Covalent Immobilization of Proteins for the Single Molecule Force Spectroscopy
Summary
This protocol describes the covalent immobilization of proteins with a heterobifunctional silane coupling agent to silicon-oxide surfaces designed for the atomic force microscopy based single molecule force spectroscopy which is exemplified by the interaction of RrgA (pilus-1 tip adhesin of S. pneumoniae) with fibronectin.
Abstract
In recent years, atomic force microscopy (AFM) based single molecule force spectroscopy (SMFS) extended our understanding of molecular properties and functions. It gave us the opportunity to explore a multiplicity of biophysical mechanisms, e.g., how bacterial adhesins bind to host surface receptors in more detail. Among other factors, the success of SMFS experiments depends on the functional and native immobilization of the biomolecules of interest on solid surfaces and AFM tips. Here, we describe a straightforward protocol for the covalent coupling of proteins to silicon surfaces using silane-PEG-carboxyls and the well-established N-hydroxysuccinimid/1-ethyl-3-(3-dimethyl-aminopropyl)carbodiimid (EDC/NHS) chemistry in order to explore the interaction of pilus-1 adhesin RrgA from the Gram-positive bacterium Streptococcus pneumoniae (S. pneumoniae) with the extracellular matrix protein fibronectin (Fn). Our results show that the surface functionalization leads to a homogenous distribution of Fn on the glass surface and to an appropriate concentration of RrgA on the AFM cantilever tip, apparent by the target value of up to 20% of interaction events during SMFS measurements and revealed that RrgA binds to Fn with a mean force of 52 pN. The protocol can be adjusted to couple via site specific free thiol groups. This results in a predefined protein or molecule orientation and is suitable for other biophysical applications besides the SMFS.
Introduction
Beside optical and magnetic tweezers, the atomic force microscope (AFM)1,2 has emerged as a useful tool to analyze and manipulate molecules and probe their properties and functions, including their response to external force3,4. In contrast to methods like the enzyme linked immunosorbent assay (ELISA), surface plasmon resonance (SPR) or quartz crystal microbalance (QCM) setups, AFM allows to measure interactions on the single molecule (SMFS)5 and single cell level (SCFS)6. These technologies yielded valuable insight into binding mechanisms like the catch bonds found for the interaction of E. coli pilus protein FimH with mannose7, or the tandem β-zipper repeats formed by Fn binding proteins from S. aureus upon binding to Fn8. We were recently able to show that the pilus-1 adhesin RrgA9,10 from the Gram-positive bacterium Streptococcus pneumoniae (S. pneumoniae)11 is able to bind to fibronectin12 with its two terminal domains. This revealed a new two-domain binding mechanism which differs from the tandem β-zipper and may enable piliated pneumococci to form and maintain a transient contact to fibronectin-containing host surfaces13.
The success of SMFS experiments critically depends on the functional and native immobilization of the biomolecules on solid surfaces and AFM tips. As high forces may occur during SMFS measurements, the proteins should preferably be covalently coupled to the surface. There are a large number of different coupling methods for the immobilization of proteins and other biomolecules, as well as whole cells on (inorganic) solid surfaces, nano- particles and other devices described in the literature14,15,16,17,18,19,20,21,22,23,24,25,26,27. These protocols often make use of hazardous substances, are difficult to perform and/or require special equipment (e.g., plasma cleaner). A simple way to couple molecules to glass is to attach a thicker polymer layer of heterobifunctional crosslinkers with a silane-reactive group on one side and an amine-reactive group on their other side. Depending on the application, the coupling agents can comprise flexible hydro-carbon chains of variable length, e.g., polyethylenglycol (PEG). They suppress non-specific interactions of the modified surfaces (e.g., hydrophobic, electrostatic and van-der-Waals interactions) and may provide the coupled molecule rotational freedom.
Here, we describe a general protocol for the covalent coupling of proteins containing one or more free amino groups (-NH2) to glass surfaces and silicon nitride AFM tips via a heterobifunctional ethoxy silane-PEG-carboxyl (-COOH). This protocol can be used in SMFS experiments, which is exemplified based on the interaction of RrgA and the extracellular matrix protein Fn (see Figure 1 for an overview).
The first step is the silanization of the surface28,29,30,31. It involves the hydrolysis of the ethoxy groups of the coupling agent in order to form highly reactive SiOH groups. These can react with SiOH groups on the substrate. In a primary condensation step, these silanols form hydrogen bonds and spread on the substrate. In a secondary condensation reaction (which usually requires heat or vacuum to remove water), siloxane bonds are formed. This results in a covalently attached organo-silane layer.
The second step is the coupling of the proteins to the functional (-COOH) groups which extend from the polymer32. First, the acid is converted to a reactive N-hydroxysuccinimid (NHS) ester intermediate, which is gained through the well-established NHS/EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimid chemistry33 and undergoes nucleophilic substitution to finally form an amide bond with primary amines on the proteins.
In this way, RrgA was coupled to silicon nitride AFM tips and human Fn to glass substrates in a random orientation and their interaction forces were analyzed on the single molecule level. Our results show that the described surface chemistry leads to a homogenous distribution of Fn on the glass surface and to an appropriate concentration of RrgA on the tip, apparent by the target value of up to 20% of interaction events during SMFS measurements. This chemistry reduces non-specific background interactions, is subject to little alteration during data acquisition and is therefore excellently suited for precise SMFS experiments.
Protocol
1. Immobilization of Proteins via Functional Silane Coupling Agents
Note: Figure 1 gives an overview over the surface chemistry applied in this protocol.
CAUTION: In the following protocol, different chemicals with corrosive and skin irritating properties are used. Wear adequate (acid-resistant) gloves, safety goggles, and laboratory coat and work under the fume hood while preparing solutions in order to avoid inhalation of vapors.
- Functionalization of glass surfaces and silicon nitride cantilever with silane coupling agents
- Remove coarse dust and contaminations from glass slides with isopropanol and lint-free precision wipes and cut the slides in desired size (optional).
Note: Beside glass, the solid surface can be silica, quartz, and the oxides of aluminum, copper, tin, titanium, iron, chromium, zirconium, nickel, and zinc.
CAUTION: Cutting the glass slides may cause sharp edges. - Place the glass slides in a staining jar filled with hydrochloric acid (33% HCl) diluted with doubly distilled water (ddH2O) to 3 – 5% (v/v), close the jar with appropriate lid and place it in an ultrasonic bath for 90 min at room temperature.
Note: The used jar has a diameter of 6 cm and an approximate size of 65-70 mL. An appropriate volume of diluted HCl for the jar is 50 mL containing 5 mL 33% HCl and 45 mL of ddH2O. The HCl effectively removes non-binding metal ions, especially sodium, potassium, and calcium and reduces the silicon in order to produce a hydroxyl saturated glass surface.
CAUTION: HCl is corrosive and skin irritating. Wear adequate acid-resistant gloves, safety goggles and laboratory coat and work under the fume hood while preparing the solution in order to avoid inhalation of vapors. - Place the silicon nitride AFM cantilever probes on a clean glass slide with the tip facing upwards and irradiate with ultra violet light from above for at least 90 min.
Note: For single molecule force spectroscopy, cantilevers with a nominal spring constant of 0.01 to 0.1 N m-1 are suitable. Irradiation of the cantilever surface with UV light will remove organic contaminants, mainly fat substances, and render it hydrophilic on one side. If the other side is heavily contaminated – which should not be the case, or if the cantilever probes are used fresh out of the suppliers’ box – it may affect the SMFS measurement. A thorough cleaning of the whole cantilever chip using piranha solution, which has been used in many studies34, may help.
CAUTION: UV light is harmful to the eyes; therefore, irradiation of the cantilever probes should be carried out in an UV light impermeable chamber. Piranha solution is highly reactive and may burn skin, paper and other organic material. Do not use plastic containers. If placed in dishes or jars even with small amounts of organic surface contaminations (e.g., from previous use), it may react rapidly. - Replace the hydrochloric acid in the staining jar with ddH2O without letting the glass surface dry and place the jar back in the ultrasonic bath for another 10 min. Replace the water two more times for 10 min respectively to properly wash off the hydrochloric acid.
- In the meantime, dissolve ethoxy (or methoxy) silane polyethylene glycol acid (Si(OC2H5)3-PEG-COOH) in a mixture of ethanol and ddH2O (v/v 95%/5%, pH 4.6 adjusted with acetic acid) to a final concentration of 0.1 mg mL-1. Store the solution hermetically sealed in order to avoid the evaporation of the ethanol.
Note: Silane coupling agents are sensitive to moisture and temperature. Therefore, they should be stored under inert gas (N2), at low temperature (-20 °C) and under dry conditions. Before opening the flask, make sure that the silanes have reached room temperature to minimize hydration and thereby passivation of reactive groups. Heterobifunctional PEG coupling agents are available with numerous different functional groups and different spacer lengths. For random immobilization of proteins via their free amino groups (NH2), as described in this protocol, the functional group additional to the ethoxy/methoxy silane must be an NHS ester. Beside the purchase of a silane agent with NHS ester, a simple way to gain such NHS ester is to activate a carboxyl group (-COOH) with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimid (EDC) and NHS (see 1.2.1. and 1.2.2.).
CAUTION: Ethanol is flammable and skin irritating. Acetic acid is flammable and corrosive. Take care not to get reactive silanes onto skin or into eyes. Wear adequate gloves, safety goggles and laboratory coat and work under the fume hood in order to avoid inhalation of vapors. - Pour the silane solution in two separate Petri dishes, place the prepared cantilever probes and glass slides in one Petri dish respectively, seal hermetically (e.g., parafilm) to avoid evaporation of the ethanol and incubate stationary for 90 min at room temperature.
Note: The optimal size of the Petri dish depends on the number of cantilever probes and the size of the glass slides which should be functionalized. A diameter size for good handling and low reagent volume is 50-60 mm. To avoid undesired bending of the cantilever while penetrating through the air water interface the cantilever should be held at a 90° angle to the air-water interface. The incubation of the glass slides (but not the cantilever probes) can optionally be carried out on an orbital shaker. - Rinse the cantilever and glass slides in three consecutive beakers containing pure ethanol to completely wash off the unbound silane compounds.
Note: To avoid undesired bending of the cantilever while penetrating through the air water interface, the cantilever should be held at a 90° angle. - Place the functionalized glass slides in the staining jar and the cantilever on a clean glass slide and cure at 110 °C for 30 min.
Note: Curing with heat induces the formation of covalent siloxane bonds and the removal of water. As the glass slides were only functionalized on the one side, make sure to properly indicate the coated side. - Store the silanized glass samples and cantilever probes in a vacuum desiccator for up to one week.
Note: The protocol can be paused here.
- Remove coarse dust and contaminations from glass slides with isopropanol and lint-free precision wipes and cut the slides in desired size (optional).
- Random immobilization of proteins on silanized glass and silicon nitride cantilever
Note: To avoid undesired bending of the cantilever, the cantilever probe should be held at a 90° angle while penetrating any air-water interfaces.- Prepare a solution containing 42 mg mL-1 of EDC and 20 mg mL-1 NHS in standard phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4).
CAUTION: EDC is corrosive and skin irritating and can cause serious eye damage. Wear adequate gloves, safety goggles and laboratory coat. - Cover the silane coated glass slides with the solution and put silanized cantilever probes in a drop of the EDC/NHS solution and incubate for 10 min at room temperature.
Note: For the incubation of the cantilever probes, the original cantilever box is suitable. To couple proteins via their free amino groups to the carboxyls (-COOH) of the heterobifunctional silane-PEG agents, the -COOH group is activated with the widely used EDC/NHS chemistry. EDC couples NHS to the carboxylic acid, forming a “stable” NHS ester which enables efficient conjugation to primary amines at physiological pH in a next step. - Rinse the cantilever and glass slides thoroughly with PBS in three consecutive beakers in order to completely wash off excessive EDC/NHS.
Note: This washing step is critical as remaining EDC/NHS can crosslink proteins and thereby alter their functionality. - Incubate the activated glass slides with and the cantilever probes in a droplet of the desired protein solution in a wet chamber at room temperature. The protein concentration and incubation time should be adapted to meet the requirements of the experiment. In general, a concentration between 0.5 to 1 mg mL-1 and incubation times from 30 min to 2 h are suitable for most proteins. In the case of fibronectin (on glass slide) and pilus-1 tip protein RrgA (on cantilever), a molar concentration of 1.5 µM and 3 µM, respectively, and an incubation time of 2 h are sufficient.
- Wash the glass slides and the cantilever probes thoroughly with PBS in three consecutive beakers in order to wash off unbound proteins.
- Saturate the remaining NHS ester with tris(hydroxymethyl)-aminomethan by placing the probes in tris-buffered saline (TBS; 50 mM Tris, 150 mM NaCl, pH 7.6) for 20 min at room temperature.
Note: This step reduces undesired covalent coupling of proteins between the functionalized surface of the AFM tip and the glass, because amino groups of Tris can bind to the remaining activated COOH groups on the cantilever and substrate surface. - Wash the glass slides and the cantilever probes thoroughly with PBS and store them in separate Petri dishes covered in PBS until use.
Note: The samples should be prepared freshly and used the same day.
- Prepare a solution containing 42 mg mL-1 of EDC and 20 mg mL-1 NHS in standard phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4).
2. Atomic Force Microscopy Based Single Molecule Force Spectroscopy
Note: In this work, an atomic force microscope from JPK Instruments was used and the set ups for obtaining force-distance curves was defined with the Force RampDesigner.
- Cantilever calibration with the thermal noise method35
Note: For cantilever calibration, follow the step in the manual of the manufacturer. Most cantilever suppliers state an approximate spring constant, which is usually calculated from the nominal cantilever shape (length, width, thickness) and is therefore not very reliable. As the correct spring constant is crucial, it is advisable to carry out the cantilever calibration described below in triplicate and use the mean values of optical lever sensitivity and spring constant. It can be useful to record the cantilever deflection in volts (V) during the experiment and convert it to force (pN) afterwards, using the optical lever sensitivity and spring constant mean values. The spring constant and optical lever sensitivity can be determined before and/or after the experiment, as long as the laser position is not changed on the cantilever (the position of the reflected laser can be readjusted on the photodiode).- Fix a clean, fresh glass slide on the AFM sample holder and cover it with PBS buffer.
Note: The calibration should be carried out on a hard surface (e.g., glass) and in the same buffer as the actual experiments. - Fix the prepared cantilever probe at the cantilever holder, place it in the AFM head and carefully wet the cantilever with a drop of PBS buffer.
Note: The wetting of the cantilever reduces the surface tension appearing during penetration in the PBS buffer on the calibration glass slide and thereby undesirable bending of the cantilever. - Slowly move the cantilever towards the calibration surface until the cantilever is completely immersed in the PBS buffer but still away from the calibration surface.
- Use the top view optical microscope of the AFM or (if available) the inverted microscope underneath the AFM to position the laser of the AFM on the backside of the cantilever. Place the laser spot near the end of the cantilever close to where the tip is located.
Note: The laser spot should be located close to the end of the cantilever, but it should still be completely on the cantilever. If no optical microscope is available, use a piece of paper for visible laser diodes or a laser detector card for infrared laser diodes, put it underneath the AFM head and move the laser spot towards the edge of the cantilever-chip, where the cantilevers are located, until you see the spot on the paper or detector card. Then move the laser parallel to the edge. When the spot disappears, it is on a cantilever arm. For linear cantilevers, move the spot towards the end of the lever arm until it appears on the paper/detector card and move it back until it is again on the lever arm (disappears from the paper/card). For triangular, place the spot in the middle between two arms of the cantilever and move it towards the end of the cantilever, until it disappears from the paper/card. Check that you are in the middle of the cantilever by moving the spot perpendicular to the long axis of the cantilever. - Adjust the position of the four quadrant detector photodiode of the AFM in such a way that the reflected laser beam is positioned in the center of the photodiode.
Note: Proceed as follows: Use the micrometer screws near the detector diode to move the diode in horizontal and in vertical direction, until the sum signal from all four quadrants is maximized. Then move the diode in vertical direction, until the vertical deflection signal is zero, and move the diode in horizontal direction, until the lateral deflection signal is zero. Silicon nitride cantilevers usually have a gold coating and are therefore a bimetal with two different coefficients of thermal expansion. This results in a thermal drift (apparent in the vertical deflection signal) especially in solution. To reduce this drift during measurements, let the whole system equilibrate for a few minutes before starting the calibration. - Open the calibration manager in the AFM software and calibrate the cantilever sensitivity and spring constant of the cantilever with the thermal noise method as follows.
- Carefully approach the substrate surface and record a force-distance curve.
- Determine the optical lever sensitivity in nm/V by fitting a straight line to the steepest part of the retraction force curve, where the tip is in contact with the substrate surface. The sensitivity enables to convert the cantilever spring constant to pN/nm.
Note: The slope of the retraction curve is the piezo travel distance vs. the change in photodiode voltage (measured in nm/V). - Record several thermal noise spectra of the cantilever with the cantilever approximately 100 µm or more away from the surface in order to exclude any surface damping.
- Determine the spring constant of the cantilever in pN/V by fitting a harmonic oscillator provided by the AFM software to the thermal noise spectra.
- Slowly retract the cantilever and withdraw it from the solution.
- Substitute the glass surface used for cantilever calibration with the sample surface containing the immobilized proteins. Make sure that the cantilever and the sample surface (and thereby the proteins) do not dry while changing the glass slides.
- Fix a clean, fresh glass slide on the AFM sample holder and cover it with PBS buffer.
- Interaction force experiments on the single protein level
- Slowly move the moist cantilever towards the sample surface until the cantilever is completely covered by PBS buffer but still away from the substrate surface.
Note: To reduce the thermal drift during the experiment, let the whole system set for a few minutes before starting the force spectroscopy measurements. - Approach the surface and record multiple force-distance curves (≥ 500) at different locations of the sample surface, with a contact force of 250 pN, a contact time of 1 s, a retraction length of 2 µm and a retraction velocity of 1 µm s-1.
Note: For general force spectroscopy adjustments, follow the manufacturer’s manual. Variations: The retraction speed can be varied between 0.1 and 5 µm s-1 to calculate kinetical data depending on the increasing force load. The interaction time can be varied to analyze time dependent bond strengthening. Instead of keeping the retraction speed constant, one may keep the force constant (force clamp mode).
- Slowly move the moist cantilever towards the sample surface until the cantilever is completely covered by PBS buffer but still away from the substrate surface.
- Data analysis
Note: Data analysis was carried out using the data processing software. Depending on the immobilized proteins, the contact time or the retraction speed, whether a footprint was incorporated or not and other variable parameters, the force-distance curves contain multiple different information. Data analysis and interpretation can vary greatly between different SMFS experiments and can therefore not be described in detail here. As for the interaction of RrgA and Fn, the following protocol can be a first step for the analysis of SMFS data.- Open the measured force curve files by selecting the Open Batch of Force Scan icon and process the force-distance curves as follows:
- Convert the cantilever deflection (V) to the directly proportional force (F) by selecting the (Re)Calibrate the V-deflection by Adjusting Sensitivity and Spring Constant icon.
Note: If cantilever calibration was carried out before the experiment, the values are saved in the force scan files and are automatically used during calibration of the V-deflection. If the calibration was carried out after the experiment, the software uses default values, which can be changed to the measured values. - Subtract the baseline of the retraction channel in a region of the force curve far from the surface to set the zero force level by selecting the icon Baseline Subtraction.
Note: In some cases, the retraction may not have the same constant force value and the curve may display a linear tilt, which can be removed by selecting Offset + Tilt. - Define the point where the tip gets into contact with the sample by selecting the Contact Point Determination icon.
- Convert the height signal to tip-sample separation by selecting the Tip-Sample Separation icon. In addition to subtracting the contact point position, this procedure subtracts the cantilever bending to calculate the distance between substrate surface and AFM-tip.
Note: For fitting polymer elastic models, like the extensible worm-like-chain model, and the determination of interaction lengths, the tip-sample separation, which is corrected for the cantilever bending, is needed. To determine the force loading rate from the slope of the force curve and the z-piezo velocity, the uncorrected force curves should be used. - Screen the force-distance traces for force peaks occurring at rupture lengths above 70 nm (length of the stretched PEG spacer)36 to sort out nonspecific interactions and apply the extensible worm-like-chain model to the selected peaks by selecting the Fit a Polymer Chain Model icon and chose the Extensible Worm-like Chain Model. The peaks in the retraction force curve will be fitted with this model and rupture forces and lengths are obtained, together with the elastic parameters of the polymer.
- Display the data as histograms showing the force and length distributions. Use at least 100 unbinding events for the histograms.
Representative Results
The protocol described here results in a covalent immobilization of proteins via their accessible primary amines with random orientation (Figure 1). Figure 2 shows an AFM image of a silanized glass surface with (left) and without (right) Fn immobilized, recorded after the dehydration of the samples under a gentle stream of nitrogen. The silane polymer layer shows only small surface corrugations with a height of approximately 2-5 nm (Figure 2, right), while on the surface functionalized with Fn, approximately 10 nm high Fn molecules are apparent (Figure 2, left). In close-ups, the dimeric structure of the Fn can be recognized. The Fn molecules seem to be compact with a height of 4 – 5 nm above the PEG surface coating and a length of ~ 120 nm (see inserts).
To investigate the interaction forces of RrgA with Fn, which was recently described in detail by our group13, RrgA was coupled to a silicon nitride AFM tip and human Fn to the glass substrate (Figure 3a). Figure 3 shows representative tip sample separation curves of the interaction of RrgA with Fn recorded at a pulling velocity of 1 µm s-1. The used surface chemistry led to a low background interaction and well-shaped single (or double) interaction events (Figure 3a), which were fitted using an extensible worm like chain (eWLC) model (red curves). Plotting the results of the fit (rupture force and -length, see Figure 4) shows that after overcoming non-specific surface interactions between AFM tip and substrate, and stretching the PEG linkers (> 70 nm), up to ~19% of the force curves showed rupture events with a mean rupture force for the RrgA – Fn interaction of ~ 52 pN at a tip-sample distances of about 100 nm. In contrast, an inapplicable surface chemistry (here, omitted quenching with Tris buffered saline Figure 3b) will hinder a clear evaluation of single interaction events due to unspecific interactions, multiple protein binding (trace 2 and 3) and/or covalent coupling of proteins between the sample surface and the AFM cantilever tip. This leads to high rupture forces (trace 1) possibly accompanied by unfolding of the protein (Fn) domains (trace 4 and 5).
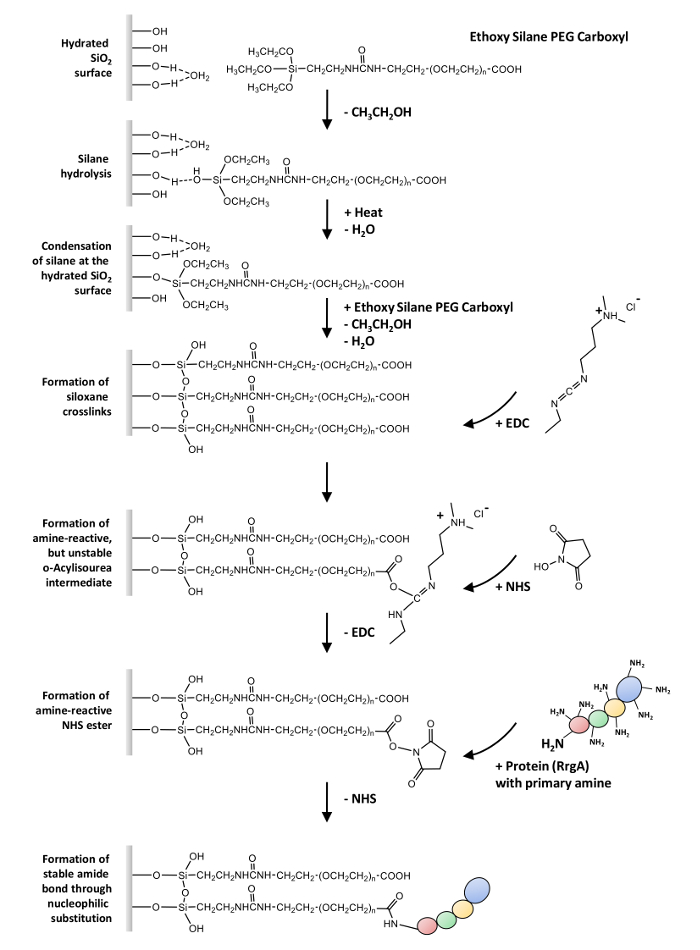
Figure 1: Overview over the surface chemistry. The hydrolysis of ethoxy silane-PEG-carboxyl is followed by its condensation at the hydrated glass surface and the formation of siloxane crosslinks. The reaction of EDC with the carboxyl groups results in a reactive o-acylisourea, an amine-reactive intermediate with an extremely short half-life in aqueous solution (hydrolysis). The intermediate is stabilized by the formation of an NHS ester which undergoes nucleophilic substitution to finally form an amide bond with primary amines on the proteins. Please click here to view a larger version of this figure.
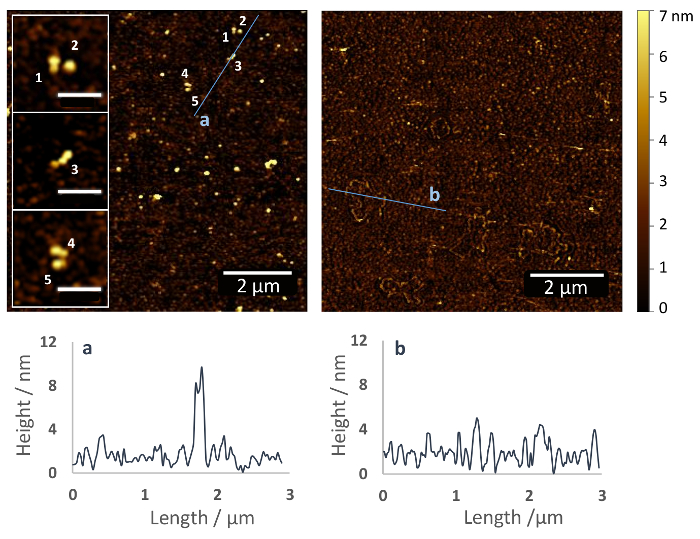
Figure 2: Immobilization of fibronectin on a glass substrate via heterobifunctional ethoxy silane PEG carboxyl coupling agent. AFM images of functionalized glass surfaces with (left) and without (right) Fn. Numbers indicate individual Fn molecules, which are distributed homogeneously on the substrate surface (inserts). The molecules adopt a dimeric and compact structure with a height of 4-5 nm above the PEG coating and a length of >100 nm. This resembles the structure of Fn in solution and is consistent with previous AFM data on other surfaces, e.g., mica (scale bar of inlays = 500 nm)37. Below the AFM images are height profiles along the lines indicated in the AFM images. Please click here to view a larger version of this figure.
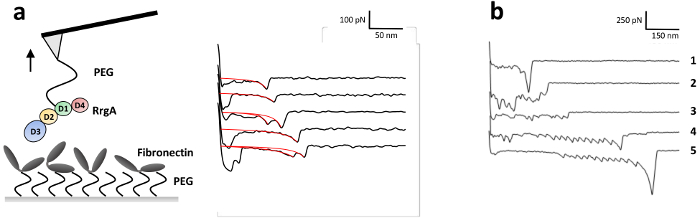
Figure 3: Illustration of a SMFS experiment and representative force distance curves of the RrgA – Fn interaction. (a) RrgA and Fn were covalently linked via the heterobifunctional ethoxy silane PEG carboxyl coupling agent to a silicon nitride AFM cantilever tip and a glass surface, respectively. Representative SMFS force distance curves obtained for RrgA – Fn interaction at a retraction velocity of 1 µm s-1 with the described immobilization of RrgA and Fn are shown. Red curves represent the extensible worm-like chain fits applied to obtain rupture forces and lengths. The figure has been modified from Becke, et al., ACSnano 201813. (b) Representative SMFS force distance curves obtained for the RrgA – Fn interaction without quenching with Tris buffered saline. In this case, the primary amine of Tris was absent so that remaining active NHS esters were left unsaturated during the experiment. This led to multi protein binding (trace 2 and 3) and the clamping of proteins between the surface and the AFM tip which resulted in high rupture forces accompanied by (Fn-) domain unfolding (trace 1, 4 and 5; note the different scales). Please click here to view a larger version of this figure.
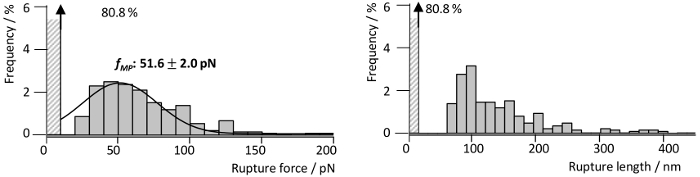
Figure 4: Force and length distribution of single RrgA – Fn interactions. Rupture force and corresponding rupture length histograms obtained from RrgA – Fn SMFS interaction measurements (n = 1400) at a retraction velocity of 1 µm s-1. The histograms reveal a most probable rupture force fMP of 51.6 pN (Gauss fit, black line) and an accumulation of rupture lengths around 100 nm. The figure has been modified from Becke, et al., ACSnano, 201813. Please click here to view a larger version of this figure.
Discussion
Since the introduction of AFM based SMFS, it evolved into a widely used technique to directly probe intra and intermolecular forces of individual proteins, nucleic acids and other biomolecules3,4,5. For successful SMFS experiments, an appropriate surface coupling strategy is a prerequisite. To probe the intramolecular forces in natural and synthetic polymers, the polymers may be directly coupled to the substrate surface and AFM tip36,38,39,40,41. For the investigation of inter-molecular interactions, such as molecular bonds, however, it is advisable to use flexible linker molecules such as hetero-bifunctional PEG linkers, or polypeptide chains, to attach the interaction partners to the AFM tip and the substrate surface, in order to allow for the correct orientation of the binding partners, to overcome short-range surface forces and to avoid denaturation and unfolding of proteins21,22,23,24,25,26,27,42. We therefore described a simple and straight forward protocol for the covalent immobilization of proteins via their accessible primary amines using hetero-bifunctional PEG spacers.
We demonstrated its applicability with the investigation of the interaction forces between adhesin RrgA from S. pneumoniae and the extracellular matrix protein Fn, as recently described in detail elsewhere13.
The surface chemistry is well established and analyzed and similar approaches have been successfully used in multiple SMFS experiments19,42,43,44,45. The silylether used for coupling the silane polymer to the surface, is subject to hydrolysis. The degree of hydrolysis depends on the amount of formed siloxane bonds, which can be controlled during the silanization process. If high interaction forces (≥ 1000 pN) are expected during SMFS measurements, the silanization should be performed via vapor-phase deposition30 which results in the formation of a continuous layer of siloxanes. As for many experiments (e.g., many protein – protein interactions), the interaction forces are in the range of a few hundred pN, and the described procedure, in which siloxane formation is carried out by deposition from an aqueous phase and unbound organo-silanes are thoughtfully washed off with ethanol (step 1.1.7) followed by curing with heat (step 1.1.8), is sufficient.
Another critical step is to wash the remaining EDC and NHS molecules off the surface (step 1.2.3), as leftovers will lead to the activation of carboxyl groups on the proteins. This may either result in crosslinking of proteins on the same surface, which can alter their functionality or covalently couple activated proteins to other proteins on the opposite surface. This may lead to clamping of the proteins between the surface and the AFM tip, which results in high rupture forces possibly accompanied by domain unfolding (see Figure 3b, trace 1, 4 and 5, unfolding of Fn domains)46. The same problem can occur, if the active NHS esters of the PEG spacer are left unsaturated. Therefore, the incubation with Tris buffered saline is recommended (step 1.2.6), as the primary amine of Tris quenches the remaining amino reactive groups.
Following the protocol stepwise leads to a homogenous distribution of Fn on the silanized glass surface (see Figure 2), leaving a dimeric form of the protein. This resembles Fn´s structure in solution and is consistent with previous AFM data on other sample surfaces37. In addition, an appropriate concentration of RrgA on the AFM tip is obtained, which generates a target value of ~20% of well-defined interaction events during SMFS measurements (Figure 3 and Figure 4). Another elegant way to control the amount of molecules coupled to the sample substrate and cantilever tip besides varying the protein concentration and/or incubation times, is the combination of silane-agents with different secondary functional groups. By changing the ratio of protein reactive groups extending from the PEG-polymer, the number of immobilized proteins can be controlled15,16,17,18.
The protocol described here can also be used to immobilize other -NH2 containing molecules or be adjusted to couple proteins to other silicon-oxide surface besides glass and silicon nitride. Depending on the protein design, the amine reactive carboxyl group can be changed to a sulfhydryl reactive group (e.g., maleimide or ortho-pyridyl disulfide) to couple the protein via its free –SH groups. For Fn, this results in a predefined orientation13,17,20.
In summary, this protocol can be adjusted to serve different requirements and is suitable for other biophysical applications besides single molecule force spectroscopy experiments.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
TB and HG acknowledge financial support through the European Research Council "Cellufuel, Advanced Grant No. 294438". HCS acknowledges financial support from the Federal Ministry for Education and Research through the Innovationsallianz Technofunktionale Proteine (TeFuProt), SS acknowledges financial support from the Bavarian State Ministry for Science and Education through the research focus "Herstellung und biophysikalische Charakterisierung dreidimensionaler Gewebe – CANTER". We thank Conny Hasselberg-Christoph and Martina Hörig for technical support
Materials
| Material | |||
| 2-Propanol | Carl Roth | 6752 | |
| 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide | Sigma-Aldrich | 03450 | EDC |
| Acetic acid | Carl Roth | 3738 | 100 %; analytical purity |
| Doubly distilled water | |||
| Ethanol | Carl Roth | 9065 | ≥ 99.8 %; analytical purity |
| Ethoxy silane polyethylene glycol acid | Nanocs | PG2-CASL-5k | 5 kDa; COOH-PEG-Si(OC2H5)3 |
| Hydrochloric acid | Carl Roth | X896 | 32 % |
| N-Hydroxysuccinimid | Merck | 804518 | NHS; for synthesis |
| Phosphate Buffered Saline – Dulbecco | Biochrom | L1825 | PBS |
| Probe molecule e.g. Fibronectin, human plasma | Sigma-Aldrich | F1056 | |
| Probe molecule e.g. RrgA | Produced in laboratory | ||
| Sodiumchlorid | Carl Roth | 9265 | NaCl |
| Tris(hydroxymethyl)-aminomethan | Carl Roth | AE15 | ≥ 99,3 %; TRIS; Buffer Grade |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Beakers | |||
| Glass cutter | |||
| Glass slides | Carl Roth | 0656 | |
| Inert gas desiccator | Sicco | ||
| Inverted Microscope – Zeiss Axiovert 200 | Zeiss | ||
| JPK NanoWizard 1 | JPK Instruments | ||
| JPK NanoWizard SPM and DP software | JPK Instruments | ||
| Laboratory oven | Binder | ||
| Magnetic stirrer | IKA | ||
| Micro spatula | |||
| Microcentrifuge tubes | |||
| Microsoft Excel | Microsoft | ||
| Parafilm M | Brand | 701606 | |
| Petri dishes | |||
| pH-meter | Knick | ||
| Pipettes | Starlab | 10-100 µl, 50-200 µl, 100-1000 µl | |
| Precision balance | Acculab | ||
| Silicon nitride cantilever – MLCT | Bruker AXS S.A.S | Spring constant ≤ 100 pN/nm | |
| Sonication bath | Bandelin | ||
| Staining jar | |||
| Stereo microscope – Zeiss Stemi | Zeiss | ||
| Stir bar | |||
| Kimtech science precision wipes | Kimberly-Clark | ||
| Twezzers | |||
| UV PenRay | UVP, LLC | 90-0012-01 | Mercury spectrum with the primary energy at 254 nm |
| Vacuum desiccator | |||
| Vacuum pump | |||
| Vortex mixer | VWR | ||
| Weighing paper | Carl Roth | TP64 |
Referenzen
- Binnig, G., Quate, C. F., Gerber, C. Atomic Force Microscope. Physical Review Letters. 56, 930-933 (1986).
- Neuman, K. C., Nagy, A. Single-Molecule Force Spectroscopy: Optical Tweezers, Magnetic Tweezers and Atomic Force Microscopy. Nature Methods. 5 (6), 491-505 (2008).
- Dufrene, Y. F. Atomic Force Microscopy, a Powerful Tool in Microbiology. Journal of Bacteriology. 184 (19), 5205-5213 (2002).
- Muller, D. J., Dufrene, Y. F. Atomic Force Microscopy as a Multifunctional Molecular Toolbox in Nanobiotechnology. Nature Nanotechnology. 3 (5), 261-269 (2008).
- Hinterdorfer, P., Dufrene, Y. F. Detection and Localization of Single Molecular Recognition Events Using Atomic Force Microscopy. Nature Methods. 3 (5), 347-355 (2006).
- Dufrene, Y. F. Sticky Microbes: Forces in Microbial Cell Adhesion. Trends in Microbiology. 23 (6), 376-382 (2015).
- Yakovenko, O., et al. FimH Forms Catch Bonds That Are Enhanced by Mechanical Force Due to Allosteric Regulation. The Journal of Biological Chemistry. 283 (17), 11596-11605 (2008).
- Casillas-Ituarte, N. N., et al. Amino Acid Polymorphisms in the Fibronectin-Binding Repeats of Fibronectin-Binding Protein A Affect Bond Strength and Fibronectin Conformation. The Journal of Biological Chemistry. 292 (21), 8797-8810 (2017).
- Hilleringmann, M., et al. Molecular Architecture of Streptococcus Pneumoniae TIGR4 Pili. The EMBO Journal. 28 (24), 3921-3930 (2009).
- Izore, T., et al. Structural Basis of Host Cell Recognition by the Pilus Adhesin from Streptococcus Pneumoniae. Structure. 18 (1), 106-115 (2010).
- Henriques-Normark, B., Tuomanen, E. I. The Pneumococcus: Epidemiology, Microbiology, and Pathogenesis. Cold Spring Harbor Perspectives in Medicine. 3 (7), a010215 (2013).
- Henderson, B., Nair, S., Pallas, J., Williams, M. A. Fibronectin: a Multidomain Host Adhesin Targeted by Bacterial Fibronectin-Binding Proteins. FEMS Microbiology Reviews. 35 (1), 147-200 (2011).
- Becke, T. D., et al. Single Molecule Force Spectroscopy Reveals Two-Domain Binding Mode of Pilus-1 Tip Protein RrgA of Streptococcus Pneumoniae to Fibronectin. ACS nano. 12 (1), 549-558 (2018).
- Herman-Bausier, P., Pietrocola, G., Foster, T. J., Speziale, P., Dufrene, Y. F. Fibrinogen Activates the Capture of Human Plasminogen by Staphylococcal Fibronectin-Binding Proteins. mBio. 8 (5), e01067 (2017).
- Vitry, P., Valotteau, C., Feuillie, C., Bernard, S., Alsteens, D., Geoghegan, J. A., Dufrene, Y. F. Force-Induced Strengthening of the Interaction between Staphylococcus aureus Clumping Factor B and Loricrin. mBio. 8 (6), e01748 (2017).
- Milles, L. F., Schulten, K., Gaub, H. E., Bernardi, R. C. Molecular Mechanism of Extreme Mechanostability in a Pathogen Adhesin. Science. 359 (6383), 1527-1533 (2018).
- Jobst, M. A., Schoeler, C., Malinowska, K., Nash, M. A. Investigating Receptor-Ligand Systems of the Cellulosome with AFM-Based Single-Molecule Force Spectroscopy. Journal of Visualized Experiments. (82), e50950 (2013).
- Stetter, F. W., Kienle, S., Krysiak, S., Hugel, T. Investigating Single Molecule Adhesion by Atomic Force Spectroscopy. Journal of Visualized Experiments. (96), e52456 (2015).
- Schmidt, S. W., Christ, T., Glockner, C., Beyer, M. K., Clausen-Schaumann, H. Simple Coupling Chemistry Linking Carboxyl-Containing Organic Molecules to Silicon Oxide Surfaces under Acidic Conditions. Langmuir: the ACS journal of surfaces and colloids. 26 (19), 15333-15338 (2010).
- Zimmermann, J. L., Nicolaus, T., Neuert, G., Blank, K. Thiol-Based, Site-Specific and Covalent Immobilization of Biomolecules for Single-Molecule Experiments. Nature Protocols. 5 (6), 975-985 (2010).
- Ott, W., Jobst, M. A., Schoeler, C., Gaub, H. E., Nash, M. A. Single-Molecule Force Spectroscopy on Polyproteins and Receptor-Ligand Complexes: The Current Toolbox. Journal of Structural Biology. 197 (1), 3-12 (2017).
- Ott, W., et al. Elastin-like Polypeptide Linkers for Single-Molecule Force Spectroscopy. ACS nano. 11 (6), 6346-6354 (2017).
- Ebner, A., et al. A New, Simple Method for Linking of Antibodies to Atomic Force Microscopy Tips. Bioconjugate Chemistry. 18 (4), 1176-1184 (2007).
- Kufer, S. K., et al. Covalent Immobilization of Recombinant Fusion Proteins with hAGT for Single Molecule Force Spectroscopy. European Biophysics Journal with Biophysics Letters. 35 (1), 72-78 (2005).
- Hinterdorfer, P., Baumgartner, W., Gruber, H. J., Schilcher, K., Schindler, H. Detection and Localization of Individual Antibody-Antigen Recognition Events by Atomic Force Microscopy. Proceedings of the National Academy of Sciences of the United States of America. 93 (8), 3477-3481 (1996).
- Hinterdorfer, P., Schilcher, K., Gruber, H. J., Schindler, H. Conjugation of Biomolecules to Tip and Probe Surfaces for Molecular Recognition in Atomic Force Microscopy. European Journal of Cell Biology. 74, 72 (1997).
- Riener, C. K., et al. Heterobifunctional Crosslinkers for Tethering Single Ligand Molecules to Scanning Probes. Analytica Chimica Acta. 497 (1-2), 101-114 (2003).
- Metwalli, E., Haines, D., Becker, O., Conzone, S., Pantano, C. G. Surface Characterizations of Mono-, Di-, and Tri-Aminosilane Treated Glass Substrates. Journal of Colloid and Interface Science. 298 (2), 825-831 (2006).
- Beyer, D., Knoll, W., Ringsdorf, H., Elender, G., Sackmann, E. Covalently Attached Polymer Mono- and Multilayers on Silanized Glass Substrates. Thin Solid Films. 284, 825-828 (1996).
- Hermanson, G. T. Chapter 13 – Silane Coupling Agents. Bioconjugate Techniques. 3, 535-548 (2013).
- Howarter, J. A., Youngblood, J. P. Optimization of Silica Silanization by 3-aminopropyltriethoxysilane. Langmuir: the ACS Journal of Surfaces and Colloids. 22 (26), 11142-11147 (2006).
- Hermanson, G. T. Chapter 6 – Heterobifunctional Crosslinkers. Bioconjugate Techniques. 3, 299-339 (2013).
- Sehgal, D., Vijay, I. K. A Method for the High Efficiency of Water-Soluble Carbodiimide-Mediated Amidation. Analytical Biochemistry. 218 (1), 87-91 (1994).
- Xue, Y., Li, X., Li, H., Zhang, W. Quantifying Thiol-Gold Interactions Towards the Efficient Strength Control. Nature Communications. 5, 4348 (2014).
- Butt, H. J., Jaschke, M. Calculation of Thermal Noise in Atomic Force Microscopy. Nanotechnology. 6, 1-7 (1995).
- Oesterhelt, F., Rief, M., Gaub, H. E. Single Molecule Force Spectroscopy by AFM Indicates Helical Structure of Poly(ethylene-glycol) in Water. New Journal of Physics. 1 (1), 6 (1999).
- Gugutkov, D., Gonzalez-Garcia, C., Rodriguez Hernandez, J. C., Altankov, G., Salmeron-Sanchez, M. Biological Activity of the Substrate-Induced Fibronectin Network: Insight Into the Third Dimension Through Electrospun Fibers. Langmuir: the ACS Journal of Surfaces and Colloids. 25 (18), 10893-10900 (2009).
- Rief, M., Oesterhelt, F., Heymann, B., Gaub, H. E. Single Molecule Force Spectroscopy on Polysaccharides by Atomic Force Microscopy. Science. 275 (5304), 1295-1297 (1997).
- Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M., Gaub, H. E. Reversible Unfolding of Individual Titin Immunoglobulin Domains by AFM. Science. 276 (5315), 1109-1112 (1997).
- Marszalek, P. E., Oberhauser, A. F., Pang, Y. P., Fernandez, J. M. Polysaccharide Elasticity Governed by Chair-Boat Transitions of the Glucopyranose Ring. Nature. 396 (6712), 661-664 (1998).
- Clausen-Schaumann, H., Rief, M., Tolksdorf, C., Gaub, H. E. Mechanical Stability of Single DNA Molecules. Biophysical Journal. 78 (4), 1997-2007 (2000).
- Schmidt, S. W., Filippov, P., Kersch, A., Beyer, M. K., Clausen-Schaumann, H. Single-Molecule Force-Clamp Experiments Reveal Kinetics of Mechanically Activated Silyl Ester Hydrolysis. ACS nano. 6 (2), 1314-1321 (2012).
- Schmidt, S. W., Beyer, M. K., Clausen-Schaumann, H. Dynamic Strength of the Silicon-Carbon Bond Observed Over Three Decades of Force-Loading Rates. Journal of the American Chemical Society. 130 (11), 3664-3668 (2008).
- Schmidt, S. W., Kersch, A., Beyer, M. K., Clausen-Schaumann, H. Mechanically Activated Rupture of Single Covalent Bonds: Evidence of Force Induced Bond Hydrolysis. Physical Chemistry Chemical Physics. 13 (13), 5994-5999 (2011).
- Schmidt, S. W., Pill, M. F., Kersch, A., Clausen-Schaumann, H., Beyer, M. K. Mechanically Induced Silyl Ester Cleavage Under Acidic Conditions Investigated by AFM-Based Single-Molecule Force Spectroscopy in the Force-Ramp Mode. Faraday Discussions. 170, 357-367 (2014).
- Rief, M., Gautel, M., Gaub, H. E. Unfolding Forces of Titin and Fibronectin Domains Directly Measured by AFM. Advances in Experimental Medicine and Biology. 481, 129-136 (2000).

