Direct Injection of a Lentiviral Vector Highlights Multiple Motor Pathways in the Rat Spinal Cord
Summary
This protocol demonstrates injection of a retrogradely transportable viral vector into rat spinal cord tissue. The vector is taken up at the synapse and transported to the cell body of target neurons. This model is suitable for retrograde tracing of important spinal pathways or targeting cells for gene therapy applications.
Abstract
Introducing proteins of interest into cells in the nervous system is challenging due to innate biological barriers that limit access to most molecules. Injection directly into spinal cord tissue bypasses these barriers, providing access to cell bodies or synapses where molecules can be incorporated. Combining viral vector technology with this method allows for introduction of target genes into nervous tissue for the purpose of gene therapy or tract tracing. Here a virus engineered for highly efficient retrograde transport (HiRet) is introduced at the synapses of propriospinal interneurons (PNs) to encourage specific transport to neurons in the spinal cord and brainstem nuclei. Targeting PNs takes advantage of the numerous connections they receive from motor pathways such as the rubrospinal and reticulospinal tracts, as well as their interconnection with each other throughout spinal cord segments. Representative tracing using the HiRet vector with constitutively active green fluorescent protein (GFP) shows high fidelity details of cell bodies, axons and dendritic arbors in thoracic PNs and in reticulospinal neurons in the pontine reticular formation. HiRet incorporates well into brainstem pathways and PNs but shows age dependent integration into corticospinal tract neurons. In summary, spinal cord injection using viral vectors is a suitable method for introduction of proteins of interest into neurons of targeted tracts.
Introduction
Viral vectors are important biological tools that can introduce genetic material into cells in order to compensate for defective genes, upregulate important growth proteins or manufacture marker proteins that highlight the structure and synaptic connections of their targets. This article focuses on direct injection of a highly efficient retrogradely transportable lentiviral vector into the rat spinal cord in order to highlight major motor pathways with fluorescent tracing. This method is also highly appropriate for axonal regeneration and regrowth studies to introduce proteins of interest into diverse populations of neurons and has been used to silence neurons for functional mapping studies1,2.
Many of the anatomical details of spinal motor pathways were elucidated through direct injection studies with classical tracers such as BDA and fluoro-gold3,4,5,6,7,8. These tracers are considered gold standard but may have certain disadvantages such as uptake by damaged axons, or axons in passage in the white matter surrounding an injection site9,10,11. This could lead to incorrect interpretations of pathway connectivity and may be a drawback in regeneration studies where dye absorption by damaged or severed axons could be mistaken for regenerating fibers during later analysis12.
Lentiviral vectors are popular in gene therapy studies, as they provide stable, long-term expression in neuronal populations13,14,15,16,17,18,19. However, traditionally packaged lentiviral vectors can have limited retrograde transport and may trigger immune system response when used in vivo4,20,21. A highly-efficient retrograde transport vector termed HiRet has been produced by Kato et al. by modifying the viral envelope with a rabies virus glycoprotein to create a hybrid vector that improves retrograde transport22,23.
Retrograde tracing introduces a vector into the synaptic space of a target neuron, allowing it to be taken up by that cell’s axon and transported to the cell body. Successful transport of HiRet has been demonstrated from neuronal synapses into the brains of mice and primates23,24 and from the muscle into motor neurons22. This protocol demonstrates injection into the lumbar spinal cord, specifically targeting the synaptic terminals of propriospinal interneurons and brainstem neurons. PNs receive connections from many different spinal pathways and can thus be utilized to target a diverse population of neurons in the spinal cord and brainstem. Labeled neurons in this study represent circuits innervating motor neuron pools relating to hindlimb motor function. Robust labeling is seen in the spinal cord and brainstem, including high fidelity details of dendritic arbors and axon terminals. We have also used this method in previous studies within the cervical spinal cord to label propriospinal and brainstem reticulospinal pathways25.
This protocol demonstrates injection of a viral vector into the lumbar spinal cord of a rat. As seen in Movie 1, the incision is targeted by identifying the L1 vertebra located at the last rib. This is used as a caudal landmark for a 3-4 cm incision that exposes musculature over the L1-L4 spinal cord. Laminectomies of the dorsal aspects of the T11-T13 vertebrae are performed and a beveled glass needle is directed 0.8 mm lateral from the midline and lowered 1.5 mm deep into the gray matter to inject virus.
Protocol
All of the following surgical and animal care procedures have been approved by the Animal Care and Use Committee of Temple University.
1. Pre-surgical preparations
- Prepare pulled glass needles for viral injection a few days before surgery using 3.5 nanoliter glass capillary pipettes designed for nanoliter injectors. Pull each pipette on a two-step needle puller according to the manufacturer’s instructions to create two needle templates.
- Refine the tip of the needle templates by cutting off approximately 1-2 mm of excess glass with microscissors. Measure approximate aperture size under a microscope with a microscope calibration slide to isolate needles with 30-40 µm apertures.
- With the needle positioned at 30°, use a micropipette beveller to create a tip with a 30-40 µm aperture and a 45° beveled angle. Verify aperture width with the Vernier scale on the calibration slide. Pass water and ethanol through the glass needle using a syringe with a flexible needle attachment to wash away debris and mark the needle at regular intervals with a black marker.
- Place the needles in a covered Petri dish previously cleaned with 70% ethanol and sterilize for 30 min in a Biosafety hood under UV light.
- Prepare HiRet lentivirus by removing a suitable volume from the freezer immediately prior to the procedure.
NOTE: A suitable volume includes the amount needed for injection (1 µL per injection x number of injections) plus a small amount of extra volume to account for pipetting and loading losses. Transport and store the virus on ice when not in use. - Prepare the injector by plugging it into the micropump and placing it into a micromanipulator with a Vernier scale.
- To prepare the glass needle, carefully load a colored dye such as red oil with a syringe outfitted with a flexible needle. Ensure that no bubbles remain in the needle. Use aseptic technique when handling the needle, and refrain from touching the tip.
- Insert the glass needle into the injector, ensuring that the needle is seated correctly into the washers, the injector cap is screwed on tight, and the steel injector needle is extended approximately ¾ the length of the glass needle. Virus can be loaded into the needle in a later step.
2. Anesthesia and surgical site preparation
- Weigh the animal on a digital scale. Record the pre-operative weight to determine the volume of anesthetic required and to allow for monitoring of weight post-surgery. Female Sprague-Dawley rats approximately 200–250 g were used in this protocol.
- Anesthetize the rat using either isoflurane inhalation or an injected ketamine/xylazine solution (k/x). Here, ketamine is injected intraperitoneally at a 67 mg/kg and xylazine at a 6.7 mg/kg dosage.
- Confirm an appropriate anesthetic plane by pinching the foot firmly. If reflexive withdrawal occurs, wait several additional minutes before proceeding.
NOTE: Also observe the whiskers, eyes and breathing rate for signs of consciousness. If the whiskers are twitching, the eye blinks when touched gently, or breathing is rapid and shallow, wait until the anesthetic plane is deeper to proceed with the protocol. Also monitor these signs throughout the laminectomy and injection surgery. If the animal displays a shallow anesthetic plane, administer a booster shot of ketamine-only equal to ½ the original k/x dosage. - Shave the rat along the dorsal midline from the hips to the inferior angle of the scapulae. Pull the skin of the animal taut for an easier and more precise shave.
- Apply ophthalmic ointment to both eyes.
- Apply antiseptic to the shaved area to sterilize the site. For the first scrub, soak sterile gauze with a 5% iodine solution and wipe away all hair and debris. Follow this with a unidirectional swipe with sterile gauze soaked in 70% ethanol, so that no area is contacted twice. Use this same technique with alternating iodine and ethanol-soaked gauze twice more.
3. Surgical field and instrument preparation
- Prepare a set of autoclaved surgical tools that include a scalpel, rongeurs, rat tooth forceps, spring scissors, hemostats, medium point curved forceps and retractors or weighted hooks by unwrapping the sterile wrap to create a sterile field.
- Open a package of sterile surgical gloves and place the sterile glove wrap on the table. Use this as an additional sterile field for used tools to prevent contamination of the sterile wrap.
- Drop a #10 scalpel blade onto the sterile field. Secure the blade to a handle with hemostats. Position sterile saline, 4.0 chromic catgut suture, and materials to control bleeding such as a cauterizer, sterile gauze, sterile cotton-tipped applicators (for muscle bleeds), or gelfoam or bonewax (for bone bleeds) in an accessible place.
- Retrieve the animal and set it on a sterile cloth. Place gauze underneath the bladder to collect urine. Prop up the target area with a rolled towel under the abdomen. If available, place a surgical heating pad underneath the sterile cloth, especially for longer procedures.
NOTE: Sterility is important during survival surgery. Keep a spray bottle of 70% ethanol on hand to maintain sterility of gloved hands, and a use bead sterilizer if instrument sterility is compromised, or between individual surgeries.
4. Exposing the vertebral column and identifying the laminectomy site
- Identify the area where a skin incision will be made by pressing the fingers gently at the last rib to locate the L1 vertebra. Using this as a landmark, make a 3-4 cm skin incision with a #10 surgical scalpel ending just inferior to L1 to expose the muscle. Hold the skin taut by gentle spreading and press firmly with the scalpel blade to ensure a clean incision.
- Cut and spread superficial fat with forceps and scissors if necessary. (Depending on target vertebra, there may or not be a large fat pad superficial to the muscle).
- Feel for the spinous processes with the flat of the scalpel blade or a finger. Often the midline area will be outlined by a “V” of white fascia on either side. Make a small rostral cut to allow room to grab securely onto an upper process with rat tooth forceps, then make 2 long, deep cuts as close to the processes as possible. At the deepest point of the cut, the dorsal surface of the vertebrae can be felt with the scalpel blade.
- Hold the lateral muscles aside with retractors or weighted hooks to improve visibility. Clear muscle around the processes with a scalpel, spring scissors or rongeurs to determine the shape of their heads.
NOTE: Remember that the spinal cord does not extend the full length of the vertebral column, as spinal cord tissue stops growing earlier in development than bone. This means that the target spinal level may be underneath a differently named vertebra. - Locate the T11 and the adjoining T12 and T13 processes.
NOTE: Assistance in targeting correct vertebral levels can be found in a rat spinal cord atlas and previous studies outlining landmarks in the mouse, which has a very similar vertebral structure6,33. Leave a rostral spinous process such as T9 undisturbed to give a midline landmark.
5. Performing a laminectomy
- Once the target area has been correctly identified, perform laminectomies of the dorsal aspects of T11-T13. Gently spread the vertebrae to reveal intervertebral ligaments, which are good sites to insert rongeurs for the initial bite of bone. Hold the rongeurs in a half-closed position to increase fine control.
- Remove the spinous processes and the dorsal aspect of the vertebrae by taking small bites with the rongeurs. Be careful not to damage the spinal cord or disturb the dura. Lift slightly with the rat tooth forceps to help pull the spinal cord away from the vertebrae and decrease the tendency to hit spinal cord tissue.
- Clear bone away from the midline so that the midline blood vessel can be observed. Leave a window that clearly shows the spinal cord tissue and is free of debris.
- Gently touch the spinal cord with forceps. Some animals may reflexively jump even if their anesthetic plane is deep. Apply a few drops of a numbing agent such as lidocaine directly to the spinal cord to prevent jumping during the injection procedure.
- Secure the animal in a spinal holder by fastening stabilizing forceps to spinous processes rostral and caudal to the laminectomy window. Raise the abdomen of the animal using the spinal holder to negate the effect of breathing movements. This will increase needle stability and ensure appropriate depth of injection.
6. Loading virus and positioning the injector
- Load virus into the injector by pipetting approximately 5 µL onto a piece of parafilm and positioning the needle so that the tip is inside the drop.
- Use the micropump to withdraw up to 4 µL of virus at a rate of 20–100 nL/s.
- Set the controller to inject and release a small amount of virus from the needle to ensure the tip of the needle is not blocked. Wipe off excess virus with a laboratory wipe.
NOTE: A Hamilton syringe with a steel needle may be used as an alternative to pulled glass pipettes. - Position the micromanipulator so that the Vernier scale is visible and position the needle at the midline of the spinal cord.
NOTE: The midline can sometimes be located by a large blood vessel running on the anterior surface of the spinal cord. However, this can vary in individual rats, and midline targeting should be confirmed by comparison with an intact spinous process. - Direct the needle laterally by 0.8 mm using the Vernier scale on the micromanipulator.
- Lower the needle to the spinal cord until it is indenting, but not puncturing, the dura. Using a quick twisting motion, puncture the dura with the needle until it has sunk to a depth of 1.5 mm.
7. Injecting virus into the spinal cord
- Once the needle is in place, program the injector to inject at a rate of 400 nL/min. Confirm that virus is entering the spinal cord by observing the progress of the dye front. There should be no obvious leakage or bulging of spinal cord tissue. If leakage is observed, this can sometimes be alleviated by reducing the injection speed to 200 nL/min.
- Once the injection is finished, allow the needle to rest in the spinal cord for 2–5 min (depending on volume injected) to facilitate diffusion of the virus.
- Slowly withdraw the needle and move to the next injection site. Inject 1 µL of virus into each of 6 evenly spaced sites approximately 1 mm apart along the length of the L1-L4 spinal tissue. The same needle may be used for each injection as long as it continues to function properly.
8. Wound closure and post-operative care
- Remove the animal from the spinal holder and take out retractors or hooks used to spread lateral muscle. Ensure that the wound is clear of all debris before closing.
- Suture the muscle using a 4.0 chromic catgut suture. Cut suture threads close to the knot to reduce likelihood of internal skin irritation.
- Staple the skin closed using 9 mm wound clips. To allow for optimal healing, line up the edges of the skin before stapling.
- Place the animal on a water convection warming pad and monitor until wakeful.
- Inject 5–10 mL of sterile saline subcutaneously to replenish fluids and an antibiotic such as cefazolin to prevent infection. When the animal is ambulatory, place it back in its home cage and provide initial analgesics. Monitor the rats for any sign of pain and distress and treat according to your IACUC approved procedure for alleviation of pain.
Representative Results
Successful injection and transport of the viral vector should result in transduction of a robust population of unilateral neurons in the spinal cord and in certain brainstem nuclei. Figure 1 demonstrates stereotypical labeling of neurons and axons in the thoracic spinal cord and in the pontine reticular formation of the brainstem at four weeks post-injection. Significant GFP expression is seen in neurons in the gray matter of the thoracic spinal cord on the side ipsilateral to the injection (Figure 1A, boxed area). A few neurons are also observed on the contralateral side, especially near the midline. In the white matter, GFP expression is observed in axons in the ipsilateral cord (Figure 1A, arrows and arrowheads), especially in areas typical to propriospinal axons (arrowheads). Figure 1A’ shows a higher magnification of the boxed area in A, demonstrating typical expression in neuronal cell bodies and dendrites. GFP expression in neurons can also be observed in brainstem nuclei such as the pontine reticular formation (Figure 1B, higher magnification of the boxed area in Figure 1B’).
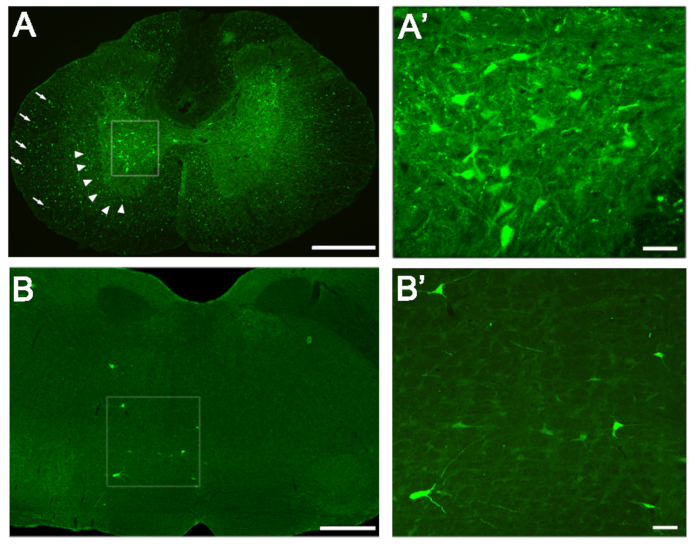
Figure 1: Transduction of Neurons in the Spinal Cord and Brainstem. (A) GFP expression in neurons (boxed area), axons of propriospinal neurons (arrowheads) and axons of other tracts (arrows) in the thoracic spinal cord. (A’) Higher magnification of neuronal expression in the boxed area of A. (B) The pontine reticular formation in the brainstem expressing GFP-labeled neurons and dendrites. (B’) Higher magnification of the boxed area in B. Scale bars: (A) = 500 μm; (B) = 1 mm; (A’), (B’) = 50 μm. Please click here to view a larger version of this figure.
Movie 1: Targeting the injection into the lumbar spinal cord of the rat. This video summaries the basics of the targeting and injection of a viral vector into the rat spinal cord. Please click here to view this video. (Right-click to download.)
Discussion
Genetic manipulation of neurons in the brain and spinal cord has served to highlight sensory, motor and autonomic pathways via fluorescent tracing and to explore regrowth potential of neuronal tracts after injury27,28,29,30,31,32,33. Direct injection of a retrogradely transportable viral vector into the spinal cord can target neuronal populations via their synaptic connections, making this method an excellent choice for mapping pathways in the central nervous system. The HiRet vector specifically shows selective uptake at the neuronal synapse22,24,25, and previous tracing with similarly structured vectors showed no spread into injured axons or unrelated cells34,35, which is consistent with results featuring the HiRet construct22,23,25. Thus, direct injection of HiRet as a retrograde tracer is an ideal method for exploring interneuronal circuits that undergo plasticity after injury.
There are two critical concepts involved in successful injection of a viral vector into the spinal cord. The first is establishing the correct target with the glass needle, and the second is ensuring adequate flow of the virus from the needle into the tissue. As this protocol involves retrograde tracing, targeting the correct area necessitates a thorough understanding of the synaptic connections of the neuronal population of interest. Propriospinal and reticulospinal neurons connecting to the lumbar spinal cord are targeted here. These neurons are spread throughout the brainstem and cervical and thoracic spinal cord segments, with the majority of PNs localized to laminae V-VII within the gray matter. To ensure transduction of an adequate population of neurons, six viral injections are made over an area spanning four spinal segments. The L1-L4 segments are chosen due to the presence of the central pattern generators, and their known connections to other areas of the spinal cord. Once the correct spinal segment(s) and laminae are known, physical location of these targets via anatomical landmarks is crucial. Atlases showing details of anatomical structure and vertebral shape can be helpful to confirm targeting landmarks8,36. It should be noted that the majority of this protocol remains the same whether one injection or six are made; the difference is only in the number of spinal segments exposed via laminectomy, and the fact that you will need to reload the glass needle with additional virus if injecting more than 4 µL. The protocol can also be easily adapted for the mouse. Due to its smaller size, the volume of virus injected into the cord should be adjusted downward and the measurements needed to hit the correct spinal laminae adjusted. Several videos of similar surgeries on the mouse have previously been published37,38.
The next consideration is adequate diffusion into the tissue. Careful preparation of the needle is necessary to ensure that the aperture is sufficient for the viral suspension to flow outward and that debris does not block the tip, and visualization of the flow from the needle into the spinal cord via a front of colored dye is helpful to confirm that the suspension is penetrating the tissue. Once the suspension has been distributed into the tissue, maintaining the needle within the spinal cord for 2-5 minutes will ensure adequate diffusion.
Successful transduction of a target population can also depend on intrinsic properties of the virus injected such as titer, serotype and infection efficiency. We find a genomic copy titer of at least 1010 GC/mL for HiRet lentivirus to work well for in vivo experimentation. Higher titers or injection volumes might show higher labeling index, but care needs to be taken since virus could diffuse out of the intended area or into the contralateral spinal cord. Consideration should also be given to the type of vector appropriate for labeling cells of interest based on their tropism. Certain viral serotypes may have better infection and transduction rates in different populations of neurons39. Traditional lentiviral vectors are known to infect a broad range of cell types due to the VSV-G envelope protein, however modifications to this vector may influence its tropism40. The HiRet construct modifies the envelope by pseudotyping with a fusion glycoprotein (FuG-B) of rabies virus, which allows for highly-efficient retrograde transport22,23 and is effective in transducing propriospinal and reticulospinal tracts, with neuronal numbers comparable to tract tracing via methods such as fluorogold and microruby4,41 (for details of construction of the HiRet vector see Hirano et al.)22. However, it did not sufficiently label the adult corticospinal tract in these experiments, though it does label the CST in neonates with high efficiency in other studies1. It is possible that receptors necessary for HiRet uptake at the targeted synapses, such as NCAM or p75NTR, are only weakly expressed on adult CST neurons42,43, though this is still being investigated. In any case, this demonstrates the importance of determining whether the vector being used is appropriate for the targeted cells. In this experiment, brain and spinal cord tissue were processed and probed after four weeks to ensure abundant time for amplification and transport from the lumbar cord to all brain areas. HiRet is transported via fast retrograde axonal transport, and thus a shorter experimental period may be appropriate if the distance traveled is lesser, such as if the injection area is in the cervical spinal cord.
Direct injection surgery is a useful tool for introduction of viral vector technology into the spinal cord. Use of HiRet for genetic experimentation is advantageous as it permits stable, long lasting transgene expression, and is non-toxic to neurons. In this experiment, no GFP labeling was seen in neurons that do not make direct synaptic connections to the injection area. This was also true in previous studies in animals with a thoracic contusion injury25. Additionally, HiRet-GFP was unable to label spinal motor neurons or dorsal root ganglion neurons when injected into the transiently demyelinated sciatic nerve (unpublished observations). Together, these data suggest that HiRet does not readily enter through axons and efficiently transduces neurons by uptake of the vector at synapses, providing a more detailed and high-fidelity map of neuronal connections of the targeted population. This is an advantage over other retrogradely transportable viral vectors such as retrograde adeno-associated virus (rAAV-retro), which is known to be taken up by axons in passage44 and makes HiRet especially useful in studies mapping regenerating and reconnecting circuitry in the injured spinal cord. HiRet’s advantages may also allow for specific targeting of neuronal populations for silencing or ablation studies25,44,45,46,47.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was funded by a grant from the National Institute of Neurological Disorders and Stroke R01 R01NS103481 and the Shriners Hospital for Pediatric Research grants SHC 84051 and SHC 86000 and the Department of Defense (SC140089).
Materials
| #10 Scalpel Blades | Roboz | RS-9801-10 | For use with the scalpel. |
| 1 mL Syringes | Becton, Dickinson and Company | 309659 | For anesthetic IP injection, potential anesthetic booster shots, and antibiotic injections. |
| 10mL Syringes | Becton, Dickinson and Company | 309604 | For injecting saline into the animal, post-surgery. |
| 4.0 Chromic Catgut Suture | DemeTECH | NN374-16 | To re-bind muscle during closing. |
| 48000 Micropipette Beveler | World Precision Instruments | 32416 | Used to bevel the tips of the pulled glass capillary tubes to form functional glass needles. |
| 5% Iodine Solution | Purdue Products L.P. | L01020-08 | For use in sterilzation of the surgical site. |
| 70% Ethanol | N/A | N/A | For sterilization of newly prepared glass needles, animal models during surgical preparation, and surgeon's hands during surgery, as well as all other minor maintainances of sterility. |
| Anesthetic (Ketamine/Xylazine Solution) | Zoetis | 240048 | For keeping the animal in the correct plane of consciousness during surgery. |
| Antibiotic (Cefazolin) | West-Ward Pharmaceuticals | NPC 0143-9924-90 | To be injected subcutaneously to prevent infection post-surgery. |
| Bead Sterilizer | CellPoint | 5-1450 | To heat sterilize surgical instruments. |
| Bonewax | Fine Science Tools | 19009-00 | To seal up bone in the case of bone bleeding. |
| Cauterizer | Fine Science Tools | 18010-00 | To seal any arteries or veins severed during surgery to prevent excessive blood loss. |
| Digital Scale | Okaus | REV.005 | For weighing the animal during surgical preparation. |
| Flexible Needle Attachment | World Precision Instruments | MF34G-5 | For cleaning glass needles and loading red oil into glass needles. |
| Gelfoam | Pfizer | H68079 | To seal up bone in the case of bone bleeding. |
| Glass Capillary Tubes | World Precision Instruments | 4878 | For pulled glass needles – should be designed for nanoliter injectors. |
| Hair Clippers | Oster | 111038-060-000 | For clearing the surgical site of hair. |
| Hemostats | Roboz | RS-7231 | For general use in surgery. |
| Kimwipes | Kimtech | 34155 | For general use in surgery. |
| Medium Point Curved Forceps | Roboz | RS-5136 | For general use in surgery. |
| Micromanipulator with a Vernier Scale | Kanetec | N/A | For precise targeting during surgery. |
| Microscissors | Roboz | RS-5621 | For cutting glass whisps off of freshly pulled glass capillary tubes. |
| Microscope with Light and Vernier Scale Ocular | Leitz Wetzlar | N/A | Used to visualize and measure beveling of pulled glass capillary tubes into functional glass needles. |
| MicroSyringe Pump Controller | World Precision Instruments | 62403 | To control the rate of injection. |
| Nanoliter 2000 Pump Head Injector | World Precision Instruments | 500150 | To load and inject virus in a controlled fashion. |
| Needle Puller | Narishige | PC-100 | To heat and pull apart glass capillary tubes to form glass needles. |
| Ophthalamic Ointment | Dechra Veterinary Products | RAC 0119 | To protect the animal's eyes during surgery. |
| Parafilm | Bemis | PM-996 | To assist with loading virus into the nanoinjector. |
| PrecisionGlide Needles (25G x 5/8) | Becton, Dickinson and Company | 305122 | For use with the 1mL and 10 mL syringes to allow injection of the animal model. |
| Rat Tooth Forceps | Roboz | RS-5152 | For griping spinous processes. |
| Red Oil | N/A | N/A | To provide a front for visualization of virus entering tissue during injection. |
| Retractors | Roboz | RS-6510 | To hold open the surgical wound. |
| Rimadyl Tablets | Bio Serv | MP275-050 | For pain management post-surgery. |
| Rongeurs | Roboz | RS-8300 | To remove muscle from the spinal column during surgery. |
| Scalpel Blade Handle | Roboz | RS-9843 | To slice open skin and fat pad of animal model during surgery. |
| Scissors | Roboz | RS-5980 | For general use in surgery. |
| Stainless Steal Wound Clips | CellPoint | 201-1000 | To bind the skin of the surgical wound during closing. |
| Staple Removing Forceps | Kent Scientific | INS750347 | To remove the staples, should they be applied incorrectly. |
| Sterile Cloth | Phenix Research Products | BP-989 | To provide a sterile surface for the operation. |
| Sterile Cotton-Tipped Applicators | Puritan | 806-WC | To soak up blood in the surgical wound while maintaining sterility. |
| Sterile Gauze | Covidien | 2146 | To clean the surgical area and surgical tools while maintaining sterility. |
| Sterile Saline | Baxter Healthcare Corporation | 281324 | For use in blood clearing, and for replacing fluids post-surgery. |
| Surgical Gloves | N/A | N/A | For use by the surgeon to maintain sterile field during surgery. |
| Surgical Heating Pad | N/A | N/A | For maintaining the body temperature of the animal model during surgery. |
| Surgical Microscope | N/A | N/A | For enhanced visualization of the surgical wound. |
| Surgical Stapler | Kent Scientific | INS750546 | To apply the staples. |
| T/Pump Heat Therapy Water Pump | Gaymar | TP500C | To pump warm water into the water convection warming pad. |
| Water Convection Warming Pad | Baxter Healthcare Corporation | L1K018 | For use in the post-operational recovery area to maintain the body temperature of the unconscious animal. |
| Weighted Hooks | N/A | N/A | To hold open the surgical wound. |
Referenzen
- Wang, X., et al. Deconstruction of corticospinal circuits for goal-directed motor skills. Cell. 171 (2), 440-455 (2017).
- Kinoshita, M., et al. Genetic dissection of the circuit for hand dexterity in primates. Nature. 487 (7406), 235-238 (2012).
- Brichta, A. M., Grant, G. Cytoarchitectural organization of the spinal cord. The rat nervous system. Vol. 2, hindbrain and spinal cord. , (1985).
- Liang, H., Paxinos, G., Watson, C. Projections from the brain to the spinal cord in the mouse. Brain Structure & Function. 215 (3-4), 159-186 (2011).
- Rexed, B. The cytoarchitectonic organization of the spinal cord in the cat. The Journal of Comparative Neurology. 96 (3), 414-495 (1952).
- Schmued, L. C., Fallon, J. H. Fluoro-gold: A new fluorescent retrograde axonal tracer with numerous unique properties. Brain Research. 377 (1), 147-154 (1986).
- Veenman, C. L., Reiner, A., Honig, M. G. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. Journal of Neuroscience Methods. 41 (3), 239-254 (1992).
- Watson, C., Paxinos, G., Kayalioglu, G., Heise, C. Atlas of the rat spinal cord. The spinal cord. , 238-306 (2009).
- Brandt, H. M., Apkarian, A. V. Biotin-dextran: A sensitive anterograde tracer for neuroanatomic studies in rat and monkey. Journal of Neuroscience Methods. 45 (1-2), 35-40 (1992).
- Geed, S., van Kan, P. L. E. Grasp-based functional coupling between reach- and grasp-related components of forelimb muscle activity. Journal of Motor Behavior. 49 (3), 312-328 (2017).
- Reiner, A., Veenman, C. L., Medina, L., Jiao, Y., Del Mar, N., Honig, M. G. Pathway tracing using biotinylated dextran amines. Journal of Neuroscience Methods. 103 (1), 23-37 (2000).
- Steward, O., Zheng, B., Banos, K., Yee, K. M., et al. Response to: Kim et al., "axon regeneration in young adult mice lacking nogo-A/B." neuron 38, 187-199. Neuron. 54 (2), 191-195 (2007).
- Brown, B. D., et al. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 110 (13), 4144-4152 (2007).
- Lo Bianco, C., et al. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 101 (50), 17510-17515 (2004).
- Malik, P., Arumugam, P. I., Yee, J. K., Puthenveetil, G. Successful correction of the human cooley’s anemia beta-thalassemia major phenotype using a lentiviral vector flanked by the chicken hypersensitive site 4 chromatin insulator. Annals of the New York Academy of Sciences. 1054, 238-249 (2005).
- Pawliuk, R., et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 294 (5550), 2368-2371 (2001).
- Wang, G., et al. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. The Journal of Clinical Investigation. 104 (11), R55-R62 (1999).
- Liang, H., Paxinos, G., Watson, C. The red nucleus and the rubrospinal projection in the mouse. Brain Structure & Function. 217 (2), 221-232 (2012).
- Abdellatif, A. A., et al. delivery to the spinal cord: comparison between lentiviral, adenoviral, and retroviral vector delivery systems. Journal of Neuroscience Research. 84 (3), 553-567 (2010).
- DePolo, N. J., et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Molecular Therapy. 2 (3), 218-222 (2000).
- Higashikawa, F., Chang, L. Kinetic analyses of stability of simple and complex retroviral vectors. Virology. 280 (1), 124-131 (2001).
- Hirano, M., Kato, S., Kobayashi, K., Okada, T., Yaginuma, H., Kobayashi, K. Highly efficient retrograde gene transfer into motor neurons by a lentiviral vector pseudotyped with fusion glycoprotein. PLoS One. 8 (9), e75896 (2013).
- Kato, S., et al. A lentiviral strategy for highly efficient retrograde gene transfer by pseudotyping with fusion envelope glycoprotein. Human Gene Therapy. 22 (2), 197-206 (2011).
- Kato, S., et al. Selective neural pathway targeting reveals key roles of thalamostriatal projection in the control of visual discrimination. The Journal of Neuroscience. 31 (47), 17169-17179 (2011).
- Sheikh, I. S., Keefe, K. M., et al. Retrogradely transportable lentivirus tracers for mapping spinal cord locomotor circuits. Frontiers in Neural Circuits. 12, 60 (2018).
- Harrison, M., et al. Vertebral landmarks for the identification of spinal cord segments in the mouse. NeuroImage. 68, 22-29 (2013).
- Tang, X. Q., Heron, P., Mashburn, C., Smith, G. M. Targeting sensory axon regeneration in adult spinal cord. The Journal of Neuroscience. 27 (22), 6068-6078 (2007).
- Cameron, A. A., Smith, G. M., Randall, D. C., Brown, D. R., Rabchevsky, A. G. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. The Journal of Neuroscience. 26 (11), 2923-2932 (2006).
- Liu, Y., Keefe, K., Tang, X., Lin, S., Smith, G. M. Use of self-complementary adeno-associated virus serotype 2 as a tracer for labeling axons: Implications for axon regeneration. PLoS One. 9 (2), e87447 (2014).
- Chamberlin, N. L., Du, B., de Lacalle, S., Saper, C. B. Recombinant adeno-associated virus vector: Use for transgene expression and anterograde tract tracing in the CNS. Brain Research. 793 (1-2), 169-175 (1998).
- Filli, L., et al. Bridging the gap: A reticulo-propriospinal detour bypassing an incomplete spinal cord injury. The Journal of Neuroscience. 34 (40), 13399-13410 (2014).
- Williams, R. R., Pearse, D. D., Tresco, P. A., Bunge, M. B. The assessment of adeno-associated vectors as potential intrinsic treatments for brainstem axon regeneration. The Journal of Gene Medicine. 14 (1), 20-34 (2012).
- Smith, G. M., Onifer, S. M. Construction of pathways to promote axon growth within the adult central nervous system. Brain Research Bulletin. 84 (4-5), 300-305 (2011).
- Morcuende, S., Delgado-Garcia, J. M., Ugolini, G. Neuronal premotor networks involved in eyelid responses: Retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. The Journal of Neuroscience. 22 (20), 8808-8818 (2002).
- Ugolini, G. Specificity of rabies virus as a transneuronal tracer of motor networks: Transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. The Journal of Comparative Neurology. 356 (3), 457-480 (1995).
- Gelderd, J. B., Chopin, S. F. The vertebral level of origin of spinal nerves in the rat. The Anatomical Record. 188 (1), 45-47 (1977).
- Inquimbert, P., Moll, M., Kohno, T., Scholz, J. Stereotaxic injection of a viral vector for conditional gene manipulation in the mouse spinal cord. Journal of Visualized Experiments. 73, e50313 (2013).
- Carbajal, K. S., Weinger, J. G., Whitman, L. M., Schaumburg, C. S., Lane, T. E. Surgical transplantation of mouse neural stem cells into the spinal cords of mice infected with neurotropic mouse hepatitis virus. Journal of Visualized Experiments. 53, e2834 (2011).
- Snyder, B. R., et al. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Human Gene Therapy. 22 (9), 1129-1135 (2011).
- Cronin, J., Zhang, X. Y., Reiser, J. Altering the tropism of lentiviral vectors through pseudotyping. Current Gene Therapy. 5 (4), 387-398 (2005).
- Reed, W. R., Shum-Siu, A., Onifer, S. M., Magnuson, D. S. Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neurowissenschaften. 142 (4), 1195-1207 (2006).
- Mao, X., Schwend, T., Conrad, G. W. Expression and localization of neural cell adhesion molecule and polysialic acid during chick corneal development. Investigative Ophthalmology & Visual Science. 53 (3), 1234-1243 (2012).
- Charles, P., et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proceedings of the National Academy of Sciences of the United States of America. 97 (13), 7585-7590 (2000).
- Tervo, D. G., et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 92 (2), 372-382 (2016).
- Tohyama, T., et al. Contribution of propriospinal neurons to recovery of hand dexterity after corticospinal tract lesions in monkeys. Proceedings of the National Academy of Sciences of the United States of America. 114 (3), 604-609 (2017).
- Liu, Y., et al. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron. 95 (4), 817-833 (2017).
- Kinoshita, M., et al. Genetic dissection of the circuit for hand dexterity in primates. Nature. 487 (7406), 235-238 (2012).

