A Pre-Clinical Porcine Model of Orthotopic Heart Transplantation
Summary
Here, we describe a pre-clinical large-animal (porcine) model of orthotopic heart transplantation that has been firmly established and utilized to investigate novel cardioprotective strategies.
Abstract
Fifty-years following the first successful report, cardiac transplantation remains the gold-standard treatment for eligible patients with advanced heart failure. Multiple small-animal models of heart transplantation have been used to study the acute and long-term effects of novel therapies. However, few are tested and demonstrated success in clinical trials. It is of critical importance to evaluate new therapies in a clinically relevant large-animal model for efficient and reliable translation of basic studies' findings. Here, we describe a pre-clinical large-animal (porcine) model of orthotopic heart transplantation that has been firmly established and previously used to investigate novel cardioprotective strategies. This procedure focuses on acute ischemia-reperfusion injury and is a reliable method to investigate novel interventions which have been tested and validated in smaller experimental models, such as the murine model. We demonstrate its usefulness in assessing cardiac performance during the early post-transplantation period and other potential possibilities enabled by the model.
Introduction
Fifty-years following the first successful report, cardiac transplantation remains the gold-standard treatment for eligible patients with advanced heart failure1. Although ischemic times of up to four hours are tolerated adequately, an ischemic time of greater than six hours is associated with inferior outcomes2. Primary graft dysfunction remains the principal cause of early morbidity and mortality following transplantation2,3. The causes of primary graft dysfunction are multifactorial and include the use of marginal organs, recipient pulmonary vascular disease, hyperacute rejection, and ischemia-reperfusion injury sustained at the time of transplantation3.
Multiple studies have investigated novel methods for donor heart preservation to reduce the incidence of primary graft dysfunction4,5,6,7. It is common practice to assess new techniques and treatments in murine models of ischemia-reperfusion injury or heterotopic heart transplantation. Additionally, small animal models permit survival models and long-term follow-up to investigate the development of rejection and cardiac allograft vasculopathy11,12,13. However, most of these strategies fail initial clinical pilot trials or never reach this stage. It is of paramount importance to evaluate new therapies in a clinically relevant large-animal model for efficient and reliable translation of basic studies' findings.
The porcine heart is often considered the most anatomically similar to the human heart when using large-animal models. As such, it is an ideal platform to perform cardiac surgical research. However, there are several important factors to consider when using a porcine model. First, the tissue is typically described as fragile and friable, especially in the right atrium and the pulmonary artery, being prone to tears14. Additionally, the pig heart is considered sensitive to manipulation and prone to arrhythmias, which is why one should routinely administer an anti-arrythmetic to each animal at the beginning of the experiment. An important anatomical difference between the porcine model and clinical heart transplantation is the left hemiazygous vein in the swine which drains directly into the coronary sinus. This has to be ligated during the recipient procedure to avoid continuous bleeding. Finally, the porcine model is very sensitive to ischemia, but it is still appropriate for acute studies in heart transplantation15.
This manuscript describes a pre-clinical large-animal (porcine) model of orthotopic heart transplantation that has been firmly established and utilized to investigate novel cardioprotective strategies5,6,8,9.
Protocol
The institutional animal care committee approved all experimental protocols and animals were treated following the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, 1996. Male Yorkshire pigs (40–50 kg) were used to perform the orthotopic heart transplants (animal size can vary according to the investigators discretion and experimental goals).
1. Donor Procedure
- Anesthetic induction and animal preparation:
- Premedicate the animal using an intramuscular injection of Ketamine (20 mg/kg), Midazolam (0.3 mg/kg), and Atropine (0.04 mg/kg). Perform anesthetic induction and maintenance using inhalational Isoflurane (end tidal concentration: 1%–3%) + 3 L/min O2 via face mask.
- Confirm anesthetic adequacy by ensuring a relaxed jaw tone and an absence of pain during toe pinch. Anesthetic adequacy needs to follow institutional guidelines.
- Once anesthetic adequacy is confirmed, perform an orotracheal intubation using an endotracheal tube of size 6.5–8 mm.
- Place an oxygen saturation monitor on the ear or bottom lip for continuous monitoring. Place the cautery electrode pad on the animal’s back.
- Insert a peripheral intravenous access via ear vein (e.g. 20 g Angiocath). Start a maintenance infusion (e.g. 0.9% NaCl). Administer 2 g of Magnesium Sulfate to prevent arrhythmias.
- Insert a percutaneous central venous sheath introducer using the Seldinger technique into the right jugular vein if right-sided heart catheterization and cardiac output measurements are to be performed. Place the animal in Trendelenburg position to facilitate venous access. Alternatively, this can be done in the left internal jugular vein. If access cannot be established, this step may be performed following midline sternotomy by dissecting the internal jugular vein (left or right) and inserting the sheath directly.
- Donor heart procurement:
- Perform a midline sternotomy from the mid cervical region to below the xiphoid process using a cautery pen. Open the sternum with a bone saw. Ensure adequate hemostasis throughout the entire procedure (e.g. cauterize sternum, and/or application of bone wax).
- In the cervical region, retract the sternocleidomastoid muscle to the center and dissect the right carotid artery (alternatively, this can be done with the left carotid artery). Place an arterial access line (e.g. 20 g Angiocath) into the right carotid artery for invasive arterial pressure monitoring.
- Dissect and remove the thymus from over the pericardium. Gently lift the thymus from the pericardium, using the cautery, and dissect the structure from the pericardium. To prevent bleeding, cauterize the small vessels that originate from the aorta and superior vena cava (SVC) to irrigate the thymus.
- Open the pericardium. Dissect the aortopulmonary space using the cautery. For this, have the assistant retract the right ventricular outflow track inferiorly and the pulmonary artery to the left and have the surgeon retract the aorta to the right. Do this carefully to avoid direct lesions to the pulmonary artery.
- Clear the anterior aspect of the ascending aorta from connective tissue. Carefully retract the right ventricular outflow tract and place a purse-string suture using a 4-0 prolene on the proximal ascending aorta adventitia (avoid full thickness sutures). Secure this suture with a tourniquet.
- Administer 30,000 units of heparin (>300 U/Kg) to achieve systemic anticoagulation. Insert the cardioplegia delivery cannula (e.g. DLP aortic root cannula) into the ascending aorta between the purse-string suture previously placed and secure by tightening the tourniquet. Prepare the cardioplegic solution that will be used and connect to the delivery cannula.
- Open the SVC and inferior vena cava (IVC), and the left inferior pulmonary vein to ensure adequate cardiac venting. Place the aortic cross-clamp on the distal ascending aorta (above the cardioplegia cannula). Alternatively, the left ventricle can be vented by opening the left atrial appendage and placing suction.
- Initiate cardioplegia infusion targeting an aortic root pressure of 80 to 100 mmHg.
NOTE: The authors applied a model using 1.5 L of a standard extracellular hyperkalemic cardioplegic solution at 4 °C. Different solutions and volumes can be used according to the experimental set-up. - Place ice slush (0.9% NaCl) in the thoracic cavity and over the organ for cooling following initiation of cardioplegia. After the cardioplegia infusion is finished, proceed with cardiectomy in a traditional manner. Section the aorta and the pulmonary artery after the innominate artery and at the bifurcation, respectively, to ensure sufficient length for implant.
- After removal, place the organ in an organ bag with at least 500 mL of preservation solution (standard extracellular hyperkalemic solution). Place this on ice and keep at 4 °C. This step can be modified according to the experimental design and objective.
2. Recipient Procedure:
- Anesthetic induction and animal preparation:
- Perform anesthesia and monitoring as described in the donor procedure (steps 1.1.1 to 1.1.6).
- After inserting the percutaneous central venous sheath introducer into the right jugular vein, insert a central venous catheter (e.g. double-lumen) into the left jugular vein using the Seldinger technique. Alternatively, this can be done after the midline sternotomy as described above.
- Cardiopulmonary bypass (CPB):
- Perform the midline sternotomy and expose the heart and great vessels as described in the donor procedure (steps 1.2.1 to 1.2.4).
- Dissect between the SVC and the innominate artery, and the IVC and pericardium using the Metzenbaum and “right-angle” forceps. Encircle the SVC and IVC using an umbilical tape (alternatively a simple O silk suture can be used). Secure each tape/suture with a tourniquet.
- Place 2 concentric purse-string sutures using a 4-0 prolene suture on the distal ascending aorta adventitia (avoid full thickness sutures). Place purse-string sutures using 4-0 prolene on the IVC and the SVC at the level of the pericardial reflection. Secure these sutures with tourniquets.
- During CPB preparation, have an assistant setup and prime the system according to the investigators’ and experimental needs. The current procedure uses the same setup used as the institution’s Cardiovascular Surgery Division and employs the help of a trained perfusionist. The bypass system is primed with 2 litres of crystalloid solution (e.g. Plasmalyte) with 500 mg of Solumedrol.
- Administer 30,000 units of heparin (>300 U/Kg) to achieve systemic anticoagulation. Activated clotting time (ACT) should be above 300 s, if the test is available.
- Cannulate the aorta with a 17 to 21 F arterial cannula. Use an extracorporeal membrane oxygenator (ECMO) cannula (e.g. EOPA arterial cannula) inserted using the Seldinger technique to facilitate this step and avoid blood loss. Alternatively, a standard bypass arterial cannula can be used.
- Connect the cannula to the arterial line of the bypass circuit using a 3/8-3/8 connector. Ensure complete deairing to avoid air embolism.
- Perform a bicaval cannulation. For this, cannulate the SVC and then the IVC using 24 to 28 F right angled single-stage venous cannula (e.g. DLP single lumen angled venous cannula).
- First, make a small incision (5 mm) at the center of the purse-string suture. Dilate the incision with a small angled instrument (e.g. right angle or a snap). Insert the cannula directing the angle superiorly in the SVC and inferiorly in the IVC (away from the heart). Secure by tightening the tourniquet holding the purse-string suture.
- Between each step, cover the incision with a finger to avoid excessive bleeding. Connect the cannulas to the venous line of the bypass circuit using a 3/8-3/8-1/2 Y connector. Ensure deairing to avoid an airlock in the system.
- Initiate CPB. Adjust flowrates to maintain an arterial pressure above 50 mmHg (approximately 4 L/min). Maintain normothermia throughout the procedure.
NOTE: These settings can be modified according to the experimental design. Vasoactive medications should only be administered during CPB if needed to aid in pressure regulation (e.g. epinephrine infusion).
- Donor heart implantation:
- After initiation of CPB, open the left pleura and retract the native heart to the right. Dissect and encircle the left hemiazygous vein using a sharp dissection instrument (e.g. Metzenbaum) and a right-angle, respectively. Ligate distally with an O Silk tie. Only one ligature is needed as the native heart will be removed.
- Cross-clamp the recipient aorta proximally to the arterial cannula. Snare both vena cava with the tourniquets previously placed using O silk ties. Remove the recipient’s native heart. Alternatively, the heart can be arrested using standard hypothermic blood-based cardioplegia.
- During cardiectomy, make sure to maintain large cuffs in the recipient to facilitate donor heart implant. For this, section the aorta and pulmonary artery proximally, close to their roots. Similarly, the left and right atriums must be kept with large cuffs. Leave the right and left atrial appendages in the recipient cuffs, which may be needed during the anastomoses.
- Prepare the donor heart for implant.
- Dissect the pulmonary artery off the left atrium and separate the pulmonary artery completely from the aorta. Leave at least 2-3 cm of each vessel to be trimmed during implant as needed. Ligate both vena cava with an O silk tie. Unite all pulmonary veins, creating a single left atrial cuff to be anastomosed.
- Compare left atrial cuff sizes (donor and recipient) and trim each as needed to become similar sizes. The recipient’s left atrial appendage can be shortened, or the donor’s left atrial roof and appendage can be opened for this.
- Deliver the first cardioplegia dose to the donor heart using the previously placed cardioplegia cannula as described in step 1.2.6. Cardioplegic protective solution consists of 500 mL of a 2:1 mixture of blood:crystalloid containing 24 mEq of potassium and delivered at 10°C. Achieve the desired potassium concentration by adding potassium chloride to the cardioplegic mixture.
- After the completion of each anastomosis, deliver an additional dose of 300 mL of cardioplegia at 10 °C containing 8 mEq of potassium.
- Following all anastomoses and before removal of the aortic cross-clamp, administer an additional dose of 500 mL of warm (37 °C) blood cardioplegia with 8 mEq of potassium.
- Remove the donor heart from storage and implant with the standard biatrial anastomotic technique in the following sequence: left atrium, right atrium, pulmonary artery, and aorta. Use a 4-0 prolene suture with a SH needle for the left and right atrium, and the aorta and a 5-0 prolene suture with a BB needle for the pulmonary artery.
- Left atrium: place a 4-0 prolene suture at the junction between the left atrium and the IVC (right inferior margin) and another at 180° from the first, connecting the donor and recipient cuffs. Complete the posterior wall anastomosis. Complete the anterior wall anastomosis. This is performed from the superior suture to the inferior suture.
- Right atrium: open the donor right atrium from the appendage towards the IVC, creating a donor cuff that matches the recipient cuff size. Starting at the inferior angle (junction between IVC and right atrium), complete the interior wall anastomosis and then the lateral wall.
- Pulmonary artery: trim the edges of both recipient and donor pulmonary arteries to create matching sizes. Place a 5-0 prolene suture at the left lateral wall uniting donor and recipient vessels and another at the right lateral edge. Complete the inferior wall anastomosis and then the anterior wall anastomosis.
- Aorta: trim as described with the pulmonary artery. Place a suture on the left lateral wall connecting donor and recipient vessels. Complete the inferior wall and then the anterior wall anastomosis.
- Perform single layered anastomoses, except for the pulmonary artery, where a double layered anastomosis is required. The porcine tissue is extremely fragile and should be handled carefully to avoid tears. Importantly, the pulmonary artery anastomosis is the most delicate step of the implant and must be done with extreme care. The implant technique can be modified according to the surgeons’ preference and the experimental design.
- Following completion of all anastomoses and warm cardioplegia dose delivery as described above, remove the aortic cross-clamp. Inspect all anastomoses for sites of bleeding, they should be corrected at this point.
- Reperfuse the donor heart for 60 min on CPB. Ventricular arrhythmias can be treated with internal defibrillation (20-50 J). If required, ventricular pacing can be used to maintain a heart rate of 100 beats per min. Antiarrhythmic drugs (e.g. Amiodarone, Lidocaine, or Magnesium Sulphate) can be used at the investigators discretion, if required.
- After 60 min of reperfusion, administer 1 g of calcium chloride. Initiate weaning from CPB by decreasing the flow to half, then one-quarter and then off. The central venous line can be used to monitor central venous pressure, targeting 10 mmHg. Initiate infusion of vasoactive and inotropic medications (e.g. dobutamine, epinephrine, norepinephrine, and vasopressin) according to the experimental design or the investigators’ discretion.
- Weaning is deemed successful if the animal maintains a systolic arterial pressure above 60 mmHg for over 30 min after discontinuation of CPB. As this is not a survival model, do not reverse the heparin; continuous bleeding can occur from needle holes and dissected structures (e.g sternum). Donor hearts respond well to small and repeated doses of volume replacement using the CPB system. Additionally, the porcine model responds well to dobutamine.
NOTE: The recipient management should be tailored to the investigators’ experience and the experimental design. A cardiac anesthesiologist may aid in this manner.
3. Graft Assessment:
- Functional assessment:
- This large animal model has the advantage of having an open chest approach at all times, which facilitates direct functional assessment. To measure cardiac contractility, use pressure-volume (PV) loop analyses, echocardiography, and/or right-sided catheterization.
- Pressure-volume loops10: Place an umbilical tape around the IVC, and insert a PV conductance catheter into the left ventricle through a small apical ventriculotomy to permit continuous measurements of left ventricular PV relations. Obtain steady-state recordings to generate volume-dependent parameters (e.g. developed pressure and stroke work) and then obtain occlusion recordings in triplicate by occluding the IVC to generate volume-independent parameters (e.g. preload recruitable stroke work).
- Echocardiography: have cardiac anesthesiologists obtain epicardial images using a standard transesophageal probe.
- Right-sided catheterization: Insert a Swans-Ganz catheter through the venous sheath placed at the beginning of the procedure and guid towards the pulmonary artery. This enables the measurement of central venous pressure, right ventricular pressure, pulmonary artery pressures, pulmonary capillary wedge pressure, and cardiac output using the thermodilution technique.
- Perform contractile evaluation at baseline and following 2 and 3 h post-reperfusion of the donor heart in the recipient. This can be modified by the investigators according the experimental design.
- Metabolic assessment:
- For metabolic assessments, collect arterial and venous (alternatively: mixed venous) blood samples and store the plasma for subsequent analyses. Real-time blood gas analyses and lactate levels should also be obtained.
- Collect these samples at baseline in the donor, before procurement in the donor, at baseline in the recipient, and at 15, 30 and 60 min of reperfusion of the donor heart (after removing cross-clamp). This can be modified according to the experimental design.
- Experiment termination and euthanasia:
- Once all assessments are finished, exsanguinate the recipient animal into the venous reservoir of the CPB circuit by opening the venous line clamp. Alternatively, exsangination can be achieved by harvesting the cardiac alograft to collect samples (i.e. myocardial biopsies).
Representative Results
This pre-clinical model has been used successfully since 19945,6,8,9. Table 1 demonstrates representative results from pressure-volume relationships and echocardiographic parameters taken at baseline, and 3 h post-transplantation in a set of 5 experiments. Although we see a decrease in myocadial contractility following transplantation, this was not statistically significant.
Figure 1 shows representative pressure-volume loops collected from one experiment at the same time-points. During "steady-state" assessments (Figure 1, top row). volume-dependent parameters are recorded, such as maximum and minimum rate of developed pressure. Volume-independent parameters are obtained by intermittent occlusion of the IVC. With this, the volume of the left ventricle progressively decreases and different relationships can be calculated. In the middle row of Figure 1, we see the end-systolic and end-diastolic pressure-volume relationships being recorded, which represent the relationship between the end-systolic or end-diastolic pressures, respectively, with the corresponding end-diastolic volume. In the bottom row od Figure 1, we see the recording of preload recruitable stroke work, which is the relationship between the stroke work and the corresponding end-diastolic volume.
Finally, as seen in Figure 2, various other metabolic (e.g., lactate levels and pH) and functional parameters (e.g., cardiac output) can be measured with this model to test different hypotheses.
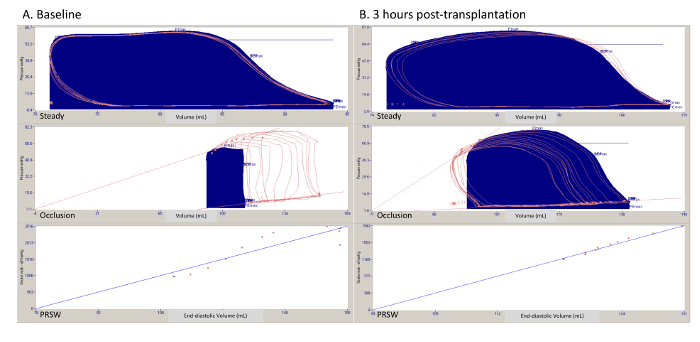
Figure 1. Representative pressure-volume loops in a steady state, during Interior Vena Cava (IVC) occlusion, and relationships (preload recruitable stroke work). (A) One experiment at baseline. (B) One experiment following 3 h of reperfusion. PRSW = preload recruitable stroke work. Please click here to view a larger version of this figure.
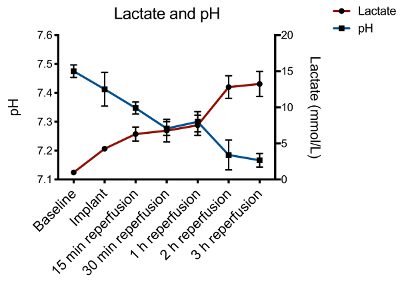
Figure 2. Lactate and pH trends during the heart transplantation protocol. Following reperfusion, there is a significant increase in lactate and decrease in pH. This can be managed by maintaining adequate perfusion pressure with proper volume replacement and vasoactive drug use. Please click here to view a larger version of this figure.
| Baseline | 3 hours post-transplant | p-value | |
| Pulmonary artery catheter | |||
| Cardiac Index (L/min) | 3.7 ± 0.8 | 2.8 ± 0.3 | 0.485 |
| Pressure-volume analysis | |||
| PRSW (erg∙cm-3∙103) Max dP/dt (mmHg∙s-1) Min dP/dt (mmHg∙s-1) |
62.1 ± 7 2500 ± 425 -1537 ± 238 |
53.8 ± 10 1815 ± 410 -1427 ± 317 |
0.841 0.309 0.547 |
| Echocardiography | |||
| LV EF (%) LV FAC (%) RV FAC (%) |
47.3 ± 3.0 53.8 ± 3.6 39.2 ± 1.3 |
37.0 ± 4.2 46.4 ± 2.9 32.8 ± 3.6 |
0.095 0.222 0.309 |
Table 1. Representative pressure-volume relationships and echocardiographic parameters from a set of 5 transplants performed at baseline and following 3 h of reperfusion. Data presented as mean ± standard error and compared using the Wilcoxon Signed Rank Test. EF = ejection fraction. FAC = fractional area change. LV = left ventricle. Max dP/dt = maximum rate of pressure change in the left ventricle. Min dP/dt = minimum rate pf pressure change in the left ventricule. PRSW = preload recruitable stroke work. RV = right ventricle.
Discussion
This manuscript describes a large-animal pre-clinical model of orthotopic heart transplantation. Various small animal models of heterotopic heart transplantation have been successfully used to study the effects of novel treatments to improve organ preservation and decrease ischemia-reperfusion injury11,12,13. Additionally, small animal models permit survival models and long-term follow-up to investigate the development of rejection and cardiac allograft vasculopathy11,12,13. However, most of these novel therapies fail in or never make it to clinical trials. In order to facilitate and streamline clinical translation, a reliable and clinically relevant large-animal model is needed.
This protocol was designed to investigate different treatment and organ preservation strategies to prevent or decrease primary graft dysfunction and ischemia-reperfusion injury. As mentioned above, this model has been used since 1994. Authors previously demonstrated the beneficial effects of hypertonic saline infusion in the donor8 or recipient9 prior to organ procurement or implant, respectively. Furthermore, authors investigated different preservation protocols and strategies, such as the use of donor shed blood infusions during cold storage6 and the effect of insulin supplementation into the cardioplegic solution5.
The major limitation of the technique described here is the short-term follow-up. A long-term survival porcine heart transplant model would be resource-intense and involve high-costs. The procedure described here focuses on acute ischemia-reperfusion injury and is a reliable pre-clinical method to investigate novel interventions which have been tested and validated in smaller experimental models, such as the murine model. In addition, this technique can easily by adapted for longer-term follow-up experiments. This would involve adequate heparin reversal, animal decannulation, adequate hemostasis, and chest closure.
The porcine heart is often considered the most anatomically similar to the human heart when using large-animal models. As such, it is an ideal platform to perform cardiac surgical research. However, it is important to note that the tissue is typically described as fragile and friable, especially in the right atrium and the pulmonary artery, being prone to tears14. Additionally, the pig heart is considered sensitive to manipulation and prone to arrhythmias, which is why magnesium sulfate must be routinely administered to each animal at the beginning of the experiment. An important difference between the porcine model and clinical heart transplantation is the left hemiazygous vein in the swine, which drains directly into the coronary sinus. This has to be ligated during the recipient procedure to avoid continuous bleeding. Finally, the porcine model is very sensitive to ischemia, which seems appropriate for acute studies in heart transplantation15.
Recipient management following transplantation can be challenging at times. It is important to revise all anastomoses and ensure no bleeding. A particularly troublesome area is around the posterior pulmonary artery. As mentioned above, the porcine tissues and fragile and can easily tear; if this happens, the surgeon can quickly go back on CPB to correct the issue and attempt weaning once again. Ventricular fibrillation usually occurs during initial reperfusion; if this does not resolve with simple defibrillation, pharmacological interventions, such as 2 g of magnesium sulphate or 1 mg/kg of lidocaine, can be administered and a following defibrillation should be applied. Normal sinus rhythm can be easily achieved in under 3 min.
This procedure requires at least one trained surgeon to be performed; further, 3 to 5 experiments are needed to optimize the protocol within each research group. Additionally, the team should allocate one member to exclusively perform animal anesthesia and recipient management as needed (e.g. inotropic support). Due to the important considerations regarding the porcine model described above, the following steps are critical in this procedure: anesthetic induction and intubation (important to avoid prolong hypoxemic periods), cardiac manipulation during assessment, cannulation for cardiopulmonary bypass, and right atrial and pulmonary artery manipulation and anastomosis. However, as these are routine steps performed in clinical practice, they should be carried out with care and attention to detail. Consistency and repetition will lead to an optimized and reliable model for various uses.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| Amiodarone | Purchased from institutional pharmacy | ||
| Angiocath 20G | BD | 381704 | |
| Calcium Chloride 1g/10ml | Purchased from institutional pharmacy | ||
| Cardioplegia solution | This should be chosen at the investigators discretion. | ||
| Cautery Pencil | Covidien | E2515H | |
| Central Venous Catheter double-lumen | Cook Medical | C-UDLM-501J-LSC | |
| CPB pack | Medtronic | Custom-made cardiopulmonary bypass perfusion circuit. | |
| D5W 5% 250ml | Baxter | JB1064 | |
| DLP Aortic Root Cannula/stabber | Medtronic | 12218 | |
| DLP single-lumen venous cannula (24F or 28F) | This should be chosen at the investigators discretion. | ||
| Dobutamine | Purchased from institutional pharmacy | ||
| Electrode Polyhesive | Covidien | E7507 | |
| EOPA arterial cannula (17F or 21F) | This should be chosen at the investigators discretion. | ||
| Epinephrine | Purchased from institutional pharmacy | ||
| Eppendorf Tubes, 1.5 mL | Sarstedt | 72.690.001 | |
| Gloves, nitrile, medium | Fischer | 27-058-52 | |
| Heparin 1000 IU/ml | Purchased from institutional pharmacy | ||
| Ketalean (Ketamine) inj. 100mg/ml, 50ml/vial | Health Canada | Requires health canada approval | |
| Lidocaine/Xylocaine 1% | Purchased from institutional pharmacy | ||
| Magnesium Sulfate 5g/10ml | Purchased from institutional pharmacy | ||
| Midazolam inj. USP 5mg/ml vial/10ml | Health Canada | Requires Health canada approval | |
| MPS Quest delivery disposable pack | Quest medical | 5001102-AS | |
| NACL 0.9% 1L | Baxter | JB1324 | |
| Organ Bag | CardioMed | 2990 | |
| Pipette Tips, 1 mL | Fisherbrand | 02-707-405 | |
| Propofol 1mg/ml | Purchased from institutional pharmacy | ||
| Rocuronium | Purchased from institutional pharmacy | ||
| Set Admin Prim NF PB W/Checkvalve | Smith Medical | 21-0442-25 | Intravenous infusion pump line. Researchers should choose infusion lines compatible with the infusion pump available at their facilities |
| Set Intro Sheath 8.5FRx 10CM | Arrow | SI-09880 | |
| Sofsilk 0 wax coated | Covidien | S316 | |
| Solumedrol 500mg/5ml | Purchased from institutional pharmacy | ||
| Suction tip | Covidien | 8888501023 | |
| Suction Tubing 1/4" x 120" | Med-Rx | 70-8120 | |
| Suture 5.0 Prolene BB | Ethicon | 8580H | |
| Suture Prolene Blum 4-0 SH 36 | Ethicon | 8521H | |
| Sutures 2.0 Prolene Blu M SH | Ethicon | 8523H | |
| Sutures BB 4.0 Prolene | Ethicon | 8881H | |
| Tracheal Tube, 6.5mm | Mallinckrodt | 86449 | |
Referenzen
- Lund, L. H., Edwards, L. B., et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. The Journal of Heart and Lung Transplantation. 35 (10), 1158-1169 (2016).
- Lund, L. H., Edwards, L. B., et al. The Registry of the International Society for Heart and Lung Transplantation_ Thirty-first Official Adult Heart Transplant Report-2014; Focus Theme_ Retransplantation. The. Journal of Heart and Lung Transplantation. 33 (10), 996-1008 (2014).
- Cosío Carmena, M. D. G., Gómez Bueno, M., et al. Primary graft failure after heart transplantation: characteristics in a contemporary cohort and performance of the RADIAL risk score. The Journal of Heart and Lung Transplantation. 32 (12), 1187-1195 (2013).
- Fedak, P. W. M., Rao, V., et al. Combined endothelial and myocardial protection by endothelin antagonism enhances transplant allograft preservation. The Journal of Thoracic and Cardiovascular Surgery. 129 (2), 407-415 (2005).
- Ramzy, D., Rao, V., et al. Cardiac allograft preservation using donor-shed blood supplemented with L-arginine. The Journal of Heart and Lung Transplantation. 24 (10), 1665-1672 (2005).
- Rao, V., Feindel, C. M., Weisel, R. D., Boylen, P., Cohen, G. Donor blood perfusion improves myocardial recovery after heart transplantation. Journal of Heart and Lung Transplantation. 16 (6), 667-673 (1997).
- Wicomb, W. N., Cooper, D. K., Barnard, C. N. Twenty-four-hour preservation of the pig heart by a portable hypothermic perfusion system. Transplantation. 34 (5), 246-250 (1982).
- Badiwala, M. V., Ramzy, D., et al. Donor pretreatment with hypertonic saline attenuates primary allograft dysfunction: a pilot study in a porcine model. Circulation. 120, 206-214 (2009).
- Ribeiro, R. V. P., Badiwala, M. V., Ramzy, D., Tumiati, L. C., Rao, V. Recipient Hypertonic Saline Infusion Prevents Cardiac Allograft Dysfunction. The Journal of Thoracic and Cardiovascular Surgery. , (2018).
- Townsend, D. Measuring Pressure Volume Loops in the Mouse. Journal of Visualized Experiments. (111), e53810 (2016).
- Ratschiller, T., Deutsch, M. -. A., et al. Heterotopic Cervical Heart Transplantation in Mice. Journal of Visualized Experiments. (102), e52907 (2015).
- Fukunaga, N., Bissoondath, V., Rao, V. Submandibular Gland-preserving Technique for Heterotopic Cervical Heart Transplantation in Mice. Transplantation. 1, (2018).
- Gong, W. Mouse Heterotopic Abdominal Heart Transplant Model. Rodent Transplant Medicine. , 107-118 (2014).
- Robinson, N., Souslian, L., Gallegos, R. P., Rivard, A. L., Dalmasso, A. P., Bianco, R. W. Animal Models for Cardiac Research. Handbook of Cardiac Anatomy, Physiology, and Devices. , 469-491 (2015).
- Bianco, R. W., Gallegos, R. P., Rivard, A. L., Voight, J., Dalmasso, A. P. Animal Models for Cardiac Research. Handbook of Cardiac Anatomy, Physiology, and Devices. , 393-410 (2009).

