Data obtained from this practice can help to interpret variations between experiments, as well as to provide novel (sometimes unexpected) knowledge regarding the contribution of astrocytes versus microglia under a particular experimental condition. In addition, seasonal variations on microglial content have been observed. This may be an additional factor to consider when planning large sets of experiments. Furthermore, while neurons usually will not survive under our culture condition, as NeuN (a neuronal- marker)-positive cells have not been observed in our glial cultures after immunohistochemical staining, the culture may contain limited numbers of oligodendrocytes (this has not been routinely quantified). One should decide whether this population needs to be examined depending on the specific experimental conditions.
As with many experimental methods, this method can be modified according to the needs of individual researchers. It is essential to perform pilot experiments to fully test the specific modifications prior to using them routinely. Furthermore, it should be noted that the mixed glia can be used to obtain microglia-depleted and microglia-enriched cultures to study the responses of astrocyte-dominant versus microglia-dominant cells after specific stimulations, as previously described for neonatal mixed glial cells3, 4. However, the yield of microglia-enriched cells will be limited unless large numbers of spinal cords are used to establish the culture at the beginning. This is a limitation of this method, particularly if mice are used. When rat spinal cords are used, one individual spinal cord (instead of 4 mouse spinal cords) can be used to set up one 12-well plate. Finally, the microglial content does not appear to be significantly different between mouse and rat adult spinal cord glial cultures4, 5. Both mouse and rat adult spinal cord glial cultures produce a robust response to LPS stimulation in terms of cytokine production; however, differential responses may be observed when alternative stimuli are used.
Altogether, this rodent adult glial culture system provides an alternative method to study glial cells in vitro. Results obtained from this culture system more closely reflect what would be observed under in vivo conditions compared to results obtained from neonatal cultures or cell lines. In the current method, glial cells are collected from adult rodents, as adult glia respond to stimuli very differently when compared to neonatal glia4 (Figure 1). The cells are not manipulated significantly through immortalization procedures, which would be used to generate and maintain cell lines. Although this method was initially developed to be used in neuropathic pain studies, it can be used to study other neurological diseases that involve pathological changes within the spinal cord, such as ALS and MS.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Adult spinal cord mixed cultures were first developed using rats with grant support from an NIH/NIDA R01 award (PI De Leo) and an NIH T32 training grant (PI Green). These methods were further adapted for mouse spinal cords with support from the following grants: NIH/NIDA 5K01DA023503 (PI Cao), NIH/NINDS 5R21NS066130 (PI Cao), and NIH/NIGMS P20GM103643 (PI Meng). The authors would also like to thank Dr. Alejandro M. S. Mayer, Department of Pharmacology, Chicago College of Osteopathic Medicine, for his technical help in obtaining microglia-rich mixed glial cells.
Materials
| Dulbecco’s modification of Eagle’s media (DMEM) with 4.5g/L glucose | Lonza | 12-709F | The cDMEM media is a standard, widely used culture media. Individual researcher can decide where to purchase the DMEM and all other component used to make cDMEM media. |
| L-Glutamine (100x) | Lonza | 17-605E | The L-glutamine is a standard, widely used component for various culture media. Individual researcher can decide where to purchase L-glutamine. |
| Antibiotic-Antimycotic Solution (100x) | Corning-Mediatech | 30-004-CI | This is a combination of penicillin, streptomycin and Amphotericin formulated to contain 10,000 units/ml penicillin G, 10 mg/ml streptomycin sulfate and 25 µg/ml amphotericin B. Individual researcher can decide where to purchase individual component. |
| 2-mercaptoethanol (2-ME) | Sigma-Aldrich | M3148 | A BioReagent, suitable for cell culture, molecular biology and electrophoresis. |
| Papain dissociation system | Worthington Biochemical Corporation | LK003150 | Individual components of this kit can be purchased separately. |
| Percoll | GE Health Care | 17-0891-01 | Percoll is sold as sterile solution. Undiluted Percoll can be re-autoclaved if needed. |
| Lab-line incubator/shaker | Barstead/Lab-line | MaxQ4000 | This is the incubator/shaker we have currently. Other types of shakers can be used instead. |
| Lipopolysaccharides, Salmonella Minnesota Re595 | Sigma-Aldrich | L-9764 | Other strains of LPS can also induce glial responses. The magnitude of the responses may vary. |
| Mouse IL-6 DuoSet ELISA | R&D Systems | DY406 | Each individual Researcher can select the ELISA kit he or she prefers. |
| Mouse TNF-alpha DuoSet ELISA | R&D Systems | DY410 | Each individual Researcher can select the ELISA kit he or she prefers. |
| Mouse IP-10 (CXCL10) DuoSet ELISA | R&D Systems | DY466 | Each individual Researcher can select the ELISA kit he or she prefers. |
| Mouse MCP-1 (CCL2) ELISA Opteia set | BD Biosciences | 555260 | Each individual Researcher can select the ELISA kit he or she prefers. |
| Mouse chemokine Q-Plex | Quansys Biosciences | 120251MS | This product was available for in-house assay at the time. Instead, we sent out samples to the manufacturer for analysis. |
| APC-anti-mouse-CD45 (clone 30-F11) | eBioscience | 17-0451 | Antibody of the same clone but from other vendors can be used. |
| FITC-anti-mouse-CD11b (clone M1/70) | eBioscience | 11-0112 | Antibody of the same clone but from other vendors can be used. |
| Accuri C6 flow cytometer | BD Biosciences | BD Accuri C6 | Each individual Researcher can use any flow cytometer he or she prefers. |
| FlowJo software | Tree Star, Inc. | FlowJo7.6.5 | Each individual Researcher can use any analysis software he or she prefers. |
Referenzen
- Gaskin, D. J., Richard, P. The economic costs of pain in the United States. J Pain. 13 (8), 715-724 (2012).
- Ji, R. R., Berta, T., Nedergaard, M. Glia and pain: Is chronic pain a gliopathy?. Pain. 154 (01), S10-S28 (2013).
- Ni, M., Aschner, M., Maines, M. D. Neonatal rat primary icroglia: isolation, culturing and selected applications. Curr Protoc Toxicol. , (2010).
- Cao, L., Fei, L., Chang, T. T., DeLeo, J. A. Induction of interleukin-1beta by interleukin-4 in lipopolysaccharide-treated mixed glial cultures: microglial-dependent effects. J Neurochem. 102 (2), 408-419 (2007).
- Malon, J. T., Maddula, S., Bell, H., Cao, L. Involvement of calcitonin gene-related peptide and CCL2 production in CD40-mediated behavioral hypersensitivity in a model of neuropathic pain. Neuron Glia Biol. 7 (2-4), 117-128 (2011).
- Blasi, E., Barluzzi, R., Bocchini, V., Mazzolla, R., Bistoni, F. Immortalization of murine microglial cells by a v-raf / v-myc carrying retrovirus. J Neuroimmunol. 27 (2), 229-237 (1990).
- Bocchini, V., et al. An immortalized cell line expresses properties of activated microglial cells. J Neurosci Res. 31 (4), 616-621 (1992).
- Alliot, F. O., Marty, M. C., Cambier, D., Pessac, B. A spontaneously immortalized mouse microglial cell line expressing CD4. Dev Brain Res. 95 (1), 140-143 (1996).
- Alliot, F. O., Pessac, B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 306 (1-2), 283-291 (1984).
- Malon, J. T., et al. Microglial content-dependent inhibitory effects of calcitonin gene-related peptide (CGRP) on murine retroviral infection of glial cells. J Neuroimmunol. 279, 64-70 (2015).
- Cao, L., DeLeo, J. A. CNS Infiltrating CD4(+) T lymphocytes Contribute to Murine Spinal Nerve Transection-Induced Neuropathic Pain. Eur J Immunol. 38 (2), 448-458 (2008).
- Mayer, A. M., et al. Effect of a short-term in vitro exposure to the marine toxin domoic acid on viability, tumor necrosis factor-alpha, matrix metalloproteinase-9 and superoxide anion release by rat neonatal microglia. BMC Pharmacol. 1, 7 (2001).
- Lawson, L. J., Perry, V. H., Dri, P., Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neurowissenschaften. 39 (1), 151-170 (1990).
- Rock, R. B., et al. Role of Microglia in Central Nervous System Infections. Clin Microbiol Rev. 17 (4), 942-964 (2004).
- Yang, I., Han, S. J., Kaur, G., Crane, C., Parsa, A. T. The Role of Microglia in Central Nervous System Immunity and Glioma Immunology. J Clin Neurosci. 17 (1), 6-10 (2010).

