Summary
Photodynamic therapy (PDT) is a medical procedure that involves incubation of an exogenously applied photosensitizer (PS) followed by visible light photoactivation to induce apoptosis. We present an in vitro PDT protocol designed to simulate PDT that may be used to study differences in PS incubation and light treatment parameters.
Abstract
Photodynamic therapy (PDT) is a medical procedure that involves incubation of an exogenously applied photosensitizer (PS) followed by visible light photoactivation to induce cell apoptosis. The Federal Drug Administration has approved PDT for the treatment of actinic keratosis, and clinical guidelines recommend PDT as a treatment for certain non-melanoma skin cancers and acne vulgaris. PDT is an advantageous therapeutic modality as it is low cost, non-invasive, and associated with minimal adverse events and scaring. In the first step of PDT, a PS is applied and allowed to accumulate intracellularly. Subsequent light irradiation induces reactive oxygen species formation, which may ultimately lead to cell apoptosis, membrane disruption, mitochondrial damage, immune modulation, keratinocyte proliferation, and collagen turnover. Herein, we present an in vitro method to study PDT in an adherent cell line. This treatment protocol is designed to simulate PDT and may be adjusted to studying the use of PDT with various cell lines, photosensitizers, incubation temperatures, or photoactivation wavelengths. Squamous cell carcinoma cells were incubated with 0, 0.5, 1.0, and 2 mM 5-aminolevulinic acid (5-ALA) for 30 min and photoactivated with 417 nm blue light for 1,000 s. The primary outcome measure was apoptosis and necrosis, as measured by annexin-V and 7-aminoactinomycin D flow cytometry. There was a dose-dependent increase in cell apoptosis following thirty-minute incubation of 5-ALA. To achieve high inter-test validity, it is important to maintain consistent incubation and light parameters when performing in vitro PDT experiments. PDT is a useful clinical procedure and in vitro research may allow for the development of novel PSs, optimization of protocols, and new indications for PDT.
Introduction
Photodynamic therapy (PDT) is a medical procedure that involves incubation of an exogenously applied photosensitizer (PS) followed by visible light photoactivation to induce cell apoptosis. The Federal Drug Administration (FDA) has approved PDT for the treatment of actinic keratosis (AK), and clinical guidelines recommend PDT as a treatment for certain non-melanoma skin cancers and acne vulgaris1,2. Emerging non-dermatological uses for PDT include treatment of gastrointestinal, prostate, and gynecological cancers3. PDT is an advantageous therapeutic modality as it is low cost, non-invasive, and associated with minimal adverse events and scarring2.
In the first step of PDT, a PS is applied and allowed to accumulate intracellularly2. In the United States, topical 5-aminolevulinic acid (5-ALA) is commonly used to treat AKs. During the incubation phase, 5-ALA is converted into protoporphyrin IX (PP-IX) via the heme biosynthesis pathway in incorporated cells3. Cancer and pre-cancer cells have decreased ferrochelatase activity, which converts protoporphyrin IX (PP-IX), into the end product, heme. As a result, cancers cells accumulate PP-IX compared to normal cells4,5. This accumulation of PP-IX in cancer and pre-cancer cells relative to the surrounding tissue allows for PDT to be a targeted approach with minimal adverse events. Light irradiation leads to reactive oxygen species (ROS) generation when oxygen accepts excited electrons from PP-IX. PP-IX has a peak absorption in the ultraviolet spectrum with smaller peaks throughout the visible light spectrum, including blue and red light2. ROS formation may ultimately lead to cell apoptosis, membrane disruption, mitochondrial damage, immune modulation, keratinocyte proliferation, and collagen turnover6,7,8,9,10.
PDT was developed in the 1970s and is an evolving therapeutic modality3. The FDA-approved protocol for the treatment of AKs recommends 5-ALA skin incubation for 14–18 h, but 1 to 2 h incubations are commonly used in clinical practice2. In vitro, we have shown that shorter 15 min 5-ALA incubations may increase fibroblast cell apoptosis compared to untreated fibroblasts11,12. Additionally, novel chlorin, phthalocyanine, and nanoparticle-based PSs are being studied to improve PDT efficacy3. Herein, we present an in vitro method to study PDT in an adherent squamous cell carcinoma cells. In this protocol, SCC-13 cells are incubated with 0, 0.5, 1.0, and 2 mM 5-ALA for 30 min and photoactivated with 417 nm blue light for 1,000 s. The primary outcome measure is apoptosis and necrosis, as measured by annexin-V and 7-aminoactinomycin D (7-AAD) flow cytometry. This treatment protocol is designed to simulate PDT and may be adjusted to studying the use of PDT with other cell lines, photosensitizers, incubation temperatures, or photoactivation wavelengths. Preparation of additional control groups may be necessary, including unstained, single fluorophore stained, negative, and positive controls for flow cytometry. A comprehensive review of theory, protocols, experimental design, and gating for apoptosis/necrosis flow cytometry can be found elsewhere13.
Protocol
1. Preparation of Cells
- Plate 20,000 cells in 2 mL of culture medium into each well of a 6-well plate using sterile technique in a biosafety cabinet.
- Place 6-well plates in a humidified incubator (37 °C, 5% CO2) for 24 h to allow the cells to adhere to plates.
2. Preparation of Photosensitizer for Treatment of Cells
- Prepare 0.5, 1, and 2 mM 5-ALA solutions in culture medium. If fetal bovine serum (FBS) is an ingredient of culture medium, prepare and treat cells with 5-ALA in culture medium solution with 0.1% FBS for incubations.11,12 In this case, SCC-13 cells do not require FBS, so 5-ALA was added directly to keratinocyte serum-free medium supplemented with bovine pituitary extract and epidermal growth factor.
- Aspirate the culture medium and wash the cells with at least 2 mL of phosphate buffered saline (PBS).
- Aspirate PBS and add 2 mL of 0, 0.5, 1, or 2 mM 5-ALA solutions into separate plates or wells.
- Incubate the cells with 0, 0.5, 1, or 2 mM 5-ALA on a 36 to 37 °C heating block for 30 min. Protect the cells from light exposure during incubation using aluminum foil.
- Aspirate the 0, 0.5, 1, and 2 mM 5-ALA solutions and wash the cells with 2 mL of PBS.
- Aspirate PBS and add 2 mL of culture medium for blue light irradiation.
3. Blue Light Phototherapy
- Before irradiating the cells, turn on the blue light device (e.g., light-emitting diode, fluorescent, or halogen light) and run for one cycle (1,000 s) to warm up the device. The blue light device should have an output wavelength of 417 +/- 5 nm. Allowing the device to run for one cycle before treatments ensures that the blue light wavelength and irradiance are consistent throughout the photoactivation phase.
- Use a photometer to measure irradiance at the cell surface. The blue light irradiance at the cell surface should be 10 mW/cm2. The distance between the light and the cells may need to be adjusted to achieve an irradiance of 10 mW/cm2 depending on the intensity of the light source.
- Place 0, 0.5, 1, and 2 mM ALA treatment groups on a black surface under the blue light source. Irradiate the cells with blue light for 1,000 s (16 min and 40 s) for a total fluence of 10 J/cm2. Following blue light photoactivation, the cells are ready for analysis.
4. Collection and Staining
- Prepare flow buffer according to manufacturer guidelines (see Table of Materials).
- Aspirate culture medium and wash cells with 2 mL of PBS.
- Add 1 mL of 0.25% trypsin-EDTA and allow cells to detach for approximately 3 to 5 min. The cells may be examined under the microscope to confirm cell detachment.
- Add 1 mL of culture medium (or PBS) with 10% fetal bovine serum to deactivate the trypsin.
- Collect cell suspension into labeled 5 mL flow tubes.
- Spin 5 mL flow tube in a centrifuge at 201 x g for 5 min. Aspirate solution without removing cell pellet.
- Add 200 µL of flow buffer with conjugated annexin-V antibody (1 µL of annexin-v per 39 µL of flow buffer) to each sample.
- Resuspend the cells and place in the incubator (37 °C, 5% CO2) for 20 min to allow annexin-V binding.
- Add 3 µL of 7-AAD to each sample. Incubate for 5 min at room temperature.
5. Flow Cytometric Analysis
- Follow flow cytometer manufacturer guidelines for set-up (see Table of Materials).
- Collect and analyze samples with flow cytometry.13
- Perform statistical comparison of treatment and control groups using analysis of variance (ANOVA)14. Compare the means of the treatment groups to the mean of the control with a Dunnett's test for post hoc analysis.
Representative Results
After SCC-13 cells were incubated for 30 min with 0, 0.5, 1, and 2 mM 5-ALA and irradiated with 1,000 s of blue light, there was a dose-dependent increase in cell apoptosis. Total apoptosis (mean ± standard error of mean) was 3.94 ± 0.34, 7.90 ± 0.52, 23.86 ± 0.52, and 38.33 ± 1.81 after 30 min of incubation with 0, 0.5, 1, and 2 mM 5-ALA, respectively, and 1000 seconds of blue light (Figure 1). We compared the mean percentage of apoptotic cell among 0, 0.5, 1, and 2 mM 5-ALA incubated groups using ANOVA. Cells treated with 1 and 2 mM 5-ALA had a significant increase in cell apoptosis compared to 0 mM 5-ALA treated cells. Figure 2A shows representative flow cytometric gating of forward scatter (FSC) versus side scatter (SSC) for SCC-13 cells incubated with 0, 0.5, 1, and 2 mM 5-ALA. The circle gating encloses the SCC-13 cell population. For this experiment, 7500 events were collected and the SCC-13 cell population gate captured at least 90% of events. Figure 2B shows a representative plot of the 7-AAD on the x-axis and annexin-V on the y-axis. The cell populations are gated into four quadrants (Q1 to Q4) to discriminate apoptotic cells. Q1 (high annexin-v, low 7-AAD) represents cells undergoing early apoptosis. Q2 (high annexin-v, high 7-AAD) represent cells undergoing late apoptosis or necrosis. Q3 (low annexin-v, high 7-AAD) represents cells undergoing necrosis. Q4 (low annexin-v, low 7-AAD) represents cells not undergoing apoptosis or necrosis. To calculate the percentage of cells undergoing apoptosis, we added the percentage of cells in Q1 and Q2. Inconsistency in 5-ALA incubation period length, incubation temperature, or photoactivation irradiation dose may alter PDT efficacy. Figure 3 demonstrates a representative annexin-V and 7-AAD flow cytometric plot when the incubation temperature is varied (i.e., 27, 36, or 42 °C). There was a temperature dependent increase in apoptosis.
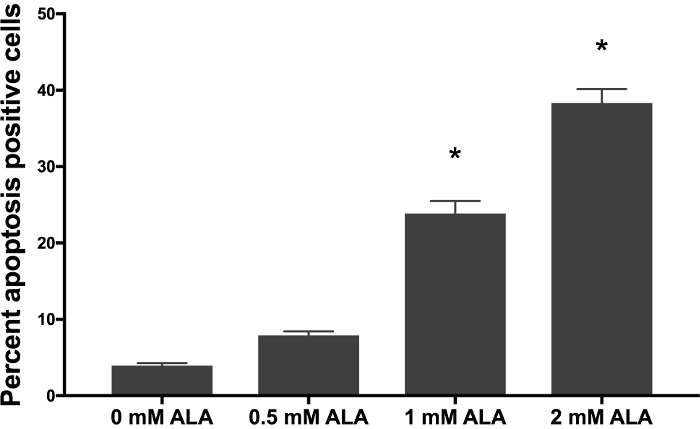
Figure 1: Dose-dependent increase in apoptosis after thirty-minute incubation of 5-ALA. 5-ALA incubated at 36 °C for thirty minutes followed by 1,000 s of blue light in SCC-13 cells. Bars represent average percent annexin-V positive cells in each treatment and control group. Experiments were performed in technical triplicate. Statistical significance was determined by ANOVA, with p <0.05 denoted by an asterisk (*). Error bars represent the mean ± standard error of the mean. Please click here to view a larger version of this figure.
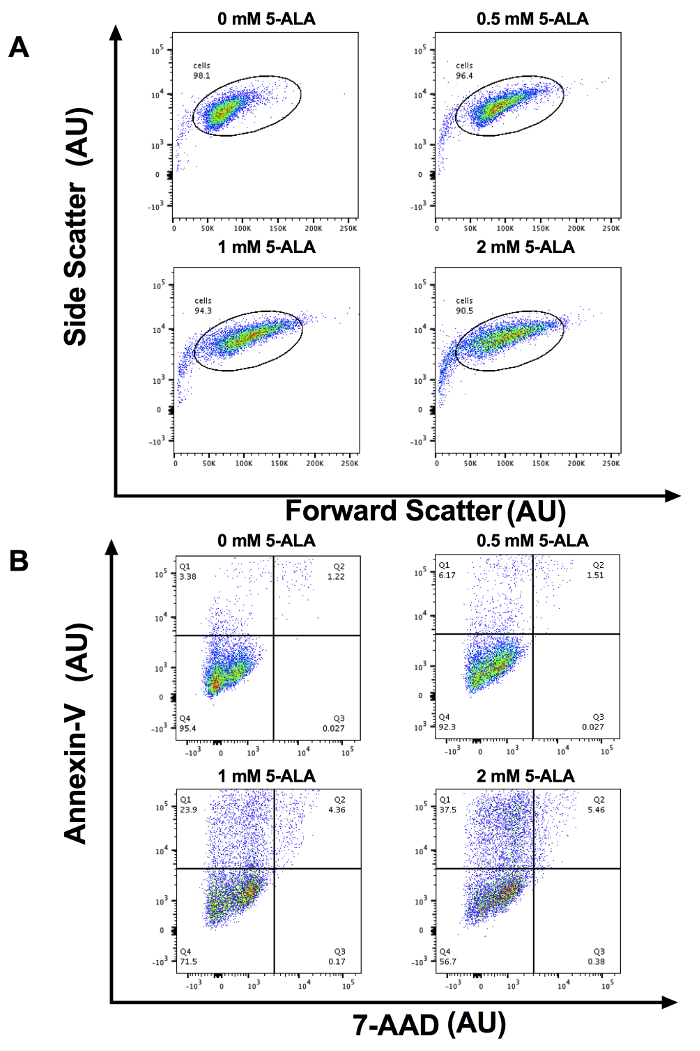
Figure 2: Representative Flow Cytometry plots. (A) Representative forward scatter vs. side scatter cytometry plots in Arbitrary Units (AU) for x- and y-axis for 0, 0.5, 1, and 2 mM 5-ALA incubated cells. For this experiment, 7500 events were collected and the SCC-13 cell population gate captured 90% of events. (B) Representative annexin-V vs. 7-AAD flow cytometry plots in AU for x- and y-axis for 0, 0.5, 1, and 2 mM 5-ALA incubated cells. Q1: early apoptotic cells, Q2: late apoptotic/necrotic cells, Q3: necrotic cells, and Q4: viable cells. Please click here to view a larger version of this figure.
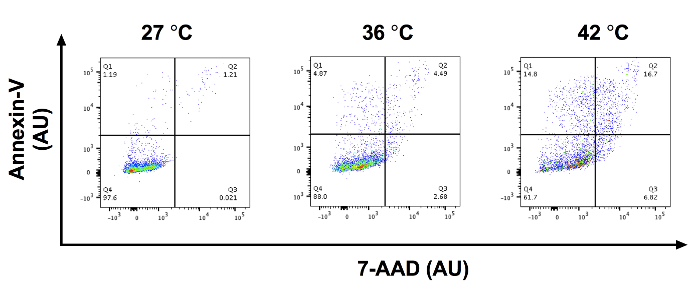
Figure 3: Incubating 5-ALA at different temperatures may alter PDT efficacy. Representative annexin-V vs. 7-AAD flow cytometry plots in AU for x- and y-axis for SCC-13 cells incubated with 0.5 mM 5-ALA at 27, 36, and 42 °C. Q1: early apoptotic cells, Q2: late apoptotic/necrotic cells, Q3: necrotic cells, and Q4: viable cells. Please click here to view a larger version of this figure.
Discussion
There was a significant increase in SCC-13 apoptosis following 30 min incubations of 0.5, 1, and 2 mM 5-ALA followed by 1,000 s of blue light. These results are consistent with our previously published research demonstrating that 5-ALA incubations of 30 min followed by blue light activation leads to a dose-dependent increase in the percentage of fibroblast cells undergoing apoptosis12,14.
This experimental approach to studying PDT has advantages and limitations. The described methods conform to clinical practice as a commercially available PS and light source were used. Researchers may customize the methods with different cell types, PSs, light sources, and irradiation parameters.However, there are limitations to this approach as this protocol is designed for adherent cells cultured in a monolayer. In clinical practice, differences in tissue architecture or disease pathology (i.e., hyperkeratosis or fibrosis) may decrease PS absorption or light penetration15. As a result, the treatment doses and experimental findings may not directly correspond to clinical practice. Spheroid tumor models and microfluidic chip have been studied as methods to more closely replicate tumor microenvironments16,17,18. Spheroids have inner and outer cellular niches that may differentially incorporate the photosensitizers and represent heterogeneous tumor architecture. Other researchers have used microfluidic chips to screen treatment conditions and vascular delivery of photosensitizers. However, microfluidic chips may be costly to develop or difficult to implement for researchers unfamiliar with the technique. Furthermore, spheroids and microfluidic chips may not reflect the clinical treatment of skin cancers in which photosensitizers are directly applied to the tumor surface without vascular dissemination. Researchers may need to evaluate the pros and cons of monolayer culture, spheroid tumor models, and microfluidic chips for studying PDT in different disease systems. As there are no immune cells or extracellular matrix, it is not possible to determine how PDT affects complex cell-cell interactions. Researchers may need to confirm in vitro experimental results using animal models and clinical trials. To achieve high inter-test validity, it is important to maintain consistent incubation and light parameters when performing in vitro PDT experiments.
Temperature is known to alter the efficacy of PDT19.One study demonstrated that cell death, 5-ALA uptake, PP-IX formation, and cytokine release may be enhanced by incubating cells with 5-ALA at temperatures up to 44 °C19. Incubation temperatures above 44 °C may lead to thermal induced cell death, and incubation temperatures below 20 °C may not lead to significant 5-ALA uptake and accumulation19. We recommend that researchers perform PS incubations on a temperature-adjustable heating block at a constant temperature to control for potentially confounding thermal effects. Additionally, it is important to incubate the photosensitizer for a sufficient amount of time to allow for the conversion of 5-ALA into PP-IX. In this protocol, SCC-13 cells were incubated with 5-ALA for 30 min, which successfully induced cell apoptosis. We have previously demonstrated that a 10 min incubation of 5-ALA did not significantly increase apoptosis in fibroblast compared to untreated control fibroblasts11,14. PP-IX starts accumulating in mouse skin after 5-ALA is incubating for six minutes at 37 °C. Therefore, after ten minutes of 5-ALA incubation, there may not be sufficient accumulation of PP-IX to induce cell apoptosis20. We recommend a minimum incubation period of 20 to 30 min for 5-ALA based on our previous studies11,14. Researchers may need to optimize incubation period based on the laboratory setting, photosensitizer of interest, cell type, and clinical indication. Antibody titration and optimization experiments may be performed for best results using manufacturers guidelines. Other assays of interest may be performed following blue light photoactivation including dihydroethidium flow cytometric quantification of ROS.14
Variation in the amount of light delivered per unit of surface area (i.e., irradiance) and total irradiation dose (i.e., fluence) during the photoactivation phase may affect ROS formation and cell apoptosis21. The relationship between fluence, irradiance, and time can be described by the following equation:
Fluence (J/cm2) = Irradiance (W/cm2) x time (s)
As fluence is dependent on time, lengthening or shortening the photoactivation phases may change treatment efficacy. The irradiance is proportional to distance squared between the light source and the target tissue. As a result, if the light is too far away, there may not be sufficient light energy delivered to the targeted tissue to excite the PS. Alternatively, the irradiance may be increased by light reflecting off the surrounding surfaces. Therefore, we recommend performing light irradiations with the cell culture plates placed on a black surface to prevent light reflection. Researchers may acquire commercially available or FDA-approved blue light-emitting diodes and fluorescent devices to use in PDT experiments, but these devices may have different power outputs and light field uniformity. A wavelength-specific photometer should be used to measure the irradiance at the cell surface and uniformity of the light field before every experiment for consistent results. A commercially available diffuser may be used to enhance field uniformity, if necessary.
In summary, we have described an in vitro approach to investigating PDT by detailing theory, experimental methods, limitations, potential pitfalls, and recommendations for optimization. PDT is a useful clinical procedure and in vitro research may allow for the development of novel PSs, optimization of protocols, and new indications for PDT.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| Keratinocyte serum free medium | Thermofisher | 17005042 | Culture medium for SSC-13 cells |
| DMEM low glucose glutamax | Thermofisher | 10567022 | Possible culture medium for other cell types |
| 6-well culture plates | Thermofisher | 720083 | |
| PBS | Thermofisher | 14190250 | |
| 0.25% Trypsin-EDTA | Thermofisher | 25200056 | |
| Trypan Blue Solution, 0.4% | Thermofisher | 15250061 | For cell counting and plating |
| BLU-U light | DUSA | Request from manufacturer | Other blue LED, flourescent, or halogen lights may used |
| 5-ALA | DUSA | Request from manufacturer | |
| FBS | Atlanta Biologicals | C17032 | |
| FlowCellect Annexin Red Kit | Millipore Sigma | FCCH100108 | Propidium iodide may replace 7-AAD. Comes with annexin-V, 7-AAD, and flow buffer |
| FACSCanto II | BD | Request from manufacturer | Any flow cytometer may be used |
| Counting Chamber | Hausser | 3120 | For cell counting and plating |
| FlowJo | FlowJo | Request from manufacturer | Flow cytometry analysis software |
Referenzen
- Morton, C., et al. European Dermatology Forum guidelines on topical photodynamic therapy. European Journal of Dermatology. 25 (4), 296-311 (2015).
- Ozog, D. M., et al. Photodynamic therapy: A clinical consensus guide. Dermatologic Surgery: Official Publication for American Society for Dermatologic Surgery. 42 (7), 804-827 (2016).
- Abrahamse, H., Hamblin, M. R. New photosensitizers for photodynamic therapy. Biochemical Journal. 473 (4), 347-364 (2016).
- Josefsen, L. B., Boyle, R. W. Photodynamic therapy and the development of metal-based photosensitisers. Metal-Based Drugs. 2008, 276109 (2008).
- Ohgari, Y., et al. Mechanisms involved in δ-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: Relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochemical pharmacology. 71 (1-2), 42-49 (2005).
- Kessel, D., Luo, Y. Mitochondrial photodamage and PDT-induced apoptosis. Journal of Photochemistry and Photobiology B: Biology. 42 (2), 89-95 (1998).
- Goldberg, D. J. Photodynamic therapy in skin rejuvenation. Clinics in Dermatology. 26 (6), 608-613 (2008).
- Sakamoto, F. H., Lopes, J. D., Anderson, R. R. Photodynamic therapy for acne vulgaris: A critical review from basics to clinical practice: Part I. Acne vulgaris: When and why consider photodynamic therapy. Journal of the American Academy of Dermatology. 63 (2), 183-193 (2010).
- Boen, M., Brownell, J., Patel, P., Tsoukas, M. M. The role of photodynamic therapy in acne: An evidence-based review. American Journal of Clinical Dermatology. 18 (3), 311-321 (2017).
- Allison, R. R., Moghissi, K. Oncologic photodynamic therapy: Clinical strategies that modulate mechanisms of action. Photodiagnosis and Photodynamic Therapy. 10 (4), 331-341 (2013).
- Koo, E., Austin, E., Mamalis, A., Jagdeo, J., et al. Thermal ultra short photodynamic therapy: heating fibroblasts during sub-30-minute incubation of 5-aminolevulinic acid increases photodynamic therapy-induced cell death. Dermatologic Surgery: Official Publication for American Society for Dermatologic Surgery. , (2017).
- Koo, E., Austin, E., Mamalis, A., Jagdeo, J. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers in Surgery and Medicine. 49 (6), 592-598 (2017).
- JoVE Science Education Database. Cell Biology. Annexin V and Propidium Iodide Labeling. JoVE. , (2018).
- Mamalis, A., Koo, E., Sckisel, G., Siegel, D., Jagdeo, J. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. British Journal of Dermatology. 175 (3), 512-519 (2016).
- Gilaberte, Y., et al. Cellular intrinsic factors involved in the resistance of squamous cell carcinoma to photodynamic therapy. Journal of Investigative Dermatology. 134 (9), 2428-2437 (2014).
- Yoon, H. K., et al. Nanophotosensitizers engineered to generate a tunable mix of reactive oxygen species, for optimizing photodynamic therapy, using a microfluidic device. Chemistry of Materials. 26 (4), 1592-1600 (2014).
- Chen, Y. -. C., Lou, X., Zhang, Z., Ingram, P., Yoon, E. High-throughput cancer cell sphere formation for characterizing the efficacy of photo dynamic therapy in 3D cell cultures. Scientific Reports. 5, 12175 (2015).
- Campos, C., Inada, N., Kurachi, C. Low-dose PDT on breast cancer spheroids. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XXVII. , (2018).
- Yang, J., et al. The influence of temperature on 5-aminolevulinic acid-based photodynamic reaction in keratinocytes in vitro. Photodermatology, Photoimmunology & Photomedicine. 26 (2), 83-88 (2010).
- Juzeniene, A., Juzenas, P., Kaalhus, O., Iani, V., Moan, J. Temperature effect on Accumulation of protoporphyrin IX after topical application of 5-aminolevulinic acid and its methylester and hexylester derivatives in normal mouse skin. Photochemistry and Photobiology. 76 (4), 452-456 (2002).
- Novak, B., Heesen, L., Schary, N., Lubbert, H. The influence of different illumination parameters on protoporphyrin IX induced cell death in squamous cell carcinoma cells. Photodiagnosis and Photodynamic Therapy. 21, 385-392 (2018).

