Controllable Ion Channel Expression through Inducible Transient Transfection
Summary
Studying ion channels through a heterologously expressing system has become a core technique in biomedical research. In this manuscript, we present a time efficient method to achieve tightly controlled ion channel expression by performing transient transfection under the control of an inducible promoter.
Abstract
Transfection, the delivery of foreign nucleic acids into a cell, is a powerful tool in protein research. Through this method, ion channels can be investigated through electrophysiological analysis, biochemical characterization, mutational studies, and their effects on cellular processes. Transient transfections offer a simple protocol in which the protein becomes available for analysis within a few hours to days. Although this method presents a relatively straightforward and time efficient protocol, one of the critical components is calibrating the expression of the gene of interest to physiological relevant levels or levels that are suitable for analysis. To this end, many different approaches that offer the ability to control the expression of the gene of interest have emerged. Several stable cell transfection protocols provide a way to permanently introduce a gene of interest into the cellular genome under the regulation of a tetracycline-controlled transcriptional activation. While this technique produces reliable expression levels, each gene of interest requires a few weeks of skilled work including calibration of a killing curve, selection of cell colonies, and overall more resources. Here we present a protocol that uses transient transfection of the Transient Receptor Potential cation channel subfamily V member 1 (TRPV1) gene in an inducible system as an efficient way to express a protein in a controlled manner which is essential in ion channel analysis. We demonstrate that using this technique, we are able to perform calcium imaging, whole cell, and single channel analysis with controlled channel levels required for each type of data collection with a single transfection. Overall, this provides a replicable technique that can be used to study ion channels structure and function.
Introduction
Heterologously expressing systems is one of the most widely used techniques to study a multitude of cellular functions1. Their low endogenous protein profile, minimal maintenance requirements, reliable growth, and ability to take up and express foreign DNA have made cell lines such as Human Embryonic Kidney (HEK293) and Chinese Hamster Ovary (CHO) almost essential to biological research2,3. Areas of research using heterologous systems include membrane proteins, intracellular signaling, and enzymatic activity. Following transfection of foreign DNA into the cell, many different forms of analysis can be performed, including electrophysiology, ratiometric calcium imaging, western blot, etc.4,5.
Due to the wide array of potential applications for heterologous expression systems, many different reagents and products have been developed to utilize these cells and their qualities6. DNA delivery systems that transiently or permanently integrate foreign DNA into cells to study exogenous protein has become one of the most popular and useful tools for biological research. More specifically, transiently transfecting DNA into a cell is widely used as a simple, straight forward process that requires relatively little time and materials. Furthermore, the success rate of cells that undergo transfection is high7. This technique is very reliable when combined with a marker gene such as green fluorescent protein (GFP), and can be used for many different techniques such as calcium imaging and electrophysiology5. Unfortunately, though, transiently expressing DNA into host cells comes with some major pitfalls, not in the least that the expression level per cell is unreliable. The number of copies of plasmid DNA taken up per cell is uncontrollable, thus the expression between individual experiments can vary greatly2. This issue becomes significant when either trying to replicate physiological conditions, or performing precise data collection techniques.
As a solution to the complications mentioned above, stable transfection protocols have been designed in which a gene of interest can be inserted into the genome of a cell under the tight control of an inducible promoter, such as a tetracycline repressor expression system, ensuring a single copy of the plasmid integrates into the genome of each cell and is only expressed after induction of the transcription mechanism, for example, in the presence of doxycycline. While this solves the obstacles of inconsistent protein expression levels, this method loses the convenience of quick and relatively simple protocol of transient transfections. Establishing a stable cell line takes at least a few weeks in which one must calibrate a killing curve set by specific antibiotics to maintain the protein expression and ensure integration of the vector and skillfully select and grow cell colonies. Overall this takes significantly more time and effort with a lower success rate8.
Here, we introduce an intermediate protocol that draws on the strengths of both of the popular transfection options to provide a simple and effective way to control expression levels in any inducible cell line. While maintaining cells with an inducible tet system, we transiently transfect our gene of interest, Transient Receptor Potential cation channel subfamily V member 1 (TRPV1), ligated into a vector that can homologously combine with the repressor system. In this way, the gene can be introduced into the cells without beginning to express. Only with the addition of doxycycline does the gene begin to express, allowing us to calibrate the levels of protein expression according to the technique or levels observed in physiological conditions. Our protocol also avoids lengthy complications associated with generating a stably expressing cell line. We begin by showing the changing levels of TRPV1 activation in calcium imaging from un-induced through four hours of induction and how the rise in intracellular calcium levels correlates. We then duplicate the protocol in the whole cell configuration of the patch clamp technique, showing the increasing current with increasing time of induction. Finally, we present examples of single channel electrophysiology recordings, and show that this technique is especially useful for controlled expression when looking for precise data collection based on individual units of the protein. Through our protocol, we offer a convenient way to control protein expression in heterologous systems while avoiding lengthy cell culture complications, thus providing a way to control conditions between experiments and provide more replicable results.
Protocol
1. Ligating the Gene of Interest into the Repressible Site of Vector
- Obtain an inducible vector such as pcDNA5/FRT/TO or pcDNA4/TO.
- Analyze the gene of interest for any potentially susceptible restriction sites in the middle of the gene that are also featured in the multiple cloning site of the chosen vector by in silico DNA analysis software4.
- Using standard overlap PCR techniques4, flank the gene of interest with two selected restriction enzyme recognition sites (that are not found in the gene of interest), the first inserted before the start codon, and the second after the stop codon.
- Digest both the vector and insert with restriction enzymes (chosen in step 1.2) at enzymes' working temperature for one hour, followed by addition of one unit calf intestine phosphatase (CIP) to the vector reaction only for 30 minutes in order to avoid self-ligation.
- Load the digested DNA onto a 1% agarose gel and separate the cleaved segments by electrophoresis9.
- Using UV light, cut with a blade the pieces of gel containing the desired segments of vector and insert to be ligated.
- Extract the DNA segments from the agarose gel pieces (using commercial available DNA extraction kits) and measure their final concentration by spectrophotometer at 260 nm.
- Ligate the gene of interest into the inducible selected vector using T4 ligase enzyme at room temperature for 20 minutes. In order to increase the probability of the insert ligating with the vector, use a 3:1 molar ratio of the insert: vector. For control, prepare a ligation reaction that includes only the vector.
- For bacterial transformation, add 10 ng of vector alone and vector + gene into 50 μL competent E. coli. Incubate the tube on ice for 30 minutes followed by 45 seconds incubation at 42 °C. Immediately place back on ice for two minutes and transfer the transformed bacteria to 500 μL LB medium. Incubate for one hour at 37 °C under agitation at 220 rpm.
- For selection of successfully transformed bacteria, plate 100 μL of each reaction (as described in 1.9) on a prepared LB agar plate containing the suitable antibiotic (e.g., 100 μg/mL ampicillin when using pCDNA4/TR).
- Allow to grow overnight at 37 °C. Plates may be stored at 4 °C for up to two weeks by tightly wrapping them in plastic paraffin film.
- Lift individual colonies using a 10 μL tip, and allow to grow overnight in 3 mL LB + antibiotic medium at 37 °C under agitation at 220 rpm. Bacteria with DNA of interest may be frozen (-20 °C) for short term storage by centrifuging the LB and bacteria at 11,000 x g and aspirating out the supernatant. Long term storage requires freezing the bacteria as glycerol stocks at -80 °C.
- Extract DNA through mini-prep and measure the final concentration by spectrophotometer at 260 nm5.
- Confirm successful insertion of gene of interest by sequencing the purified construct4. Alternatively, diagnostic digestion of the construct by restriction enzymes using electrophoresis to separate the cut segments and evaluate their presence and length could also be utilized to this end4.
2. Culturing Cell Lines Expressing TetR
- Obtain a cell culture line harboring a Tet repressor plasmid incorporated into their genomic DNA. Herein the protocol will be written using Human embryonic kidney 293T cells (HEK-293T) harboring pcDNA6/TR plasmid as an example.
- Seed cells in 100 mm tissue culture plate with Dulbecco's Modified Eagles Medium (DMEM) supplemented with 10% FBS, 1% Penicillin-Streptomycin, 2 mM l-Glutamine, and 25 mM HEPES, pH 7.3 (herein: Full DMEM) and incubate O/N at 37 °C and 5% CO2. All work with cells should be done in a biological hood under sterile conditions.
- In order to maintain the expression of Tet repressor gene, aspirate the medium and replace it with Full DMEM supplemented with 5 µg/mL blasticidin. Incubate cells at 37 °C and 5% CO2.
- Once cells reach 80 – 90% confluency, aspirate the medium and gently wash the cells twice with DPBS (without calcium and magnesium) warmed to 37 °C.
- In order to gently lift the cells without causing mechanical damage, incubate the culture in DMEM containing 0.05% trypsin warmed to 37 °C for 1.5 min.
- Block the trypsin action with an equal volume of Full DMEM. Gently pipette up and down in order to lift the cells and transfer them to a sterile tube.
- Centrifuge the cells at 200 x g for 5 min.
- Aspirate the supernatant and replace it with 1 mL Full DMEM. Pipette the solution with the cells up and down until no cell clumps can be seen. Count and calculate the number of cells using a hemocytometer.
- Seed 1 – 2 x 106 cells in 100 mm tissue culture dish containing 12 mL Full DMEM with 5 µg/mL blasticidin. Split the cells twice a week as they reach 90% confluency.
3. Transfecting the Plasmid of Interest into Cells
- Using the same method of splitting cells as described above, transfer cells to wells in a 12-well plate prepared with 0.9 mL Full DMEM, sufficient to make 50% confluent (~200,000 cells) and incubate overnight at 37 °C in a CO2 incubator.
- Prepare a transfection mixture with pcDNA4/TO containing the gene of interest, an inert plasmid to bring the total DNA amount to 1 µg (if needed), 3 µL lipid transfection reagent and DMEM to make the final volume 100 µL. The optimal amount of plasmid DNA to be used varies widely as different proteins express in different efficiency. For electrophysiological experiments, include EGFP in mammalian expression plasmid in the transfection cocktail in order to visualize cells that successfully undergo transfection4.
- Incubate the transfection mixture at RT for 30 min.
- Transfect cells by pipetting the transfection mixture drop-wise onto the plated cells in the 12-well plate and rock the plate vigorously to ensure a homogenous dispersion of DNA and transfection reagent. Cells should be ~80% confluent at the time of transfection.
- Incubate the transfected cells O/N at 37 °C and 5% CO2.
- Confirm that the cells were successfully transfected by observing the fluorescence signal of EGFP under UV lamp the following morning if performing electrophysiology.
4. Plating Cells on Poly-D-Lysine (PDL) Coverslips/Wells
- Sterilize 12 mm coverslips by dousing with 70% isopropyl ethanol solution. Dry and place a single coverslip in each well of a 24-well plate for electrophysiological recording.
- Pipette a solution of 0.1 – 0.2 mg/mL PDL solution onto each coverslip or well of a calcium imaging chamber.
- Allow to sit for 30 min at 37 °C in a 5% CO2 incubator.
- Wash with cell culture grade double distilled water (DDW) three times, aspirating between each wash. Following the final wash, dry thoroughly and allow to sit at RT.
- For electrophysiology, fill each well containing a PDL coated coverslip with 500 µL Full DMEM warmed to 37 °C.
- Perform the same method of splitting the transfected cells as described above. After resuspending the cells following trypsin treatment and blocking, transfer 80 µL of cells (or approximately 30,000 cells) to the center of each PDL coated coverslip for electrophysiology recording. Allow cells to settle for at least 1.5 hours or incubate overnight at 37 °C in a 5% CO2 incubator.
- For calcium imaging transfer and spot 20 µL of cells (approximately 20,000 cells) to the center of each PDL coated calcium imaging well. Allow cells to settle for at least 30 min and add 180 µL full DMEM to each well.
5. Inducing Gene Expression
- Prepare a doxycycline stock solution of 1 mg/mL in DDW according to manufacturer's instructions. Keep stock solution protected from light at 4 °C for up to 3 weeks. For long term storage keep doxycycline stock solution at -20 °C.
- Prepare a fresh 2 µg/mL (electrophysiology) or 3 µg/mL (calcium imaging) doxycycline solution in Full DMEM and warm it to 37 °C.
- For electrophysiological recordings, pipette 500 µL of 2 µg/mL doxycycline solution to each well to make a final concentration of 1 µg/mL doxycycline. For calcium imaging, add 100 µL of 3 µg/mL doxycycline solution to each well to make a final concentration of 1 µg/mL doxycycline. Write down the hour of induction.
- Incubate for the desired amount of time for induction at 37 °C and 5% CO2.
6. Calibrating Timeline of Protein Expression
- Calibrating protein expression through Calcium Imaging5,10
- Prepare Ringer's solution (140 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 2 mM MgSO4, 20 mM HEPES, pH adjusted to 7.4 with NaOH) and warm it to 37 °C.
- Prepare saturating concentrations of channel agonist in Ringer’s solution. Because the amount of agonist is diluted when added to the calcium imaging chambers containing the cells and extracellular solution, double the amount of the desired final concentration of agonist. This also makes the amount of bath solution in each well critical to control.
- Prepare a solution of non-ionic F-127 20% W/V in DMSO. Heat the solution to 40 °C until non-ionic F-127 is dissolved. Store the non-ionic F-127 solution at RT and warm it to 40 °C before use. Non-ionic F-127 is used to help disperse acetoxymethyl (AM) esters of fluorescent ion indicators such as Fura-2 in aqueous solutions.
- Aspirate medium and replace it with Fura-2AM loading solution (Ringer's solution supplemented with 2 – 3 µM Fura-2AM, 0.02 mg/mL non-ionic F-127 and 10 mM D-glucose).
- Incubate for 60 min at RT in the dark.
- Aspirate Fura-2AM solution and wash cells with Ringer's + 10 mM D-glucose solution to remove extracellular dye. Repeat this step twice.
- Leave 200 µL Ringer's + 10 mM D-glucose solution in each well and incubate for 30 min at RT in the dark.
- Place chamber on stage above the microscope nose piece and secure it with holder.
- Turn on the lamp, camera and microscope and select Fura-2 filter to detect emission at 510 nm wavelength.
- Adjust for the desired magnification and focus cells in the selected field.
- In the dark, subtract background light and set exposure time.
- Set picture sampling at the desired rate and record fluorescence response of Fura-2 loaded cells by exciting them at 340 and 380 nm wavelengths. The ratio of 340/380 signals is an indicator for intracellular Ca2+ concentration and thus also for TRPV1 activity.
- Add 2 µM capsaicin in 200 µL Ringer's solution by pipetting to create a final saturating concentration of 1 µM capsaicin in order to evaluate the expression level of TRPV1.
- Analyze TRPV1 response according to induction time and determine protocol for the protein of interest.
- Adjusting protein expression in heterologous systems using the whole cell configuration of the patch clamp technique4,11.
- Prepare the bath solution of (mM) 140 NaCl, 2.3 KCl, 2 MgSO4, 5 HEPES, and 5 2-(N-morpholino) ethanesulfonic acid (MES), adjusted to pH 7.4 with NaOH.
- Prepare saturating concentrations of channel agonist in extracellular solution.
- Pull glass electrodes with an inner diameter (I.D) of 1.10 mm and fire-polish to a resistance of 2 – 4 MΩ.
- Prepare a pipette solution of (mM) 130 KCl, 4 NaCl, 2 MgSO4, 0.5 CaCl2, 1 EGTA and 10 HEPES adjusted to pH 7.2 with KOH. Filter the pipette solution prior to use. Before each experiment, a single, fire-polished glass pipette is back filled by submerging the tip in the pipette solution for at least a minute, and then filled using melted and stretched tip with a syringe filled with the same pipette solution.
- Gently lift a single PDL coated coverslip with the plated cells from the well to a 35 mm plastic petri dish filled with approximately 2 mL bath solution at RT. Observe under an epi-fluorescent microscope.
- Locate an EGFP positive cell, as visualized with the epifluorescent microscope, on the prepared coverslip and move to the center of the visualized field.
- Attach the filled pipette to the pipette holder of the micromanipulator, and inject a small amount of positive pressure into the system to avoid contamination of the intracellular pipette solution. Lower it to just above the GFP – positive cell, checking for proper resistance.
- As the pipette touches the cell, apply a small amount of negative pressure in order to create a GΩ seal between the glass pipette and cell membrane.
- With a short, sharp pulse of suction, break the cell membrane, allowing the pipette to come into contact with the intracellular contents of the cell.
- Switch the amplifier mode to whole cell, and lower the Bessel filter and output gain to 1 – 5 kHz and 0.5α, respectively.
- Record the current response to the saturating concentrations of channel agonist by using voltage ramps or gap free protocol.
- Because expression efficiencies change between different proteins, repeat the recordings using different induction length times.
- Determining ideal induction time for single channel recordings4.
- Prepare a solution of (mM) 150 Na-gluconate, 15 NaCl, 5 EGTA, and 10 HEPES, adjusted to pH 7.4 with NaOH, as a pipette solution. For the bath solution, use the solution from step 6.2.1.
- Repeat whole cell procedure using I.D. 0.86 mm glass pipettes fire-polished to a resistance of 10 – 12 MΩ and set holding potential to -40 mV.
- Create a seal on the membrane as described previously and rupture the membrane.
- Using the micromanipulator, lift the pipette with the membrane patch away from the cell.
- Position the perfusion system directly next to the pipette containing the membrane patch.
- Lower the Bessel filter and output gain to 2 – 5 kHz and 10α, respectively.
- Perfuse saturating concentrations of agonist onto the membrane patch, evaluating if the patch contains one or more channels according to the response.
- Analyze the number of single channel patches successfully recorded against the induction time applied to cells. Adjust the induction time according to the desired results.
Representative Results
To quickly create an inducible expression model, we made use of HEK293 cells that express Tetracycline Repressor protein (TR) (e.g., T-REx-293) and vectors that contain tetracycline operator sequences (TetO) between the CMV promoter and the multiple cloning site (e.g., pcDNA4/TO). When transfected into TREx-293 cells, the expression of the gene of interest in pcDNA4/TO is repressed, as TR binds to TetO. Adding doxycycline to the medium prevents TR-TetO interaction, thus allowing the expression of the transfected protein. To control the expression of rat TRPV1 (rTRPV1) we first inserted this channel's gene into a pcDNA4/TO vector using the above-described protocol. Next, T-REx-293 cells were transfected with the rTRPV1- pcDNA4/TO plasmid according to the steps in point 3 of the protocol. Following transfection, rTRPV1 expression was induced by incubating the cells in the presence of doxycycline (1 μg/mL) for 1 – 4 h. Importantly, different doxycycline concentrations can be used to achieve desired expression rate. We used electrophysiological and calcium imaging methods to evaluate rTRPV1 expression level at each time point by applying its agonist, capsaicin. As shown in Figure 1, using live-cell calcium imaging, intracellular calcium level after capsaicin application is increased gradually with longer induction times. Activation of uninduced cells may be attributable to a basal leak in the TR activity, overexpression of the plasmid, or residual tetracycline in the cells media components. Next, we recorded currents from cells using the whole-cell configuration of the patch clamp technique applying voltage ramps between −80 mV to +80 mV. Figure 2 shows that higher current amplitudes are obtained with longer induction times. The saturation in current amplitude at 4 h induction is consistent with the saturation seen in calcium response (Figure 1). Finally, we analyzed rTRPV1 expression levels in the outside-out configuration of the patch-clamp technique. One of the major difficulties in studying ion channel structure-function is reaching expression levels suitable for single-channel analysis. Based on the published unitary rTRPV1 conductance4, the recorded current amplitude was used to determine the number of channels in the excised patch. As shown in Figure 3, the number of channels in the patch increases proportionately to the doxycycline incubation time. After one hour of induction, we were not able to detect any channel activity (0 out of 8). However, in both two- and three-hour time points we recorded single channel activity in similar success rates. Of note, while in two hours most patches did not respond, in three hours most patches showed single-to-multi -channel activity. The chances of recording multiple channels after three hours of induction is high, thus the optimal induction time to record current from a single channel is between two to three hours under the presented conditions. Together these results demonstrate that expression of ion channels can be tightly controlled and regulated after transient transfection using this protocol.
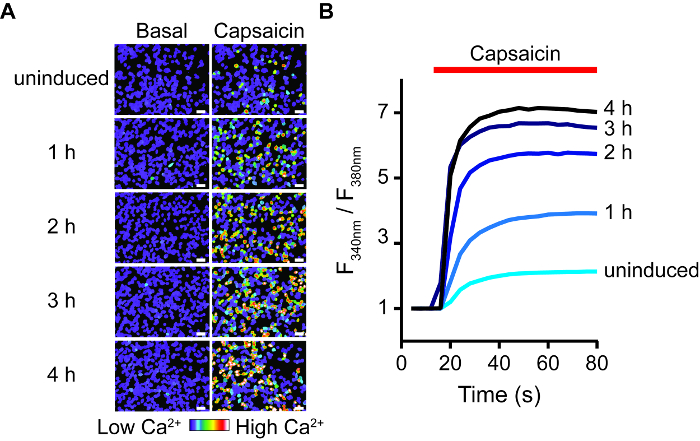
Figure 1. Response of TRPV1 Activation Increases according to Induction Time, as Visualized through Calcium Imaging. (A) Pseudo-colored images of T-REx-293 cells transiently expressing rTRPV1, before ('Basal') and after capsaicin (2 μM) application. White bars represent 30 μm. Scale bar indicates levels of intracellular calcium. (B) Changes with time of intracellular calcium levels in transfected T-REx-293 cells treated as shown in A. Each graph represents an average of 50 capsaicin sensitive cells. Note the stepwise increases in capsaicin responses in relation to induction time. Please click here to view a larger version of this figure.
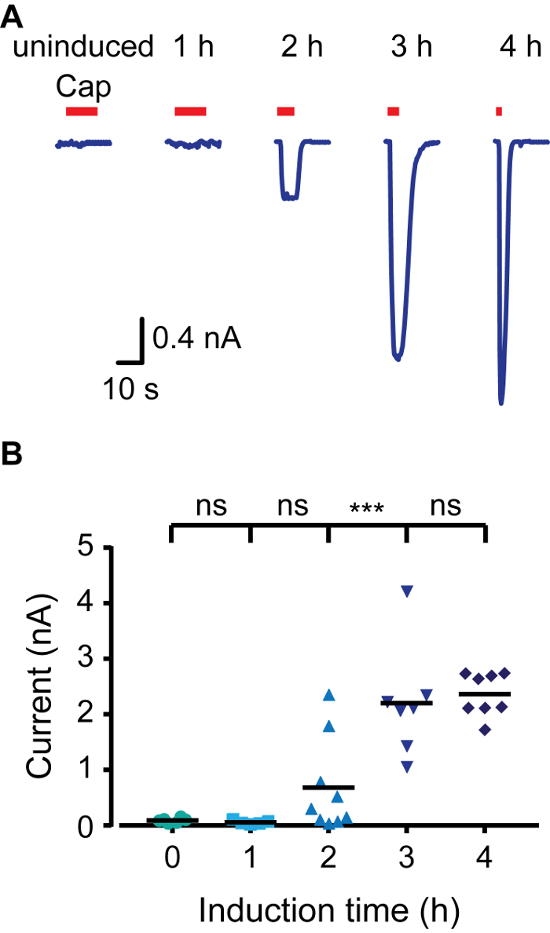
Figure 2. TRPV1 Current Increases as a Result of Increasing Induction Time in Whole Cell Patch Clamp Recordings. (A) Whole-cell recordings from T-REx-293 transiently transfected with rTRPV1 at a holding potential of -40 mV. Cells were induced with doxycycline (1 μg/mL) for the indicated time and then exposed to capsaicin ('Cap'; 1 μM; red bar). Shown is a representative trace of 6 – 11 independent recordings. (B) Mean/scatter-dot plot representing the whole-cell amplitude evoked as shown in A. Statistical significance between groups was determined with ANOVA with multiple comparisons, where *** represents p ≤0.001 and ns- not statistically significant (n = 6 – 11 cells). Please click here to view a larger version of this figure.
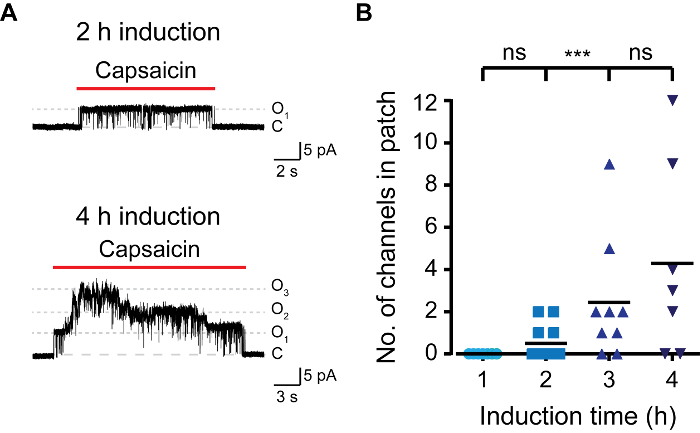
Figure 3. Success Rate of Single Channel Patch in TRPV1 Recordings is Dependent on Induction Time. (A) Outside-out recordings from T-REx-293 transiently transfected with rTRPV1 at a holding potential of +50 mV. Cells were induced with doxycycline (1 μg/mL) for the indicated time and were exposed to capsaicin ('Cap'; 1 μM; red bar). Shown is a representative trace of 6 – 9 independent recordings. (B) Mean/scatter-dot plot representing the number of channels in the excised patch as shown in A. Statistical significance between groups was determined with ANOVA with multiple comparisons, where *** represents p ≤0.001 and ns- not statistically significant (n = 6 – 9 cells). Please click here to view a larger version of this figure.
Discussion
Transfection is a widely used protocol for protein expression and research, with many different variations to improve expression consistency and stability. Transient transfection reagents offer a simple, easy to use protocol where the cell and protein of interest can be analyzed within hours to overnight from the time of transfection. Unfortunately this approach can be unpredictable when the mode of analysis requires a consistent level of protein expression, such as single channel recordings in electrophysiology2. Alternatively, stable cell lines under the tetracycline repressible expression system have been developed to offer a way to control the expression level of the protein of interest12. This approach provides consistent expression of all cells in a culture with the introduction of a tetracycline derived supplement, such as doxycycline. While this allows for consistent and precise control of protein levels, the time and effort that go in to establishing a stable cell line makes it inconvenient and disadvantageous when studying a wide variety of proteins8.
The protocol presented here offers a middle-of-the-road solution to the current transfection protocols offered. By transiently transfecting a gene under a controllable promoter into cells possessing a Tet repressor, we can achieve controllable expression while avoiding lengthy and complicated procedures. Here, we used the non-selective cation channel TRPV1 with calcium imaging and electrophysiology analysis. We found that the ideal induction time for the controlled expression of this specific protein for single channel analysis was between 2 – 3 h incubation with 1 μg/mL of doxycycline (Figures 1 – 3). On a broader scale, this technique can also be applied to any protein of interest. This protocol also offers a solution to controlling expression when a large range of different proteins or mutations in protein sequences are of interest and creating a stable cell line for each variant is impractical. Importantly, while the results here represent the optimal time and concentrations applicable for TRPV1 expression, each gene has its individual and specific translation and trafficking pace, thus the calibration and expression times of each protein will differ greatly13.
While this technique offers a convenient solution to controlled expression, it is not without limitations. Only commercially available cell lines harboring the Tet system can be used with this system, which presents a problem if these particular cell lines are unsuitable for the type of analysis that is to be performed. Another pitfall compared to stably transfected lines is that not all cells undergo transfection, and thus not all cells express the protein of interest. Overall, we offer a convenient way to control protein expression in a heterologous system that can be applied to a wide array of biomedical research.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Israel Science Foundation [Grants 1721/12, 1368/12, and 1444/16] (to A.P). A.P. is affiliated with Brettler Center and David R. Bloom Center, School of Pharmacy, The Hebrew University of Jerusalem.
Materials
| pcDNA™4/TO Mammalian Expression Vector | ThermoScientific Fisher | V102020 | |
| pcDNA™5/TO Mammalian Expression Vector | ThermoScientific Fisher | V103320 | |
| PureLink Quick PCR Purification Kit | Invitrogen | K310001 | |
| Swift™ MaxPro Thermal Cycler | Esco | n.a | |
| Restriction Enzymes | ThermoScientific Fisher | ER0501 | |
| Agarose | Lonza | 50004 | |
| PureLink Quick Gel Extraction Kit | Invitrogen | K210012 | |
| NanoDrop 2000c | ThermoScientific Fisher | ND-2000C | |
| T4 DNA Ligase | ThermoScientific Fisher | EL0011 | |
| One Shot® TOP10 Chemically Competent E. coli | ThermoScientific Fisher | C404006 | |
| Ampicillin (Sodium), USP Grade | Gold Bio | A-301-5 | |
| Tryptone for microbiology | Merck | 6.19305E+13 | |
| Yeast Extract | BD worldwide | 212750 | |
| SIF6000R Incubated Shaker | LAB COMPANION | 45H118 | |
NucleoSpin®plasmid |
Macherey Nagel | 740588.25 | |
| MS 300V Power Supply | Major Science | MP-300V | |
| Owl™ EasyCast™ B1A Mini Gel Electrophoresis System | ThermoScientific Fisher | B1A | |
| T-REx™-293 cell line | Invitrogen | R710-07 | |
| DMEM (1X), liquid (high glucose) | Gibco | 41965-039 | |
| HindIII-HF® | NEB | R3104S | |
| ApaI | NEB | R0114S | |
| CutSmart® Buffer | NEB | B7204S | |
| pcDNA™6/TR Mammalian Expression Vector | ThermoScientific Fisher | V102520 | |
| Fetal Bovine Serum, qualified, E.U.-approved, South America origin | Gibco | 10270106 | |
| HEPES Buffer Solution (1 M) | Biological Industries | 03-025-1B | |
| Penicillin-Streptomycin Solution | Biological Industries | 03-031-1B | |
| L-Alanyl-L-Glutamine (Stable Glutamine) (200 mM) | Biological Industries | 03-022-1B | |
| Heracell™ 150i CO2 Incubator | ThermoScientific Fisher | 51026406 | |
| MSC-Advantage™ Class II Biological Safety Cabinet | ThermoScientific Fisher | 51025411 | |
| Blasticidine S hydrochloride | Sigma-Aldrich | 15205-25MG | |
| Dulbecco’s Phosphate Buffered Saline Modified, without calcium chloride and magnesium chloride | Sigma-Aldrich | D8537-500ML | |
| Trypsin-EDTA (0.05%), phenol red | Gibco | 25300054 | |
| DOUBLE NEUBAUER RULED METALLIZED HEMACYTOMETER | Hausser Scientific | 31000 | |
| Opti-MEM I Reduced Serum Medium | Gibco | 31985070 | |
| TransIT®-LT1 Transfection Reagent | Mirus | MIR 2300 | |
| glass coverslips, #1 thickness, 12mm diameter round | Knittel Glass | GG-12 | |
| BioCoat™ Poly-D-Lysine | Corning | 354210 | |
| Water, Cell Culture Grade | Biological Industries | 03-055-1A | |
| Doxycycline hyclate | Sigma-Aldrich | D9891-1G | |
| Fura-2, AM ester | Biotium | BTM-50034 | |
| Pluronic® F-127 | Sigma-Aldrich | P2443-250G | |
| µ-Slide 8 Well | ibidi | 80826 | |
| (E)-Capsaicin | Tocris | 462 | |
| Olympus IX70 Fluorescence Microscope | Olympus | n.a | |
| Lambda DG-4 Wavelength Switcher | Sutter Instruments | n.a | |
| EXi Blue Fluorescence Microscopy Camera | QImaging | n.a | |
| MetaFluor Fluorescence Ratio Imaging Software | Molecular Devices | n.a | |
| Thin Walled Borosilicate Tubing | Sutter Instruments | B150-110-7.5HP | |
| Standard Walled Borosilicate Tubing | Sutter Instruments | B150-86-7.5HP | |
| Dimethyl sulfoxide anhydrous | Sigma-Aldrich | 276855 | |
| P1000 micropipette puller | Sutter Instruments | P-1000 | |
| MF-900 Microforge | NARISHIGE | n.a | |
| ValveBank perfusion sysytem | AutoMate Scientific | ||
| Digidata® 1440A Low-noise Data Acquisition System | Molecular Devices | n.a | |
| Axopatch 200B Amplifier | Molecular Devices | n.a | |
| pCLAMP 10.6 Software | Molecular Devices | n.a | |
| micromanipulator | Sutter Instruments | MP-225 |
Referenzen
- Ooi, A., Wong, A., Esau, L., Lemtiri-Chlieh, F., Gehring, C. A Guide to Transient Expression of Membrane Proteins in HEK-293 Cells for Functional Characterization. Frontiers in Physiology. 7, 300 (2016).
- Thomas, P., Smart, T. G. HEK293 cell line: A vehicle for the expression of recombinant proteins. Journal of Pharmacological and Toxicological Methods. 51 (3), 187-200 (2005).
- Xu, X., Nagarajan, H., et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nature Biotechnology. 29 (8), 735-741 (2011).
- Hazan, A., Kumar, R., Matzner, H., Priel, A. The pain receptor TRPV1 displays agonist-dependent activation stoichiometry. Scientific reports. 5, 12278 (2015).
- Kumar, R., Hazan, A., Basu, A., Zalcman, N., Matzner, H., Priel, A. Tyrosine Residue in the TRPV1 Vanilloid Binding Pocket Regulates Deactivation Kinetics. Journal of Biological Chemistry. 291 (26), 13855-13863 (2016).
- Kim, T. K., Eberwine, J. H. Mammalian cell transfection: the present and the future. Analytical and bioanalytical chemistry. 397 (8), 3173-3178 (2010).
- Preuss, A. K., Connor, J. A., Vogel, H. Transient transfection induces different intracellular calcium signaling in CHO K1 versus HEK 293 cells. Cytotechnology. 33 (1-3), 139-145 (2000).
- Dalton, A. C., Barton, W. A. Over-expression of secreted proteins from mammalian cell lines. Protein Science. 23 (5), 517-525 (2014).
- Lee, P. Y., Costumbrado, J., Hsu, C. -. Y., Kim, Y. H. Agarose gel electrophoresis for the separation of DNA fragments. J Vis Exp. (62), e3923 (2012).
- Bohlen, C. J., Priel, A., Zhou, S., King, D., Siemens, J., Julius, D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 141 (5), 834-845 (2010).
- Hamill, O. P., Marty, A., Neher, E., Sakmann, B., Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv European journal of physiology. 391 (2), 85-100 (1981).
- Jones, J., Nivitchanyong, T., et al. Optimization of tetracycline-responsive recombinant protein production and effect on cell growth and ER stress in mammalian cells. Biotechnology and Bioengineering. 91 (6), 722-732 (2005).
- Raphemot, R., Weaver, C. D., Denton, J. S. High-throughput screening for small-molecule modulators of inward rectifier potassium channels. J Vis Exp. (71), e4209 (2013).

