Forced Flowering in Mandarin Trees under Phytotron Conditions
Summary
Here, we present a protocol to force flowering in mandarin trees under phytotron conditions. Water stress, high illuminance and a simulated spring photoperiod allowed viable flowers to be obtained in a short period of time. This methodology allows researchers to have several flowering periods in 1 year.
Abstract
Phytotron has been widely used to assess the effect of numerous parameters on the development of many species. However, less information is available on how to achieve fast profuse flowering in young fruit trees with this plant growth chamber. This study aimed to outline the design and performance of a fast clear methodology to force flowering in young mandarin trees (cv. Nova and cv. Clemenules) and to analyze the influence of induction intensity on inflorescence type. The combination of a short water stress period with simulated spring conditions (day 13 h, 22 °C, night 11 h, 12 °C) in the phytotron allowed flowers to be obtained only after 68-72 days from the time the experiment began. Low-temperature requirements were adequately replaced with water stress. Floral response was proportional to water stress (measured as the number of fallen leaves): the greater the induction, the larger the quantity of flowers. Floral induction intensity also influenced inflorescence type and dates for flowering. Details on artificial lighting (lumens), photoperiod, temperatures, plant size and age, induction strategy and days for each stage are provided. Obtaining flowers from fruit trees at any time, and also several times a year, can have many advantages for researchers. With the methodology proposed herein, three, or even four, flowering periods can be forced each year, and researchers should be able to decide when, and they will know, the duration of the entire process. The methodology can be useful for: flower production and in vitro pollen germination assays; experiments with pests that affect early fruit development stages; studies on fruit physiological alterations. All this can help plant breeders to shorten times to obtain male and female gametes to perform forced-crosses.
Introduction
Phytotron has been widely used to assess the effect of numerous parameters on the development of many herbaceous and bulb plants. Species such as rice1, lily2, strawberry3 and many others4 have been evaluated under phytotron conditions. Chamber experiments on forest trees have also been carried out to evaluate ozone sensitivity on juvenile beech5,6, and to assess the influence of temperatures on frost hardening in seedlings of Scots pine and Norway spruce7. Less information is available about how to obtain fast profuse flowering in young fruit trees via growth chambers.
The flowering of citrus trees, and its relationship with many endogenous and exogenous factors, have long since been broadly studied. Temperatures8, water availability9, carbohydrates10, auxin and gibberellin contents11,12, abscisic acid13, and many other factors that affect citrus reproductive systems have been studied. Temperature and photoperiod effects on flower initiation have been studied in sweet orange (Citrus × sinensis (L.) Osbeck)14,15. In these experiments, long inductive conditions (5 weeks at 15/8 °C) were used and the temperature during shoot development influenced inflorescence type14. During citrus flowering, the term “inflorescence” has been applied to all types of flower-bearing growth that arise from axillary buds, as used by Reece16.
Having a clear precise methodology to force flowering over a short time period and at other times other than spring can provide many advantages for researchers. Save tropical areas, the flowering of fruit trees occurs only once a year, which limits the number of experiments that can be done.
Flowers obtained by forced methods can be used for a wide variety of experiments to: obtain viable pollen for in vitro growth and germination experiments in any month17; run experiments with pests that affect early fruit development stages, even before petal fall, such as Pezothrips kellyanus Bagnall18, or Prays citri Millière19; study the effect of temperatures, chemical treatments, natural predators or just insects rearing; assess the influence of numerous factors on the physiological alterations that disturb early fruit development stages, such as “creasing” in sweet orange20,21; help plant breeders to shorten times to obtain male and female gametes to perform forced-crosses.
This paper aims to outline the design and performance of a fast clear methodology to force flowering in young mandarin trees (cv. Nova and cv. Clemenules) and to analyze the influence of induction intensity on inflorescence type. To achieve this main objective, details on artificial lighting (lumens), photoperiod, temperatures, plant size and age, induction strategy, days for induction, days for sprouting, days for flowering, and the total amount of flowers per variety are provided. Water stress induction intensity was also recorded and related with inflorescence type, dates and amounts of flowers.
Protocol
1. Growth chamber characteristics and regulation requirements
- Use a growth chamber measuring 1.85 m x 1.85 m x 2.5 m (L x W x H) with a total volume of 8.56 m3 (Figure 1). A bigger or smaller growth chamber can be resorted to if necessary.
NOTE: Almost any room, or even a greenhouse, can be adapted to be used as a growth chamber. - Check if regulations such as temperature (day/night), photoperiod (day/night), light intensity and minimum relative humidity are available (Figure 2).
NOTE: Timers should allow temperature and light switch (on/off) control at least every 30 min.
2. Plant material
- Obtain the plant material from registered nurseries with a virus-free certification (e.g., six mandarin trees cv. ‘Clemenules’ and 6 mandarin trees cv. ‘Nova’).
NOTE: Mandarin trees can be young (e.g., 1- or 2-year-old varieties grafted onto rootstocks). - Use appropriate pots (e.g., a plastic pot of 22 cm x 20 cm (diameter x height) and prepare 5 L of standard substrate based on high quality white peat (50%) and coconut fiber (50%).
- Use trees that are around 1.5 m high with a well-developed spherical crown from 1 m to 1.5 m. Plants should be completely healthy, and be pest-, pathogen- or disease-free.
3. First irrigation
- Irrigate the plants for the first time as soon as they arrive from the nursery to standardize moisture content. Water by immersion. Cover the pots with water halfway for 20 min.
- Keep the plants outside in half shade without irrigation for 3-5 days (Table 1).
4. Springtime conditions in the phytotron
- Review the site’s springtime conditions to determine the average day and night temperature, photoperiod and relative humidity (e.g., at the working latitude (39° 28′ 53.95″ N, 0° 20′ 37.71″ W) with only one bloom per year the citrus tree flowering period extends from mid-March to the end of April with some annual variations. Therefore, these dates were checked in several meteorological stations (e.g. w.s. 38° 57’ 51.77″ N, 0° 15’ 02.24″ W 113 m.a.s.l.) for at least 10 years, and the average day and night temperature, photoperiod and relative humidity were determined).
- Program the growth chamber for mandarin trees with the following conditions: (i) temperature of 22 °C/11 °C (day/night); (ii) photoperiod of 13/11 h (light/dark); (iii) relative humidity around 60% and no less than 50% (Figure 3).
- Use two electronic controllers with dual output, one for day and one for night humidity. Use a timer to change from day to night humidity. Set up minimum and maximum humidity for day and night.
- For minimum humidity, press and release (single press) the Set button; SP 1 (set point 1) will appear; press and release the Set button and press the UP key or DOWN key to change the SP1 value (50%).
- For maximum humidity, press and release (single press) the Set button; SP 1 (set point 1) will appear; press the UP key or DOWN key to change to SP 2; SP 2 (set point 2) will appear; press and release the Set button and press the UP key or DOWN key to change the SP2 value (60%).
- Use an electronic controller with 2 set points and a differential set point adjustment to set up temperature. Use a timer to change from the day to night temperature.
- Set up the desired day temperature (22 °C). Press and release the Set button; SP 1 (set point 1) will appear; press the Set button; press the UP key or DOWN key to change the SP1 value.
- Set up the regulation band, for example db1 and dF1 parameters. Refrigeration will start when Set point 1 (SP1) plus db1 is reached and will stop at a temperature equal to SP1 plus db1 minus dF1. Press the Set button for 5 s; rE1 will appear; press Set; press the UP key; db1 will appear; press Set and press the UP key or DOWN key to change the db1 value (2 °C); press Set | UP; dF1 will appear; press Set and press the UP or DOWN to change dF1 value (2 °C).
- To set up the desired night temperature (11 °C), access OS1 parameter (Offset Set point 1). Press the Set button for 5 s; press DOWN 3 times; cnF will appear; press Set | DOWN; PA2 will appear; press Set; rE1 will appear; press Set; OS1 will appear; press Set and press UP or DOWN to change the OS1 value (-11 °C); press the fnc button (ESC function (exit)).
- Use two electronic controllers with dual output, one for day and one for night humidity. Use a timer to change from day to night humidity. Set up minimum and maximum humidity for day and night.
- Increase the temperature by 1 °C (23/12 °C day/night) after 4 weeks and add a half hour of light (13.5/10.5 light/dark).
NOTE: As the phytotron has variation ranges, the nighttime temperature may vary from 11 °C to 14 °C, and the daytime temperature from 19 °C to 22 °C (Figure 3). - Use two light kits with a reflector, an electric ballast sodium halide and high-pressure sodium (HPS) 600 W lamp to obtain the appropriate light intensity (Figure 4). Light intensity is essential for flowering.
- Modify the distance between the lamp and the crown to obtain the desired light intensity and set up the photoperiod with the timer.
- Check illuminance with a luxmeter. At the top of the crown, 55,000 lux (671 µmol m-2 s-1) should be attained, with 40,000 lux (488 µmol m-2 s-1) at the crown-base.
5. Placing trees inside the phytotron
- Place trees inside the phytotron and keep them for several weeks without watering them (Figure 5A).
- Distribute trees regularly so that each has the same available space and light (e.g., trees were uniformly distributed inside the growth chamber into three lines and at four positions. The distance between lines was 0.46 cm, while the distance between positions was 0.37 cm) (Figure 1).
- Distribute individuals and varieties randomly among positions (Figure 1).
6. Floral induction
- Use water stress for floral induction. After the first irrigation, do not irrigate trees until the water stress period is considered to have finished.
- Check the water stress intensity every day by looking at leaf turgidity.
- Consider enough water stress for floral induction when most leaves are flaccid, but have not started to fall (e.g., after 22 days without watering, leaves were flaccid and a few started falling) (Table 1).
NOTE: If water stress is excessive (many leaves fall), plant survival can be compromised, whereas if water stress is insufficient (not enough flaccid leaves), poor flowering may take place. - Irrigate the trees abundantly after the water stress period. For this first irrigation, water by immersion. Cover pots with water halfway for 20 min.
- Measure the water stress intensity for each individual by noting the total number of fallen leaves (Figure 5B,C). The percentage of fallen leaves is an indirect measurement of the water stress suffered by each individual. Estimate the percentage of fallen leaves by comparing the total amount of leaves before and after the water stress period.
7. Flower harvesting if necessary for other experiments
- At the beginning and the end of flowering periods, collect flowers once a day. On the days of maximum flower production, collect flowers twice a day and 7 days a week.
- Harvest flowers by hand and keep them at -20 °C in a labeled plastic bag (Figure 5D). The flower production of six mandarin trees can vary from 25 to more than 200 flowers per day.
- Choose the exact flower state when collecting.
- Use flowers for in vitro pollen germination assays or for any other purpose with a pollen viability that equals fresh pollen.
8. Other management tasks
- Water trees approximately once a week after the water-stress period depending on requirements.
- Check the presence of pests and disease every 2-3 days (e.g., only a small population of Icerya purchasi Maskell was observed in this experiment and was manually removed to avoid using chemical treatments (Figure 5E)).
- Check the temperature and humidity settings with a data logger (Figure 3).
Representative Results
The experiment was carried out in the plant growth chamber located at the Valencia Polytechnic University's Gandía Campus (municipality of Gandía) in the province of Valencia, Spain (39° 28′ 53.95″ N, 0° 20′ 37.71″ W), in autumn and winter (2017 Oct. 26 – 2018 Feb. 5) (Table 1). Six mandarin trees cv. 'Clemenules' (a bud mutation of Citrus clementina hort. ex Tanaka) and six mandarin trees cv. 'Nova' (the tangelo hybrid of C. clementina hort. ex Tanaka x [C. paradisi Macf. x C. tangerina hort. ex Tanaka.]) were used. Trees were 2-year-old varieties grafted onto rootstocks (rootstocks were 1-year-old when first grafted). Cv. Nova was grafted onto a 'Carrizo citrange' rootstock (x Citroncirus sp. = C. sinensis (L.) Osbeck 'Washington' sweet orange x Poncirus trifoliata (L.) Raf.), while cv. Clemenules was grafted onto a Citrus volkameriana Pasq. rootstock. Plant material was obtained from registered nurseries with a virus-free certification.
Flowering was forced in young citrus trees (only the 2-year-old varieties) and not in spring in a phytotron growth chamber. Bloom process was correctly triggered and lasted 24-29 days (Table 1). Flower production was plentiful in both varieties (Nova and Clemenules). Six Nova mandarin trees produced around 1488 flowers, while six Clemenules mandarin trees yielded around 1104 flowers (Table 2). Flowers were harvested daily and stored at -20 °C. They were used for in vitro pollen germination assays. The pollen of the stored flowers showed more than 60% germination, which implied good viability.
The water stress period needed for flower induction lasted 22 days, while the period between induction and the start of bud growth lasted 26-31 days. Flowers at anthesis were observed 20 days after firstly observing early flower buds (Table 1). A 68-73-day period had to pass between the time when trees arrived and the time when the first flowers were obtained.
Water stress intensity was measured for each individual by the total number of fallen leaves (Table 2). The same number of days without irrigation led to different leaf fall percentages. Three levels of water stress intensity were clearly established: (1) low intensity, 5-10% leaf fall, the six Clemenules individuals (Figure 5C); (2) medium-high intensity, 50-60% leaf fall, three Nova individuals (Nova2, Nova5, and Nova6); (3) very high intensity, 80-90% leaf fall (Figure 5B), three Nova individuals (Nova1, Nova3 and Nova4) (Table 2). In general, the Nova grafted onto Carrizo citrange suffered much more water stress than the Clemenules grafted onto C. volkameriana after the same number of days without watering.
The higher the leaf fall percentage, the more water stress and, therefore, the greater floral induction intensity. Induction intensity influenced inflorescence type, flowering date and the total amount of flowers. Individuals with a high induction (Nova 1, 3, 4) displayed mainly leafless buds with one flower or several (type A) (Figure 6 and Figure 7), while those individuals with low induction (Clemenules) exhibited mainly buds with several leaves and a few flowers (type C, more leaves than half the number of flowers) (Table 2). The individuals with intermediate induction (Nova 2, 5, 6) showed mainly buds with a balanced number of leaves and flowers (type B in Figure 6, fewer leaves than half the number of flowers), but also flower buds (A) and very few 'C' buds (Table 2 and Figure 7).
Flowering began 5-7 days earlier in the Nova than in the Clemenules mandarin trees (Table 1). Nevertheless, flowering began earlier in three Nova individuals (1, 3, and 4), which reveals that induction intensity advances flowering dates. The 'C' type shoots (mainly with leaves) needed more days to develop because they produced leaves before flowers. The highly-induced individuals produced many more flowers (274 flowers per tree on average) than the low-induced individuals (184 flowers per tree on average) (Table 2 and Figure 7).
The vast majority of flowers were complete and viable. Some small leafy flowers with very short petals were observed at the beginning of the flowering period (Figure 5F), probably due to the partial induction of some buds. At the end of the flowering period, some weak and partially infertile flowers were also observed. These flowers were smaller than regular ones, with only three petals instead of five; some were male flowers with only stamens; some were bisexual, but had a small gynoecium. Flower quality (size and fertility) diminished at the end of the flowering period for both varieties.
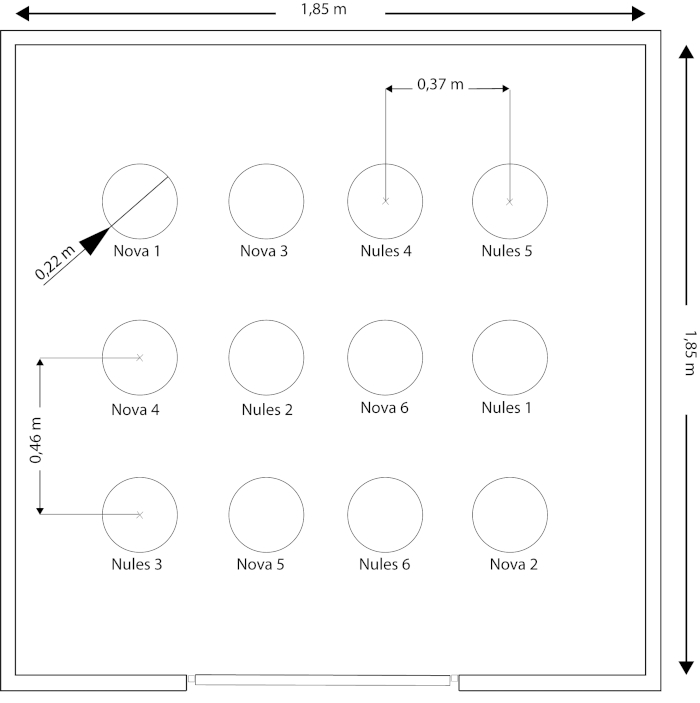
Figure 1. Growth chamber dimensions and plant distribution. Twelve trees distributed randomly into three lines spaced 0.46 m and four positions spaced 0.37 m apart. Trees were noted as Nova: cv. 'Nova' (the tangelo hybrid of C. clementina hort. ex Tanaka x [C. paradisi Macf. x C. tangerina hort. ex Tanaka.]) and Nules: cv. 'Clemenules' (a bud mutation of Citrus clementina hort. ex Tanaka). Please click here to view a larger version of this figure.
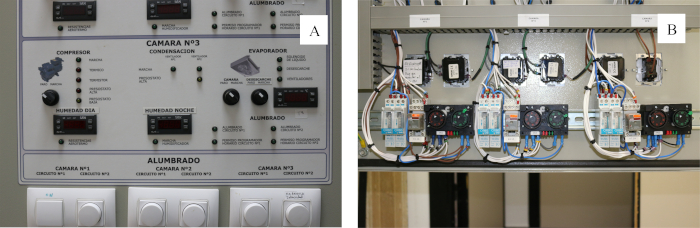
Figure 2. Phytotron control panel. (A) External control panel with temperature, light and relative humidity regulations; (B) Internal timers to switch on/off temperature and light. Please click here to view a larger version of this figure.
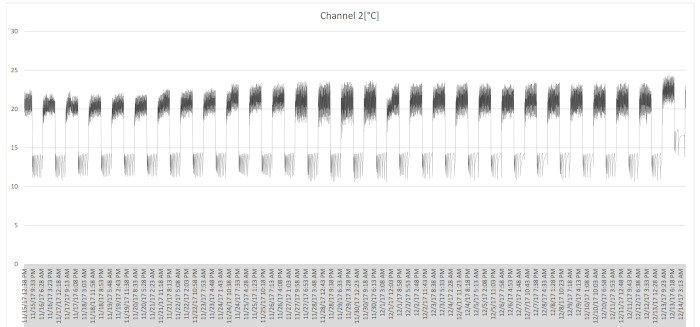
Figure 3. Data-logger temperature record. Temperatures varied from 11 °C to 14 °C at nighttime, and from 19 °C to 22 °C in the daytime. Please click here to view a larger version of this figure.

Figure 4. Light kit. Kit with a reflector, electric ballast sodium/halide and high-pressure sodium (HPS) 600W lamp. Please click here to view a larger version of this figure.
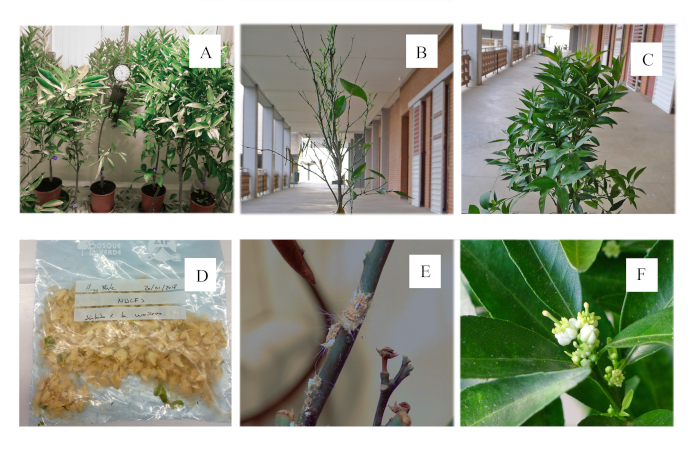
Figure 5. Photographs of the process. (A) Trees inside the phytotron; (B) Tree with 90% leaf fall; (D) Tree with 5% leaf fall; (D) Harvested flowers; (E) Icerya purchasi Maskell; (F) Leafy flowers with very short petals at the beginning of the flowering period. Please click here to view a larger version of this figure.

Figure 6. Inflorescence type. (A1,A2) Initial and more developed leafless buds with one flower or several; (B1,B2) Initial and more developed buds with a balanced number of leaves and flowers; (C1,C2) Initial and more developed buds with many leaves and a few flowers. Please click here to view a larger version of this figure.
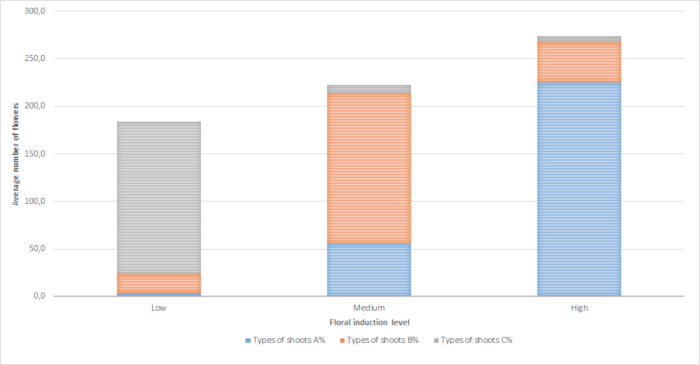
Figure 7. Average number of flowers and inflorescence type for each floral induction intensity level. (A) Shoots with all flowers; (B) Shoots with a balanced number of flowers and leaves; (C) Shoots with more leaves than flowers. Please click here to view a larger version of this figure.
| Dates | Management events | Absolute day | Periods and relative days |
| 2017 Oct. 26 | Citrus trees arrival to the University, first watering | 0 | Water stress – Floral Induction = 22 days |
| 2017 Oct. 31 | First day inside the growth chamber | 5 | |
| 2017 Nov. 17 | First irrigation day after water stress | 22 | |
| 2017 Dec. 13 | First observation of initial vegetative buds | 48 | Days since Induction to the appearance of the new buds = 26-31 days |
| 2017 Dec. 18 | First observation of initial flower buds | 53 | |
| 2018 Jan. 02 | First Nova flower at anthesis | 68 | Nova flowering period = 24 days |
| 2018 Jan. 04 | Start of harvest period for Nova flowers | 70 | |
| 2018 Jan. 07 | First Clemenules flower at anthesis | 73 | Clemenules flowering period = 29 days |
| 2018 Jan. 09 | Start of harvest period for Clemenules flowers | 75 | |
| 2018 Jan. 11 | Nova full flower production | 77 | Delay days between Nova and Clemenules = 5 – 7 days |
| 2018 Jan. 18 | Clemenules full flower production | 84 | |
| 2018 Jan. 26 | End of harvest period for Nova flowers | 92 | Days to reach full bloom by Nova = 9 days |
| 2018 Feb. 5 | End of harvest period for Clemenules flowers | 102 | Days to reach full bloom by Clemenules = 11 days |
Table 1. Schedule of the main management events
| Individual | Leave fall % | Intensity level | Types of shoots | Amount of flowers. | ||
| A% | B% | C% | ||||
| Nova 1 | 85 | 3 | 81 | 17 | 2 | 245 |
| Nova 2 | 55 | 2 | 28 | 68 | 4 | 215 |
| Nova 3 | 90 | 3 | 87 | 10 | 3 | 278 |
| Nova 4 | 82 | 3 | 79 | 19 | 2 | 298 |
| Nova 5 | 60 | 2 | 22 | 75 | 3 | 232 |
| Nova 6 | 54 | 2 | 25 | 71 | 4 | 220 |
| Nova average | 71.0 | NA | 53.7 | 43.3 | 3.0 | 248.0 |
| Nova sd | 16.4 | NA | 31.6 | 30.9 | 0.9 | 33.3 |
| Clemenules 1 | 7 | 1 | 2 | 13 | 85 | 219 |
| Clemenules 2 | 5 | 1 | 1 | 8 | 91 | 135 |
| Clemenules 3 | 9 | 1 | 2 | 11 | 87 | 185 |
| Clemenules 4 | 7 | 1 | 4 | 18 | 78 | 210 |
| Clemenules 5 | 10 | 1 | 2 | 6 | 92 | 178 |
| Clemenules 6 | 5 | 1 | 1 | 10 | 89 | 177 |
| Clemen average | 7.2 | NA | 2.0 | 11.0 | 87.0 | 184.0 |
| Clemen sd | 2.0 | NA | 1.1 | 4.2 | 5.1 | 26.6 |
| A with only flower ; B with leaves and flowers; C with many leaves and few flowers | ||||||
Table 2. Percentage of leaf fall, percentage of inflorescence type and number of flowers per individual. Individuals were classified into three intensity levels, 1: 5-10% leaf fall; 2: 50-60% leaf fall; 3: 80-90% leaf fall. Shoot types were (A) with only flower; (B) with leaves and flowers; (C) with many leaves and a few flowers.
Discussion
It was possible to force the flowering of young citrus trees (only 2 years old) quickly and at any time with profuse flower production (around 216 flowers per tree). In previous studies14,15, flower initiation was induced by low temperatures and the process lasted around 120 days. The combination of a short water stress period with spring conditions in the phytotron allowed this time to be significantly reduced, with mandarin trees (cv. Nova) flourishing after 68 days from the time the experiment began. Therefore, this protocol halves the necessary time. Trees came from the nursery after spring and summer (2017 Oct. 26) and, therefore, without inductive cool conditions. For the protocol described here, low temperatures were not necessary for floral induction, and this stimulus was adequately replaced with water stress. This result suggests that floral-promoting factors (low temperatures, photoperiod, water stress) are probably interchangeable, and can be used either alone or combined. When low temperatures were used for flower initiation, the flowering response was proportional to the amount of cold (number of weeks of 15 °C/8 °C treatment)14. Similarly in this experiment, the flowering response was proportional to the amount of water stress (% of leaf fall).
The amount and quality of flowers were influenced directly by floral induction intensity. The same drought period had different consequences on the two tested varieties. Three Nova trees lost 90% of their leaves, while the Clemenules trees lost 5-10% of their leaves after the same induction period. Therefore, Nova grafted onto Carrizo suffered much more stress than the Clemenules grafted onto C. volkameriana. Greater drought tolerance has been previously reported for the Volkamer lemon rootstock22,23. In this experiment, the variety-rootstock combination was clearly a determinant for the stress level after the same drought period. Therefore, floral intensity depends not only on ‘promoting factors’, but also on the trees’ individual characteristics. A critical step in the floral induction protocol is water stress. Severe stress can earnestly damage trees as a high percentage of leaves can fall and compromise tree viability. Therefore, water stress should be checked every day by looking at leaf turgidity. Each individual can achieve the desired water stress at a different time depending on several factors (crown-pot volume relation, rootstock, variety, etc.)
The best results were obtained with the medium-high induction (represented by 50-60% leaf fall after the induction period), where flowers developed on shoots with a balanced number of flowers and leaves (type B). To this end, the water stress period lasted until most leaves had become flaccid, but did not begin to fall. Greater inductions produced more flowers 5 to 7 days earlier, but on leafless shoots. In the field, these flowers would be less likely to become fruit as fruit set depends on carbohydrate availability24. Lower inductions produced less flowers and with some delay, but produced shoots with more leaves than flowers (type C). Consequently, the amount of flowers, inflorescence type and periods can be controlled by flower initiation intensity. The protocol can be modified with a longer or shorter drought period depending on what shoot type we need. In previous studies, the inflorescence type was influenced by temperature during shoot growth14. In our experiment, the inflorescence type was determined previously during the induction period. Therefore, the inflorescence type might be determined during both induction by intensity and later during bud development through temperatures.
The methodology described here focused on obtaining flowers for research purposes. The technique can present some limitations to obtain fruits as it is described for very young trees. For fruit production, probably bigger and more adult trees would be necessary. In any case, many of our results may be interesting for fruit production in the open field. For example, water stress can be managed to advance or improve flowering. In this case, other factors, such as fruit set and carbohydrate availability, should be taken into account.
The in vitro pollen germination assays confirmed pollen viability. Sixty percent of pollen grains germinated, which indicates an analogous viability to fresh pollen17. As a result, the methodology proved effective and useful. This methodology may be applied to other fruit trees and may offer researchers a fast and easy technique to obtain flowers several times a year and at any time. The main keys to replicate the technique are provided.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors thank José Javier Zaragozá Dolz for providing technical assistance and helping in the management tasks. This research was partially supported by the Asociación Club de Variedades Vegetales Protegidas as part of a project undertaken with the Universitat Politècnica de València (UPV 20170673).
Materials
| Data-logger | Testo | Testo 177-H1 | Testo 177-H1, humidity/temperature logger, 4 channels, with internal sensors and additional external temp |
| Data-logger sotfwae | Testo | Software Comsoft Basic Testo 5 | Basic software for the programming and reading of the data loggers Testo |
| Electronic controller differential | Eliwell | IC 915 (LX) (cod. 9IS23071) | Electronic controller with 2 set points and differential set point adjustment |
| Electronic controller dual | Eliwell | IC 915 NTC-PTC | Electronic controllers with dual output |
| Growth chamber – phytotron | Rochina | Chamber measuring 1.85 x 1.85 x 2.5 m (L x W x H) with a total volume of 8.56 m3. With temperature (day/night), photoperiod (day/night), light intensity and minimum relative humidity control. | |
| Light kit | Cosmos Grow/Bloom Light | Light kit with reflector, electric ballast sodium/halide and high-pressure sodium (HPS) 600W lamp | |
| Luxmeter | Delta OHM | HD 9221 | HD 9221 Luxmeter to measure the light intensity |
| Plant material | Beniplant S.L (AVASA) | Mandarin trees from registered nurseries with a virus-free certification | |
| Substrate | Plant Vibel | Standard substrate based on quality 50% white peat and 50% coconut fiber |
Referenzen
- Matsui, T., Omasa, K., Horie, T. The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Production Science. 4 (2), 90-93 (2001).
- Niedziela, C. E., Kim, S. H., Nelson, P. V., De Hertogh, A. A. Effects of N-P-K deficiency and temperature regime on the growth and development of Lilium longiflorum ‘Nellie White’during bulb production under phytotron conditions. Scientia Horticulturae. 116 (4), 430-436 (2008).
- Hideo, I. T. O., Saito, T. Studies on the flower formation in the strawberry plants I. Effects of temperature and photoperiod on the flower formation. Tohoku Journal of Agricultural Research. 13 (3), 191-203 (1962).
- Shillo, R., Halevy, A. H. Interaction of photoperiod and temperature in flowering-control of Gypsophila paniculata L. Scientia Horticulturae. 16 (4), 385-393 (1982).
- Nunn, A. J., et al. Comparison of ozone uptake and sensitivity between a phytotron study with young beech and a field experiment with adult beech (Fagus sylvatica). Environmental Pollution. 137 (3), 494-506 (2005).
- Matyssek, R., et al. Advances in understanding ozone impact on forest trees: messages from novel phytotron and free-air fumigation studies. Environmental Pollution. 158 (6), 1990-2006 (2010).
- Johnsen, &. #. 2. 1. 6. ;. Phenotypic changes in progenies of northern clones of Picea abies (L) Karst. grown in a southern seed orchard: I. Frost hardiness in a phytotron experiment. Scandinavian Journal of Forest Research. 4 (1-4), 317-330 (1989).
- Distefano, G., Gentile, A., Hedhly, A., La Malfa, S. Temperatures during flower bud development affect pollen germination, self-incompatibility reaction and early fruit development of clementine (Citrus clementina Hort. ex Tan.). Plant Biology. 20 (2), 191-198 (2018).
- de Oliveira, C. R. M., Mello-Farias, P. C., de Oliveira, D. S. C., Chaves, A. L. S., Herter, F. G. Water availability effect on gas exchanges and on phenology of ‘Cabula’ orange. VIII International Symposium on Irrigation of Horticultural Crops 1150. , 133-138 (2015).
- Goldschmidt, E. E., Aschkenazi, N., Herzano, Y., Schaffer, A. A., Monselise, S. P. A role for carbohydrate levels in the control of flowering in citrus. Scientia Horticulturae. 26 (2), 159-166 (1985).
- Goldberg-Moeller, R., et al. Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering-control genes in Citrus buds. Plant science. , 46-57 (2013).
- Bermejo, A., et al. Auxin and Gibberellin Interact in Citrus Fruit Set. Journal of Plant Growth Regulation. , 1-11 (2017).
- Endo, T., et al. Abscisic acid affects expression of citrus FT homologs upon floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.). Tree Physiology. 38 (5), 755-771 (2017).
- Moss, G. I. Influence of temperature and photoperiod on flower induction and inflorescence development in sweet orange (Citrus sinensis L. Osbeck). Journal of Horticultural Science. 44 (4), 311-320 (1969).
- Moss, G. I. Temperature effects on flower initiation in sweet orange (Citrus sinensis). Australian Journal of Agricultural Research. 27 (3), 399-407 (1976).
- Reece, P. C. Fruit set in the sweet orange in relation to flowering habit. Proceedings of the American Society for Horticultural Science. 46, 81-86 (1945).
- Khan, S. A., Perveen, A. In vitro pollen germination of five citrus species. Pak. J. Bot. 46 (3), 951-956 (2014).
- Planes, L., Catalán, J., Jaques, J. A., Urbaneja, A., Tena, A. Pezothrips kellyanus (Thysanoptera: Thripidae) nymphs on orange fruit: importance of the second generation for its management. Florida Entomologist. , 848-855 (2015).
- Carimi, F., Caleca, V., Mineo, G., De Pasquale, F., Crescimanno, F. G. Rearing of Prays citri on callus derived from lemon stigma and style culture. Entomologia Experimentalis et Applicata. 95 (3), 251-257 (2000).
- Jones, W., Embleton, T., Garber, M., Cree, C. Creasing of orange fruit. Hilgardia. 38 (6), 231-244 (1967).
- Storey, R., Treeby, M. T. The morphology of epicuticular wax and albedo cells of orange fruit in relation to albedo breakdown. Journal of Horticultural Science. 69 (2), 329-338 (1994).
- Rewald, B., Raveh, E., Gendler, T., Ephrath, J. E., Rachmilevitch, S. Phenotypic plasticity and water flux rates of Citrus root orders under salinity. Journal of Experimental Botany. 63 (7), 2717-2727 (2012).
- Iqbal, S., et al. Morpho-physiological and biochemical response of citrus rootstocks to salinity stress at early growth stage. Pakistan Journal of Agricultural Sciences. 52 (3), 659-665 (2015).
- Iglesias, D. J., Tadeo, F. R., Primo-Millo, E., Talon, M. Fruit set dependence on carbohydrate availability in citrus trees. Tree Physiology. 23 (3), 199-204 (2003).

