Bioluminescence Imaging of Neuroinflammation in Transgenic Mice After Peripheral Inoculation of Alpha-Synuclein Fibrils
Summary
Peripheral injection of alpha-synuclein fibrils into the peritoneum or tongue of Tg(M83+/-:Gfap-luc+/-) mice, which express human alpha-synuclein with the familial A53T mutation and firefly luciferase, can induce neuropathology, including neuroinflammation, in their central nervous system.
Abstract
To study the prion-like behavior of misfolded alpha-synuclein, mouse models are needed that allow fast and simple transmission of alpha-synuclein prionoids, which cause neuropathology within the central nervous system (CNS). Here we describe that intraglossal or intraperitoneal injection of alpha-synuclein fibrils into bigenic Tg(M83+/-:Gfap-luc+/-) mice, which overexpress human alpha-synuclein with the A53T mutation from the prion protein promoter and firefly luciferase from the promoter for glial fibrillary acidic protein (Gfap), is sufficient to induce neuropathologic disease. In comparison to homozygous Tg(M83+/+) mice that develop severe neurologic symptoms beginning at an age of 8 months, heterozygous Tg(M83+/-:Gfap-luc+/-) animals remain free of spontaneous disease until they reach an age of 22 months. Interestingly, injection of alpha-synuclein fibrils via the intraperitoneal route induced neurologic disease with paralysis in four of five Tg(M83+/-:Gfap-luc+/-) mice with a median incubation time of 229 ±17 days. Diseased animals showed severe deposits of phosphorylated alpha-synuclein in their brains and spinal cords. Accumulations of alpha-synuclein were sarkosyl-insoluble and colocalized with ubiquitin and p62, and were accompanied by an inflammatory response resulting in astrocytic gliosis and microgliosis. Surprisingly, inoculation of alpha-synuclein fibrils into the tongue was less effective in causing disease with only one of five injected animals showing alpha-synuclein pathology after 285 days. Our findings show that inoculation via the intraglossal route and more so via the intraperitoneal route is suitable to induce neurologic illness with relevant hallmarks of synucleinopathies in Tg(M83+/-:Gfap-luc+/-) mice. This provides a new model for studying prion-like pathogenesis induced by alpha-synuclein prionoids in greater detail.
Introduction
There is growing evidence that alpha-synuclein has characteristics that are similar to those of the prion protein, particularly in its capacity to self-seed and propagate misfolding between cells and along neuronal pathways. This property of alpha-synuclein is also referred to as 'prion-like' or 'prionoid', and is supported by observations in transplantation experiments, which suggest the transmissibility of misfolded alpha-synuclein from diseased neurons to newly transplanted healthy neurons1,2,3,4. Also direct injection of misfolded alpha-synuclein into the brain or the periphery, e.g. the hindlimb muscle or intestinal wall, results in a spread of alpha-synuclein pathology to distal parts of the CNS5,6,7,8,9,10. We analyzed transmission of alpha-synuclein prionoids via peripheral routes in more detail and addressed the question whether misfolded alpha-synuclein may neuroinvade the CNS after a single intraglossal or intraperitoneal injection, a feature that previously had been shown for prions but not for misfolded alpha-synuclein. After injection of prions into the tongue, neuroinvasion of the CNS is achieved via propagation along the hypoglossal nerve of the tongue that leads to the nucleus of the hypoglossal nerve, which is located in the brain stem11. As a mouse model we chose Tg(M83+/-:Gfap-luc+/-) mice that overexpress the A53T mutant of human alpha-synuclein from the prion promoter, and firefly luciferase under control of a Gfap promoter to monitor astrocytic activation by bioluminescence, as previously shown in the brain of prion-infected mice12. In our hands bigenic Tg(M83+/-:Gfap-luc+/-) mice did not develop disease until 23 months of age as has been shown by others13. A single injection of human alpha-synuclein fibrils via the intraglossal or intraperitoneal route induced neurologic disease with pathology in the brains and spinal cords of Tg(M83+/-:Gfap-luc+/-) mice supporting the hypothesis that alpha-synuclein prionoids share important characteristics with prions14.
Protocol
All procedures including animals were performed with approval of the animal protection committee of North Rhine-Westphalia State Environment Agency (LANUV). Animals were housed and cared for according to standard conditions with a 12 h light/dark cycle and free access to food and water.
1. Animal Model
- Intercross hemizygous Tg(Gfap-luc+/-) mice with hemizygous Tg(M83+/-) mice to generate hemizygous bigenic Tg(M83+/-:Gfap-luc+/-) mice15,16.
- Genotype the progeny with real-time PCR for the presence of the transgene encoding human alpha-synuclein and with standard PCR for firefly luciferase12,17.
- Inoculate the animals at an age of six-to-eight weeks.
2. Inoculum Preparation
- Prepare monomeric recombinant human alpha-synuclein with the A53T mutation in Tris-buffered saline (TBS) buffer containing 150 mM NaCl in 20 mM Tris-HCl (pH 7.2) as has been previously described14.
- Prepare fibrillar alpha-synuclein for inoculations by agitating 3 µg/µL of monomeric recombinant human alpha-synuclein in an orbital shaker at 800 rpm and 37 °C for 5 days.
- Dilute fibril assemblies in phosphate-buffered saline (PBS) to reach a final concentration of 1 µg/µL for intraperitoneal or 2 µg/µL for intraglossal inoculations.
- Fragment the alpha-synuclein fibrils with a rod sonicator for 1 min (40 pulses of 0.5 s duration with a one-second pause between each pulse and an amplitude set to 50%) on ice. To avoid contamination by cross-seeding. Use clean sonication probes to prepare different inoculum.
3. Intraglossal Injections
- Anesthetize animals with an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Pinch the animal's toe and confirm that it does not withdraw its hindlimb to ensure proper anesthesia. Use vet ointment on the animal's eyes to prevent dryness while under anesthesia.
- Fix narcotized animals carefully with adhesive tape onto a heating plate in a dorsal recumbent position with their heads facing towards the investigator.
- Fill a 27 G disposable hypodermic syringe with 5 µL of sonicated alpha-synuclein fibrils or PBS.
- Use one blunt-nosed thumb forceps with serrated tips to hold the animal’s mouth open, and a second smaller pair of forceps to carefully pull out the tongue to make the bottom side of the tongue accessible for injection.
- Inject the needle of the syringe into the right or left bottom side of the tongue in proximity to the hypoglossal nerve. Slowly inject the inoculum after 5 s. Slowly retract the needle after 5 s to ensure that the inoculum has penetrated the tissue and is not lost while retracting the needle.
- Release the animal from its fixations and leave it on the heating plate under constant monitoring until complete recovery.
4. Intraperitoneal Injections
- For intraperitoneal injections, narcotize the animals shortly in an anesthesia chamber by inhalation with isoflurane/oxygen using a flow rate of 2 L/min and the vaporizer set to 2%. Pinch the animal's toe and confirm that it does not withdraw its hindlimb to ensure proper anesthesia.
- Fill a 27 G disposable hypodermic syringe with 50 µL of sonicated alpha-synuclein fibrils or PBS.
- Directly inject into the peritoneum of the mouse and avoid penetrating the small intestine or cecum located behind the abdominal wall by holding the animal in a dorsal position with the head facing away from the investigator and downward at approximately 45°. Monitor animals until they have recovered from the anesthesia.
5. Bioluminescence Imaging
- Image Tg(M83+/-:Gfap-luc+/-) mice every 2-4 weeks using a bioluminescence imaging system.
- Prior to imaging shortly anesthetize mice in an isoflurane/oxygen chamber with a flow rate of 2 L/min and the vaporizer set to 2%. Pinch the animal's toe and confirm that it does not withdraw its hindlimb to ensure proper anesthesia.
- Shave and depilate the heads of the mice with a depilatory cream. Color the ears in black with a non-irritating marker to block unspecific bioluminescence.
- Dilute D-luciferin potassium salt, the substrate of luciferase, in PBS to a 30 mg/mL stock solution. Store this in 1 mL aliquots at -20 °C. Protect the dissolved D-luciferin from exposure to light.
- Weigh the animals prior to injection. Calculate the exact volume of D-luciferin for injection of 150 mg/kg body weight. Intraperitoneally inject D-luciferin and return the animal to the anesthesia chamber.
- Start the imaging software. After the imaging system has reached its operating temperature initialize the system by clicking on the 'Initialize' button in the control panel.
- Select the buttons 'Luminescent' and 'Photograph'. Set the exposure time to 60 s, the binning to 'Medium', the F/Stop to '1', and the EM gain to 'Off'.
- Confirm that the excitation filter is set to 'Block' and the emission filter to 'Open', and set the subject height to 1.50 cm.
- Ten min after injection with D-luciferin, place the animals onto the heating plate in the imaging chamber and ensure that their muzzles are correctly placed in the anesthesia outlet. Close the isoflurane/oxygen flow from the inhalation chamber and open the flow for the imaging chamber with a flow rate of 0.25 L/min and an evaporation of 2%. Close the door of the imaging chamber properly.
- Click on the 'Acquire' button to measure the bioluminescence, which takes 60 s. Stop the isoflurane flow and monitor the animals until they have completely recovered after returning them back to their cages.
- Use the imaging software to quantify the bioluminescence. Within the 'Tool Palette' select 'ROI Tools'. Under 'Type' within the 'ROI Tools' select 'Measurement ROI'.
- Within the 'ROI Tools' click the "circle" tool and select the number of ROIs to draw from the pull down menu – ideally '3' for three mice.
- In the image, right-click each ROI and under 'Properties' adjust the width and height to 1.25 cm. Position each ROI over the brain area that is quantified.
- In the upper left of the image, set the units panel to 'radiance (photons)'.
- Within the 'Tool Palette', select 'Image Adjust' and adjust the minimum and the maximum of the 'Color Scale', for instance, from 0.20e6 to 1.00e6.
- Under 'File' save the data with 'Save'.
6. Biochemical Analysis
- Isolate the brains and spinal cords according to established protocols18,19.
- Homogenize whole brain and whole spinal cord samples separately in PBS in the presence of protease and phosphatase inhibitors (diluted to 1x in PBS) in two 30 s cycles with 6,000 rpm in a tissue homogenizer. Ensure that the brain and spinal cord samples are homogenized to a final concentration of 20% (wt/vol).
- Sonicate the samples twice while keeping them on ice with a constant pulse of 10 s and an amplitude set to 50%.
- Adjust the homogenates to 10% in PBS and 750 mM NaCl by diluting them 1:1 with 1.5 M NaCl in PBS.
- To separate the cellular debris, centrifuge the homogenates at 1,000 x g for 5 min at 4 °C. Keep the supernatants for the next steps and discard the pellets.
- Measure the protein concentration in the homogenates using the bicinchoninic acid (BCA) assay according to the manufacturer's instructions. Incubate 1.0 mg of total protein from brain homogenates or 0.8 mg from spinal cord homogenates in N-laurylsarcosyl at a final concentration of 10% (wt/vol) for 15 min on ice.
- Overlay the homogenates onto a 3 mL sucrose cushion of 10% (wt/vol) in distilled water and ultracentrifuge them at 465,000 x g for 1 h at 4 °C.
- Resuspend the pellets in a buffer containing 4% sodium dodecyl sulfate (SDS), 2% β-mercaptoethanol, 192 mM glycine, 25 mM Tris, and 5% (wt/vol) sucrose.
- Boil the samples at 100 °C for 5 min and load them onto SDS-polyacrylamide gels for electrophoresis in a morpholineethanesulfonic acid (MES) buffer system.
- Transfer the separated proteins to polyvinylidene difluoride (PVDF) membranes in a semidry blotting system.
- Incubate the membranes in 0.4% (vol/vol) formalin solution in PBS for 30 min at room temperature on a rotor with 35 rpm to cross-link the proteins with the membrane.
- Block the membranes in TBS buffer containing 0.05% (vol/vol) Tween 20 and 5% (wt/vol) milk for 1 h at room temperature.
- Incubate the blots with the primary antibody in blocking buffer overnight at 4 °C.
- Wash the membranes three times in TBS buffer and incubate them with peroxidase- or fluorophore-conjugated secondary antibodies in TBS for 1 h at room temperature.
- Visualize the bound secondary antibodies by chemiluminescence or fluorescence according to established protocols20.
7. Immunofluorescence Analysis
- Dehydrate the dissected brains and spinal cords in a tissue processing station and embed them into paraffin using a paraffin station.
- Cut the paraffin-embedded tissues with a microtome in 8 µm thick coronal sections and mount the sections on glass slides.
- Deparaffinize the tissue sections by incubating them in two separate xylol baths for 5 to 10 min and rehydrate them through a series of graded ethanol baths (100%, 90%, 70%, 50%) and finally with H2O.
- Incubate the sections in 0.01 M citrate buffer (pH 6.0) for 5 min at room temperature and additionally boil them for 10 min in a microwave oven.
- Let the sections cool down to room temperature and incubate them with a 3% hydrogen peroxide solution for 30 min to inhibit endogenous peroxidases.
- Block the tissue sections by incubation with 20% (vol/vol) normal goat serum, 1% (vol/vol) bovine serum albumin (BSA), and 0.5% Triton X-100 in PBS for 1 h at room temperature.
- Incubate the sections with the primary antibody diluted in 1% (vol/vol) normal goat serum, 1% (vol/vol) BSA, and 0.25% Triton X-100 in PBS overnight at 4 °C.
- Wash the sections twice with 0.25% (vol/vol) Triton X-100 in PBS and once with PBS.
- Stain the sections with the corresponding fluorophore-conjugated secondary antibodies and the nuclear dye DAPI diluted in 1% (vol/vol) normal goat serum, 1% (vol/vol) BSA, and PBS for 1 h at room temperature.
- After washing twice with 0.25% (vol/vol) Triton X-100 in PBS and once with PBS, coverslip the slides with embedding media and visualize the staining with a confocal laser scanning microscope.
Representative Results
Peripheral injection of alpha-synuclein prionoids via the tongue or the peritoneum induced neuropathology in the CNS of bigenic Tg(M83+/-:Gfap-luc+/-) mice (Table 1 and Figure 1). After a single intraperitoneal injection with alpha-synuclein fibrils, four of five mice developed neurologic disease with a median incubation time of 229 ±17 days. Surprisingly, only one of five mice developed CNS disease after intraglossal injection with alpha-synuclein fibrils after 285 days. Inoculation with PBS did not cause disease within the 420 day period of the experiment.
Biochemical analysis by Western blotting and probing with the phospho-specific EP1536Y antibody against Ser129 of alpha-synuclein revealed that for both transmission routes diseased animals had accumulated aggregates of phosphorylated and sarkosyl-insoluble alpha-synuclein in their brains and spinal cords (Table 2 and Figure 2). In contrast, brains and spinal cords of PBS-injected and non-diseased animals only contained monomeric species of phosphorylated alpha-synuclein.
Immunofluorescent staining of brain slices of intraperitoneally injected and diseased mice with the phospho-specific pSyn#64 antibody revealed a wide distribution of phosphorylated alpha-synuclein deposits throughout the CNS (Table 2 and Figure 3). Moreover, phosphorylated alpha-synuclein deposits colocalized with ubiquitin and p62 indicating an aberrant protein homeostasis that might contribute to the formation of deposits.
The accumulation of alpha-synuclein aggregates in diseased animals was accompanied by neuroinflammatory changes, which was detected by immunofluorescence staining for GFAP, a marker of astrocytes that can reveal reactive astrogliosis (Figure 4). Additionally, activated microglia were also found in regions with abundant alpha-synuclein pathology by immunofluorescence staining for IBA-1 (ionized calcium-binding adapter molecule 1). In accordance, bioluminescence imaging revealed increased radiance (>2 × 106 p/s/cm2/sr) emitted from the brains of Tg(M83+/-:Gfap-luc+/-) mice injected with alpha-synuclein fibrils, shortly before they developed signs of neurologic disease. In contrast, brains of mice injected with PBS did not show any increase in radiance. Increased radiance is indicative of reactive astrogliosis, a hallmark of neuroinflammation, since luciferase expression is driven from the Gfap promoter.
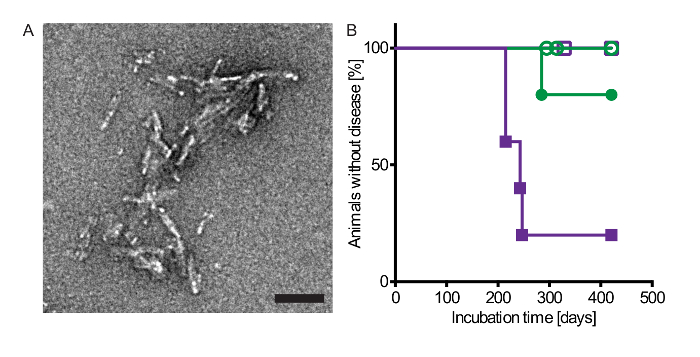
Figure 1: Injection with alpha-synuclein fibrils via the intraperitoneal or intraglossal route causes disease in bigenic Tg(M83+/-:Gfap-luc+/-) mice. (A) Electron micrograph of sonicated recombinant human alpha-synuclein fibrils that were intraperitoneally and intraglossally injected into mice. Negative staining with uranyl acetate revealed multiple clusters of short, rod-shaped aggregates. Scale bars = 100 nm. (B) Kaplan-Meier survival curves show that after intraperitoneal injection with alpha-synuclein fibrils four of five Tg(M83+/-:Gfap-luc+/-) mice developed neurologic disease in 229 ±17 days (purple closed squares), whereas none of the PBS-injected control mice developed signs of neurologic dysfunction within 420 days (purple open squares). After intraglossal inoculation with alpha-synuclein fibrils one mouse of five died after 285 days (green closed circles). In contrast, none of the PBS-injected control mice developed neurologic disease or spontaneously died (green open circles). Modified from Breid et al., 201614. Please click here to view a larger version of this figure.
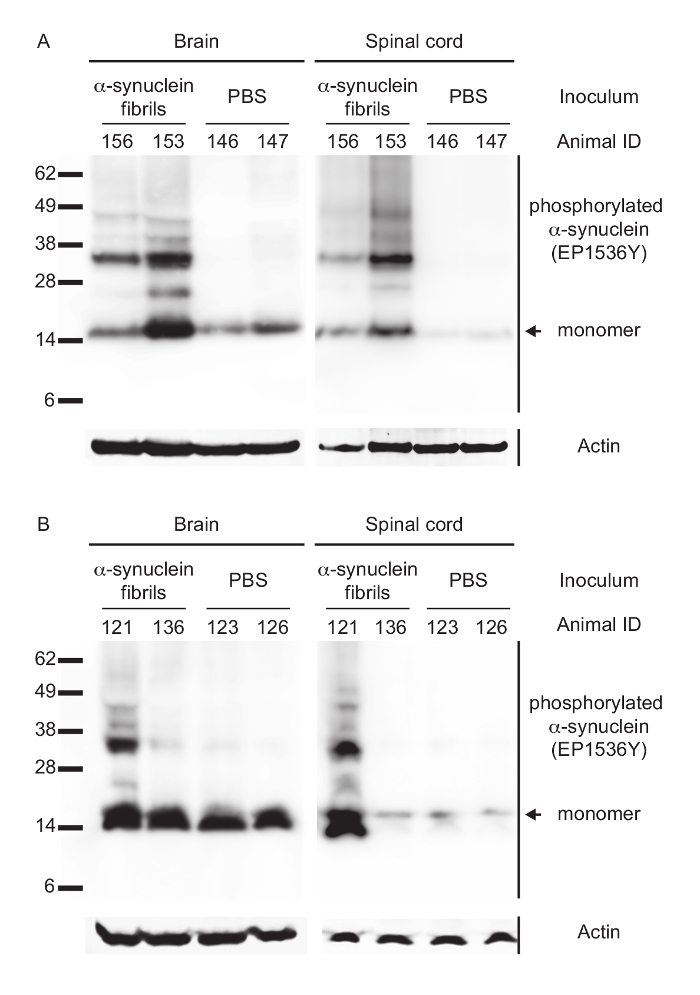
Figure 2: Biochemical analysis of phosphorylated alpha-synuclein in the CNS of peripherally injected Tg(M83+/-:Gfap-luc+/-) mice. (A) Immunoblotting with the EP1536Y antibody, recognizing phosphorylation at Ser129 of alpha-synuclein, showed that intraperitoneally injected and diseased mice accumulated high-molecular-weight species of sarkosyl-insoluble aggregates of phosphorylated alpha-synuclein in their brains and spinal cords. Control mice challenged with PBS did not accumulate aggregates of phosphorylated alpha-synuclein and showed bands only for its monomeric form. (B) After intraglossal challenge with alpha-synuclein fibrils only one mouse, animal 121, showed sarkosyl-insoluble aggregates of phosphorylated alpha-synuclein in its brain and spinal cord, whereas all other intraglossally inoculated mice remained healthy without deposits of alpha-synuclein. Molecular masses are shown in kilodaltons. Sample loading in each lane is shown by detection of actin. Modified from Breid et al., 201614. Please click here to view a larger version of this figure.
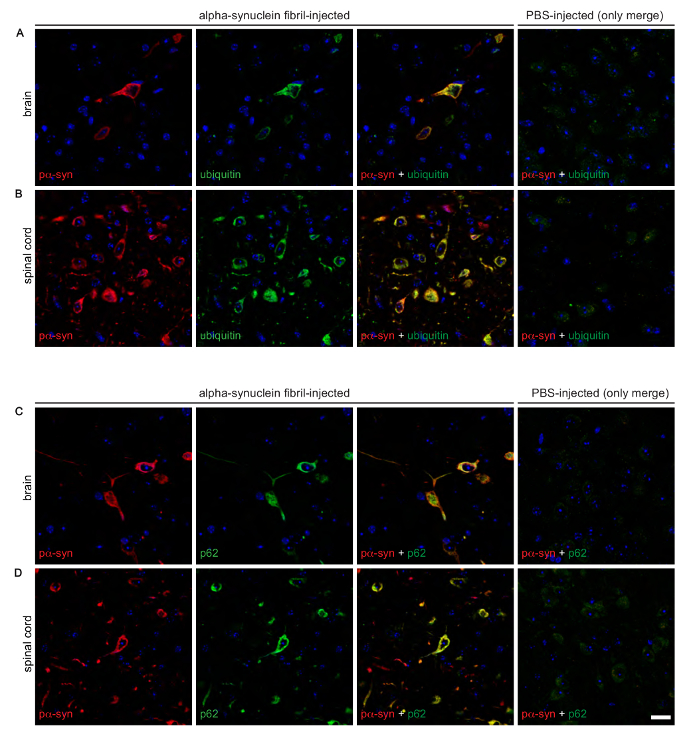
Figure 3: Immunofluorescence analysis shows that deposits of phosphorylated alpha-synuclein colocalize with ubiquitin and p62 in the brains and spinal cords of diseased Tg(M83+/-:Gfap-luc+/-) mice. Co-staining of phosphorylated alpha-synuclein, detected with the EP1536Y antibody, and ubiquitin revealed a distinct colocalization in the brains, as observed in the thalamic nucleus (A), and spinal cords, as detected in the grey matter (B), of diseased mice after intraperitoneal challenge with alpha-synuclein fibrils. Similarly, phosphorylated alpha-synuclein, detected with the pSyn#64 antibody, also colocalized with p62 in brains (C) and spinal cords (D) of diseased animals. (A auf D) PBS-injected healthy control animals did not show deposits or colocalization for any of these proteins. Nuclear staining with DAPI is shown in blue. Scale bars = 20 µm. Modified from Breid et al., 201614. Please click here to view a larger version of this figure.
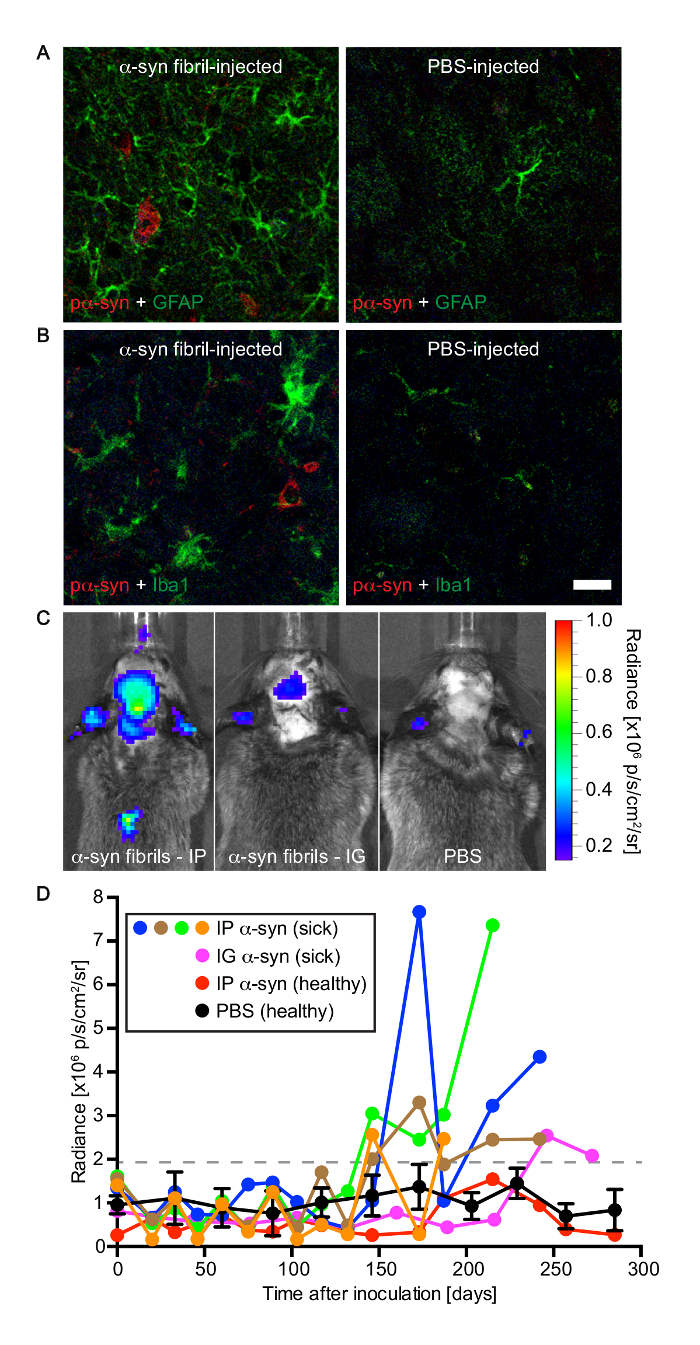
Figure 4: Tg(M83+/-:Gfap-luc+/-) mice developed gliosis after peripheral challenge with alpha-synuclein fibrils. (A) Immunofluorescence analysis of brain sections of diseased animals with an antibody against GFAP, a marker of astrocytes, revealed reactive astrogliosis in areas with deposits of phosphorylated alpha-synuclein, which were detected in the brain stem with the pSyn#64 antibody. In contrast, there was no reactive astrogliosis in brains of PBS-injected control mice. (B) Similarly, staining with an antibody to IBA-1, a marker of microglia, demonstrated microgliosis in areas with deposits of phosphorylated alpha-synuclein in diseased animals. Brains of healthy, PBS-injected control mice did not show signs of microgliosis. (C) Bioluminescence imaging revealed elevated radiance from the brains and spinal cords of Tg(M83+/-:Gfap-luc+/-) mice intraperitoneally injected with alpha-synuclein fibrils (left panel), which was caused by the activation of astrocytes, shortly before the mice developed neurologic symptoms. In the brains and spinal cords of PBS-injected control mice the basal radiance did not increase with time (right panel). After intraglossal inoculation with alpha-synuclein fibrils, one Tg(M83+/-:Gfap-luc+/-) mouse, animal 121, showed signs of reactive astrogliosis shortly before it died at 285 days (center panel). (D) After intraperitoneal challenge of Tg(M83+/-:Gfap-luc+/-) mice with alpha-synuclein fibrils, increased levels of bioluminescence (>2 × 106 p/s/cm2/sr) were measured from the brains of four mice (blue, green, brown, and orange circles) some weeks before they developed neurologic signs of disease. One of five animals also showed elevated levels of bioluminescence shortly before it died 285 days after intraglossal injection with alpha-synuclein fibrils (magenta circles). In contrast, for healthy, PBS-injected control mice (black circles; error bars show SD [n = 4]) and one animal that did not develop disease after intraperitoneal injection with alpha-synuclein fibrils (red circles), the bioluminescence signal remained below a threshold of 2 x 106 p/s/cm2/sr within the period of observation. Scale bars = 20 µm. Modified from Breid et al., 201614. Please click here to view a larger version of this figure.
| Mouse line | Inoculum (μg) | Inoculation route | No. of mice with disease/no. of mice inoculated | Mean survival time ±SD (days) |
| Tg(M83+/-:Gfap-luc+/-) | human α-synuclein fibrils (50) | intraperitoneal | 4/5 | 229 ±17/420 |
| Tg(M83+/-:Gfap-luc+/-) | PBS | intraperitoneal | 0/5 | 0/420 |
| Tg(M83+/-:Gfap-luc+/-) | human α-synuclein fibrils (10) | intraglossal | 1/5 | 285/420 |
| Tg(M83+/-:Gfap-luc+/-) | PBS | intraglossal | 0/4 | 0/420 |
Table 1: Inoculation experiments. *Modified from Breid et al., 201614
| Target [antibody clone] | Host | Immunogen | Dilution in IF | Dilution in WB |
| Actin [C4] | Mouse | – | – | 1:1,000 |
| α-synuclein, phospho S129 [pSyn#64] | Mouse | pSer129 | 1:1200 | – |
| α-synuclein, phospho S129 [EP1536Y] | Rabbit | pSer129 | 1:100 | 1:1,000 |
| Glial fibrillary acidic protein (GFAP) | Rabbit | – | 1:200 | – |
| IBA-1 | Rabbit | – | 1:500 | – |
| Sequestosome-1 (p62) | Rabbit | – | 1:100 | – |
| Ubiquitin [Ubi-1] | Mouse | – | 1:500 | – |
Table 2: Antibodies used for immunofluorescence (IF) and Western blotting (WB). *Modified from Breid et al., 201614
Discussion
Peripheral injection of alpha-synuclein fibrils into the peritoneum of Tg(M83+/-:Gfap-luc+/-) mice represents a facile method to induce neurologic disease accompanied by neuroinflammation to recapitulate important characteristics of synucleinopathies. Similarly, tongue injection represents another route for neuroinvasion by alpha-synuclein prionoids in transgenic mice but is less efficient. We chose to terminate our experiments at 420 days after injection and we cannot exclude the possibility that more or all of the fibril injected mice may have developed disease at a later time point. Additionally, the use of Tg(M83+/-:Gfap-luc+/-) mice enables non-invasive visualization of astrogliosis and represents a simple method for monitoring inflammatory responses caused by the neuroinvasion of alpha-synuclein over the entire lifespan of the mouse.
A critical step is the preparation of the inoculum. Seemingly trivial factors like aggregation time, sonication time, the abundance of endotoxins, or the amount of fibrils used for injection could influence the outcome of an experiment by affecting the seeding propensity of misfolded alpha-synuclein21. Thus it is important to always use the same conditions when preparing inocula for injection. For intraglossal injections it has to be considered that not only the hypoglossal nerve but also other cranial nerves like the glossopharyngeal nerve innervate the tongue and could be involved in the interneuronal transport of alpha-synuclein prionoids to the brain. Thus targeting different areas of the tongue could result in different spreading kinetics to the brain. We did not directly inject into the hypoglossal nerve and it is possible that targeting the hypoglossal nerve could improve CNS transmission of alpha-synuclein prionoids. For intraperitoneal injections with alpha-synuclein fibrils it is critical that the inoculum is correctly injected into the peritoneum and not accidentally into internal organs like the intestine or cecum, which could negatively affect neuroinvasion. This is also important for bioluminescence imaging when injecting D-luciferin. Mistargeting of D-luciferin to internal organs could slow down its distribution to the brain and result in lower bioluminescence. To obtain uniform results during imaging it is also critical to always freshly prepare the D-luciferin solution prior to injection and to let it exactly incubate for 10 min after intraperitoneal injection before imaging.
Bioluminescence imaging is a useful method to non-invasively measure changes in astrogliosis over time and can be applied not only after intraperitoneal or intraglossal injections but also after every type of treatment including, for example, intracerebral or intravenous injections. To monitor spreading of alpha-synuclein prionoids from the periphery to the CNS and the ensuing neuroinflammation we used bigenic Tg(M83+/-:Gfap-luc+/-) mice. Because hemizygous expression of alpha-synuclein in these animals is 40% lower relative to homozygous animals they remain free of spontaneous disease for up to 22 months of age and have a suitable genetic background for alpha-synuclein transmission studies13. However, modifications regarding the choice of the mouse model for bioluminescence imaging can be desirable. An interesting option is the use of monogenic Tg(Gfap-luc+/-) mice expressing only luciferase as a transgene, which allows an assessment of the prionoid behavior of alpha-synuclein fibrils on a background that is essentially wild-type as far as alpha-synuclein is concerned14. Moreover, crossing Tg(Gfap-luc+/-) mice to other transgenic mice, such as Tg(SOD1G93A) mice for amyotrophic lateral sclerosis or to Tg(APP23) mice for Alzheimer's disease, enables imaging of neuroinflammation in other disease models22,23.
A limitation of this protocol is that based on the site of injection the injected inoculum cannot exceed a certain volume. Thus injections into the tongue can only be performed with smaller volumes than those into the peritoneum, which may affect transmission times to the CNS. Another limitation is that the Gfap-luc transgene only allows detection of astrogliosis but not of microgliosis, which is equally important in neuroinflammation. Lastly, this model does not provide insights as to how alpha-synuclein prionoids reach the CNS after intraperitoneal injection.
The application of exogenous alpha-synuclein prionoids via injection has been shown to be a useful method to study transmission pathways that play a critical role in recapitulating characteristics of synucleinopathies. Intracerebral injections of recombinant alpha-synuclein fibrils or disease-associated mouse or human alpha-synuclein species into animal models have been used in several studies to analyze their seeding properties and transmission efficiency over time5,7,17,24,25,26. Since stereotactic injections into the brain always induce a local inflammatory response shortly after surgery, this transmission route might influence the spreading and infectivity of the inoculum caused by an alteration of brain homeostasis. More recent studies have changed the focus to peripheral or systemic applications, not primarily to avoid the surgical procedure but rather to analyze the periphery, the intestinal wall, skeletal muscle, or blood, as an initial point from where neuroinvasion to the CNS could commence6,9,14,27. For prions it has been shown that transmission through the peritoneum or the tongue leads to neuroinvasion and neurologic disease; after tongue infection, prions spread within only two weeks to the hypoglossal nucleus in the brain stem11. Since alpha-synuclein has been discussed to have neuroinvasive and seeding properties like prions, we showed that transmission via the tongue and the peritoneum represent further entrance points for alpha-synuclein prionoids to invade the CNS14. Quantification of astrogliosis by bioluminescence imaging facilitates the tracking of the neuroinflammatory process over time and represents a non-invasive alternative to histological analysis.
To obtain a deeper insight into how the CNS responds to neuroinvasion of alpha-synuclein prionoids and to other treatments that cause neuroinflammation, it would be beneficial to generate mouse models that also allow non-invasive monitoring of microgliosis, e.g. with a transgene that drives luciferase expression from a promoter specific for protein expression in microglia.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors thank Olga Sharma, Theresa Hundt, and the staff of the DZNE microscopy and animal facilities for technical support.
Materials
| anti-actin antibody | Merck Millipore | MAB1501 | |
| anti-alpha-synuclein, phospho S129 antibody [pSyn#64] | Wako | 015-25191 | |
| anti-alpha-synuclein, phospho S129 antibody [EP1536Y] | Abcam | ab51253 | |
| anti-GFAP antibody | Dako | Z0334 01 | |
| anti-IBA-1 antibody | Wako | 019-19741 | |
| anti-Sequestosome-1 (p62) antibody | Proteintech | 18420-1-AP | |
| anti-ubiquitin antibody [Ubi-1] | Merck Millipore | MAB1510 | |
| Phosphate-buffered saline (PBS) | Invitrogen | 14190169 | |
| Ketamine | Ratiopharm | 100 mg/kg | |
| Xylazine | Ratiopharm | 10 mg/kg | |
| 27-gauge syringe | VWR | 613-4900 | |
| Isoflurane | Piramal Healthcare | PZN 4831850 | |
| Depilatory cream | Veet | ||
| Secureline lab marker | Neolab | 25040 | |
| D-luciferin potassium salt | Acris | LK10000 | 30 mg/mL stock solution |
| Thermomixer | Eppendorf | 5776671 | |
| Sonopuls Mini20 sonicator | Bandelin | 3648 | |
| IVIS Lumina II imaging system | PerkinElmer | ||
| Living Image 3.0 Software | PerkinElmer | ||
| Tg(M83+/-) mice or B6;C3-Tg(Prnp-SNCA*A53T)83Vle/J mice | The Jackson Laboratory | 004479 | |
| Standard pattern forceps | Fine Science Tools | 11000-16 | |
| Narrow pattern forceps | Fine Science Tools | 11002-12 | |
| N-laurylasarcosyl | Sigma | L5125-100G | |
| Optima Max-XP ultracentrifuge | Beckman Coulter | TLA-110 rotor | |
| Thickwall polycarbonate tubes | Beckman Coulter | 362305 | |
| NuPAG Novex 4-12% Bis-Tris Midi Protein Gels | Thermo Fisher Scientific | WG1401BOX | |
| HRP conjugated antibody | Cayman | Cay10004301-1 | |
| IR Dye 680 conjugated antibody | LI-COR Biosciences | 926-68070 | |
| SuperSignal West Dura Extended Duration Substrate | Thermo Fisher Scientific | 34075 | |
| Stella 3200 imaging system | Raytest | ||
| Odyssey infrared imaging system | LI-COR Biosciences | ||
| Tween 20 | MP Biomedicals | TWEEN201 | |
| Triton X-100 | Sigma | SA/T8787 | |
| Immobilon-FL PVDF membrane | Merck Millipore | IPFL00010 | |
| Xylol | Sigma | Roth | |
| Hydrogen peroxide | Sigma | SA/00216763/000500 | working solution 3% |
| Bovine serum albumine (BSA) | Thermo Fisher Scientific | A3294-100G | |
| Goat serum | Thermo Fisher Scientific | PCN5000 | |
| 4,6-diamidino-2-phenylindole (DAPI) | Thermo Fisher Scientific | D1306 | working dilution 1:50,000 |
| Fluoromount media | Omnilab | SA/F4680/000025 | |
| LSM700 confocal laser scanning microscope | Carl Zeiss | ||
| HALT protease and phosphatase inhibitors | Thermo Fisher Scientific | 10516495 | |
| Precellys 24-Dual homogenizer | Peqlab | 91-PCS24D | |
| Alexa Fluor 488 conjugated antibody | Thermo Fisher Scientific | A31619 | |
| Alexa Fluor 594 conjugated antibody | Thermo Fisher Scientific | A11005 | |
| Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | 10741395 | |
| Microtome RM2255 | Leica | ||
| LSM700 confocal laser scanning microscope | Carl Zeiss |
Referenzen
- Aguzzi, A. Cell biology: Beyond the prion principle. Nature. 459 (7249), 924-925 (2009).
- Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B., Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 14 (5), 504-506 (2008).
- Li, J. Y., et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 14 (5), 501-503 (2008).
- Hansen, C., et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 121 (2), 715-725 (2011).
- Masuda-Suzukake, M., et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 136 (4), 1128-1138 (2013).
- Holmqvist, S., et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128 (6), 805-820 (2014).
- Luk, K. C., et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 338 (6109), 949-953 (2012).
- Sacino, A. N., et al. Induction of CNS alpha-synuclein pathology by fibrillar and non-amyloidogenic recombinant alpha-synuclein. Acta Neuropathol Commun. 1, 38 (2013).
- Sacino, A. N., et al. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A. 111 (29), 10732-10737 (2014).
- Mougenot, A. L., et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 33 (9), 2225-2228 (2012).
- Bartz, J. C., Kincaid, A. E., Bessen, R. A. Rapid prion neuroinvasion following tongue infection. J Virol. 77 (1), 583-591 (2003).
- Tamgüney, G., et al. Measuring prions by bioluminescence imaging. Proc Natl Acad Sci U S A. 106 (35), 15002-15006 (2009).
- Watts, J. C., et al. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A. 110 (48), 19555-19560 (2013).
- Breid, S., et al. Neuroinvasion of alpha-Synuclein Prionoids after Intraperitoneal and Intraglossal Inoculation. J Virol. 90 (20), 9182-9193 (2016).
- Giasson, B. I., et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 34 (4), 521-533 (2002).
- Zhu, L., et al. Non-invasive imaging of GFAP expression after neuronal damage in mice. Neurosci Lett. 367 (2), 210-212 (2004).
- Bernis, M. E., et al. Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol Commun. 3 (1), 75 (2015).
- Gunther, R., et al. Clinical testing and spinal cord removal in a mouse model for amyotrophic lateral sclerosis (ALS). J Vis Exp. (61), (2012).
- Jackson-Lewis, V., Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2 (1), 141-151 (2007).
- Mathews, S. T., Plaisance, E. P., Kim, T. Imaging systems for westerns: chemiluminescence vs. infrared detection. Methods Mol Biol. 536, 499-513 (2009).
- Kim, C., et al. Exposure to bacterial endotoxin generates a distinct strain of alpha-synuclein fibril. Sci Rep. 6, 30891 (2016).
- Keller, A. F., Gravel, M., Kriz, J. Live imaging of amyotrophic lateral sclerosis pathogenesis: disease onset is characterized by marked induction of GFAP in Schwann cells. Glia. 57 (10), 1130-1142 (2009).
- Stöhr, J., et al. Distinct synthetic Abeta prion strains producing different amyloid deposits in bigenic mice. Proc Natl Acad Sci U S A. , (2014).
- Luk, K. C., et al. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 209 (5), 975-986 (2012).
- Recasens, A., et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 75 (3), 351-362 (2014).
- Sacino, A. N., et al. Induction of CNS alpha-synuclein pathology by fibrillar and non-amyloidogenic recombinant alpha-synuclein. Acta Neuropathol Commun. 1 (1), 38 (2013).
- Peelaerts, W., et al. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 522 (7556), 340-344 (2015).

