Summary
This article describes how to perform sexual behavior tests in male mice.
Abstract
Sexual behavior is highly species-specific. Although rodents have slightly different sexual behaviors, mice and rats have a similar sexual behavioral pattern. The purpose of this article is to describe the hormone-induced estrus ovariectomized female model and the experimental procedure for the assessment of sexual behavior of male mice. The most important sexual behavioral elements are demonstrated in the video and illustrations. The critical steps, advantages, and limitations of the sexual behavior test are explained as well. Finally, the behavior parameters are presented, and mounting, intromission, and ejaculation processes in mating are distinguished. Behavioral parameters are assessed in terms of the occurred duration and counts during the test period.
Introduction
Sexual behavior in mature male mice results from the interaction of a series of related and interdependent hormonal systems and neural systems in different brain circuits1. It also requires developmental experiences, learning, context, and an appropriate partner. Behavioral analysis is an important reflection on neural or neurocrine function. Hence, sexual behavior study on animal models has been widely used in behavioral neuroscience and other related research2. The ethogram of sexual behaviors in rodents has been explained in many articles and books1,3,4. For instance, Scahs and Barfield's description of sexual behavior in the rat5 has helped understand a similar behavioral pattern in mice5. The mouse is one of the most commonly used subjects for behavioral studies. Hull et al.6 gave a detailed introduction of male mouse sexual behaviors: When a male mouse encounters a female, it starts to investigate the anogenital region of the female. Then, the male presses his front paws against the female's flanks to mount the female from the rear. The female exhibits a characteristic sexually receptive posture, bending its spine down into a bow and moving its tail to one side of the body, exposing an opening introitus for the sexual penetration of the male (i.e., lordosis). Following the mounting, the male makes rapid, shallow pelvic thrusts, followed by slow and deep vaginal thrusts. After numerous intromissions, a long-lasting thrust results in the ejaculation of semen, during which the male mouse may freeze for about 25 s before dismounting or falling off from the female6. At ejaculation the male mouse accessory glands may produce a mixture containing semen that hardens to form the copulatory plug. Finally, following ejaculation, the male begins genital grooming and displays a lack of interest in the female. In brief, the basic sequence of male sexual behavior consists of sniffing, following, mounting, intromission, ejaculation, and post-ejaculation grooming. Mouse sexual behavior exhibits strain differences. For instance, ejaculation latencies range from 594 to 6943 s, and the numbers of intromissions range from 5 to more than 100. Post-ejaculation latencies range from 17 to 60 min. However, the introduction of a novel female can decrease this time interval. In some cases, the male ejaculates on the first intromission with the new female7.
The major events for the evaluation of sexual behavior are mounting, intromission, and ejaculation. Behavioral scientists have recommended the measurement of not only the frequency of each action, but also its latency and time interval5,8. Some major measurement indicators in past studies include: number of mounts, number of intromissions, mount latency, intromission latency, ejaculation latency, post-ejaculatory mount latency (or post-ejaculatory intervals), post-ejaculatory intromission latency, number of copulatory series, and duration of copulatory series. Park et al.8 and Sachs et al.5 described how to identify each action of mounting, intromission, and ejaculation of rodents. Mounting is defined as the male mounting the female from the rear, palpating her flanks with his forelegs, and thrusting his penis rapidly and repeatedly without penile insertion. Intromission, also known as penile insertion, is identified by one or more of the following acts: a long, deep thrust after rapid shallow thrusts, a rapid kick with one hindleg, and a marked lateral withdrawal of the male from the female. Ejaculation is identified by a terminal pelvic thrust that is slower and deeper than that of an intromission and a reduction in the elevation of the hindleg. A copulatory series is identified by each sequence from mounting to ejaculation. The definitions of behavioral parameters used in the present study are listed as follows: 1) Mounting latency: the time from the introduction of the female to the first mounting of the male; 2) Intromission latency: the time from the introduction of the female to first intromission; 3) Ejaculation latency: the time from the first intromission to first ejaculation (generally following the last pelvic thrust); 4) Post-ejaculatory mount latency: the time from ejaculation to the next mounting; 5) Post-ejaculatory intromission latency: the time from ejaculation and the next intromission; 6) Number of mounts: the number of mounting times before first ejaculation; 7) Number of intromissions: the number of intromissions before the first ejaculation; 8) Number of copulatory series: the number of copulatory series during the observation period; 9) Duration of copulatory series: the time of all copulatory series during the observation period.
Sexual behavior and related behavior can be conducted in either the male's home cage or in an enclosed arena, among which an apparatus called Rissman's "No Secrets" mirrored box is introduced to observe the mating behavior3. A video camera is placed in front of the box to simultaneously record the action of the mice from a lateral view and through an inclined mirror from a ventral view. However, this method requires bright lights, which inevitably leads to longer habituation in order to eliminate environmental stress in mice. As for the measuring method, video-based behavioral analysis is recommended to record and quantify behavior4. A video recorder that has a frame-by-frame video advance option with recommended shutter speeds greater than 1/1000 s can be used to record rapid mouse movements. The high resolution infrared camera is necessary when recording in a dark environment. To analyze the film, a computer with a frame grabber to allow the individual frames of behavior to be captured for computer manipulation is needed. Mice are extremely versatile and can display compensatory behavior after almost any treatment. Ambiguity can exist about every moving body part4. Hence, the analysis of some behaviors may require still greater resolution and higher speed cameras.
Male sexual behaviors in mice are affected by many factors, including strain differences, hormone changes, and gene mutantions1,3,9,10. McGill and Blight11 illustrated the strain differences in mouse mating behaviors. For example, C57BL/6 males typically gain intromission quickly and ejaculate in about 20 min11. DBA/2 males are slow to gain intromission but ejaculate rapidly. BALB/c males are slow to achieve ejaculation (average latency of 1 h) due to a long period of courtship11. Testosterone facilitates and maintains male sexual behavior2, and changes in testosterone levels can alter sexual behavior performance12. Both surgical castration and antiandrogen treatment can reduce the level of testosterone and result in a rapid decline of sexual behaviors and even sexual motivation and sexual arousal13. Administered testosterone can restore precopulatory and copulatory behaviors in castrated mice. Lastly, knockout and knockdown mice display differences in facets of sexual behaviors compared to wild type mice. For example, male mice with targeted mutations of Adcy3, Cnga2, and Gnao exhibit a reduced ability to detect pheromones, whereas Trpc2 knockout mice show altered partner preference14,15,16. Other effects of transgenics and knockouts on the sexual behavior of mice are explained by Crawley3.
Here, one of the most common procedures to assess sexual behavior in the pairing of a male mouse with an ovariectomized female that has been hormonally primed to be receptive is described. An experimental protocol is presented for conducting sexual behavior experiments in mice. In addition, an example of changing sexual behavior patterns resulting from social isolation in CD-1 mice is shown.
Protocol
All experiments were performed in compliance with the guidelines of the Principles of Laboratory Animal Care (NIH Publication No. 80-23, revised 1996) and under the approval and supervision of the Academy of Experimental Animal Centre of the Institute of Medicinal Plant Development (China).
1. Animal husbandry
- House female and male mice at 25 °C for 12 h light/12 h dark cycles.
- Provide free access to water and a standard pelleted diet.
- Allow mice to acclimate to their environment for 7 days prior to the operation if transported from a different facility.
2. Ovariectomy in female mice
- Anesthetize the female (8 weeks postnatal, not less than 6-weeks-old) with isoflurane (~4−5% for induction, ~1−2% for maintenance) in 100% oxygen via a face cone mask.
- Check that the appropriate depth of aesthesia has been achieved by making sure that there are no voluntary movements for over 30 s, in combination with an appropriate respiratory rate (e.g., 1 breath per 2 s or longer). Alternatively, test the mouse's response to gentle pressure on the toes of the hind paws.
NOTE: The normal respiratory rate is ~180/min. A rate drop of 50% is acceptable during anesthesia17.- Use ophthalmic ointment to prevent corneal drying and eye trauma while under anesthesia.
- Keep the body temperature of the mouse at or above 36 °C. Provide supplemental heat support during the period of anesthesia when necessary.
- Sterilize and disinfect all surgical instruments and hard surfaces of the operating table with 75% ethanol prior to use.
- Place animal on a sterile drape.
- Shave fur bilaterally over the lumbar spine on the back of the mouse to expose the skin.
- Sterilize the exposed skin with 75% ethanol.
- Make a single midline incision (about 0.5 cm in length) on the back from the center of the two thigh roots toward the head of ~1 cm distance (position is shown in Figure 1).
- Use small scissors to penetrate the skin to gently free subcutaneous tissue from the underlying muscle in order to expose the muscle layer.
- Locate the ovary under the thin muscle layer and make a small incision (about 5 mm in length) to gain entry to the peritoneal cavity.
- Use small tweezers to pull the tissue slightly on the left side of the abdominal cavity to show the left-hand ovary wrapping around the white adipose tissue (a translucent, irregular mass as seen by the naked eye, see Figure 1).
- Retract the ovarian fat pad surrounding the ovary with blunt forceps to expose the oviduct.
- Perform a single ligature around the oviduct to prevent bleeding.
- Use small scissors to sever the oviduct gently and remove the ovary.
- Check the oviduct carefully to confirm that all ovarian tissues are removed. The ovary is about 5 mm × 4 mm × 3 mm with irregular nodules on the surface.
- Place the remaining part of the oviduct back into the abdominal cavity.
- Suture the muscle layer with absorbable sutures.
- Pull the skin to the right side to expose the muscle layer on the right-hand side and remove the right ovary by repeating steps 2.9–2.16.
- Close the skin incision by using absorbable sutures.
- Inject each mouse intraperitoneally with penicillin sodium (10,000 units/10 g per mouse) to prevent infection.
- Inject lidocaine (4 mg/kg, 0.4 mL/kg of a 1% solution) beneath the skin along the site of the incision. Also provide ibuprofen (50–60 mg/kg/day; 10 mL of Children's Motrin in 500 mL of water) continuously in the drinking water for 3 days for pain treatment.
- Place each mouse into a sterilized cage individually.
- Keep under close observation for approximately 1–2 h until fully recovered from anesthesia.
- Recover animals on paper towels in a clean cage without bedding. This step minimizes the risk of tracheal obstruction or pneumonia. Provide supplemental heat support during anesthetic recovery. Monitor the surgical site to prevent the rupture of the wound.
- Following the recovery period (approximately 24 h after surgery), place mice back to their home cage.
- Do not perform the experiment for at least 2 weeks after surgery.
3. Hormone-induced estrus in females
- Determine the estrous stage of the females by performing a vaginal smear as described in McLean et al.18. No estrus cycle change indicates that the ovariectomy of the female was successful.
- Inject estradiol benzoate (20 μg per mouse, dissolved in 0.1 mL of sterilized olive oil, intraperitoneally) 48 h prior to the sexual behavior test.
- Inject progesterone (500 μg per mouse, dissolved in 0.1 mL of oil, intraperitoneally) 4 h prior to the sexual behavior test.
NOTE: The eligibility of an estrus female is determined by the female accepting genital insertion of a male mouse 3 or more times, when they cohabit with a sexually active and experienced male in one cage.
4. Preparation for the sexual behavior test
- Conduct the sexual behavior test of male mice in a rectangular and open field box (40 cm x 40 cm x 40 cm) with black Plexiglass walls, except for a transparent front wall that allows for the observation of mouse movement.
- Set the general room lighting to 650 lux.
NOTE: The mice should not be illuminated directly to avoid abnormal behavior patterns. - Use a digital camera linked to a computer to videotape the movement and behavior of the mice.
- Perform the behavioral test on the male mice during the first hours of the dark cycle.
5. Habituation
- Keep the experiment room quiet.
- Place the mice to be tested in the center of the open field box, allowing them to explore the environment freely for 30 min.
- Habituate mice for 2 consecutive days prior to the testing day in the apparatus to prevent stress from the new environment.
NOTE: Mice should move and explore freely without feeling stress. Both male and female mice need to be habituated to the testing environment.
6. Behavioral assays
- Turn on the camera prior to the beginning of the test.
- Place the mouse to be tested in the center of the open field in the test box, allowing free exploration for 5 min to acclimate to the environment.
- Place a female in estrus into the test box.
- Record the social and mating behaviors and the interactions between the male and female mice for 30 min.
- Turn off the camera and confirm that the video is saved.
- Take the female out of the test box and record the formation of a vaginal plug.
NOTE: A female mouse that accepted mating cannot be employed in another sexual behavior test in one day. - Place the female back to its home cage.
- Return the male to its home cage.
- Clean the urine, feces, and padding within the apparatus.
- Remove the smell of the tested mice with 75% ethanol.
- Start the test of the next male mouse by repeating steps 6.1–6.10.
7. Behavioral data extraction
- Play back the video recording and extract the behavioral parameters (see Figure 2).
- Record the number of mounts in 30 min.
- Record the number of intromissions in 30 min. Count a pelvic thrust as an intromission.
- Record the time from the introduction of the female to the first mounting as mounting latency.
- Record the time from the introduction of the female to the first intromission as intromission latency.
- Record the time from first intromission to the first ejaculation as ejaculation latency.
- Record the time from ejaculation to the next mounting as post-ejaculatory mount latency.
- Record number of copulatory series in 30 min. A copulatory series is each sequence from mounting to ejaculation.
- Record the time of all copulatory series in 30 min as the duration of the copulatory series.
Representative Results
A comparison of sexual behavior between CD-1 mice reared in isolation and group-housed CD-1 mice is shown. Male CD-1 mice were randomly assigned into an isolation-reared group (IS, one mouse per cage, n = 30) and a group-housed group (GH, five mice per cage, n = 15). The mice underwent isolation rearing from postnatal day 23 to day 93. Then, both groups of mice were assessed for sexual behavior. Our study found that the success rate of copulation tended to be lower in the IS group than in the GH group (IS: 80.0%, GH: 86.7%), although no statistically significant difference between groups was observed (p = 0.458). Mounting latency was longer in the IS group than in the GH group (p = 0.002, Figure 3A), indicating that the former required more time to initiate sexual behavior. Intromission latency was longer in the IS group than in the GH group (p = 0.015, Figure 3B), indicating that the former required a longer time to perform the insertion of the penis into the female's vagina. No statistically significant difference between the two groups was observed in terms of ejaculation latency and post-ejaculatory mount latency. Duration of the copulatory series was shorter in the IS group than in the GH group (p = 0.002, Figure 3C). No statistically significant differences between the two groups were observed in terms of the number of mounts, number of intromissions, and number of copulatory series19 (see Table 1).
| Isolation-reared | Group-housed | t/t' | p | |
| N | 30 | 15 | ||
| Mounting latency a | 788.70 ± 262.77 | 365.03 ± 288.65 | -3.87 | 0.002 |
| Intromission latency | 937.30 ± 369.87 | 542.94 ± 352.40 | -2.75 | 0.015 |
| Ejaculation latency | 16.58 ± 9.78 | 17.37 ± 13.03 | -0.2 | 0.845 |
| Post-ejaculatory mount latency | 173.00 ± 89.84 | 192.87 ± 106.91 | 0.58 | 0.565 |
| Duration of copulatory series | 88.27 ± 52.40 | 151.65 ± 40.87 | 3.44 | 0.002 |
| Number of mounts b | 2.4±2.0 | 3.3±3.3 | 1.09 | 0.282 |
| Number of intromissions | 20.1±12.9 | 22.6±12.3 | 0.58 | 0.564 |
| Number of copulatory series | 7.0±4.3 | 9.3±4.6 | 1.55 | 0.131 |
| Mean ± SD; a unit is second (s). b unit is counts. | ||||
Table 1: Sexual behavioral parameters of isolation-reared and group-housed mice.
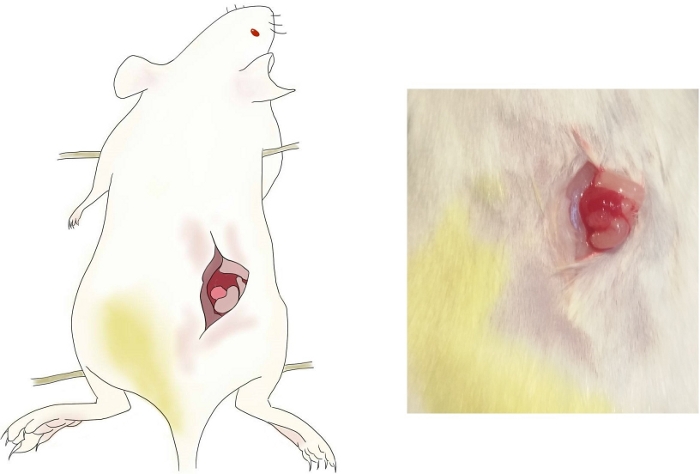
Figure 1: Ovariectomy of female mice. The position of vertical incision and the right-hand ovary are shown. Please click here to view a larger version of this figure.
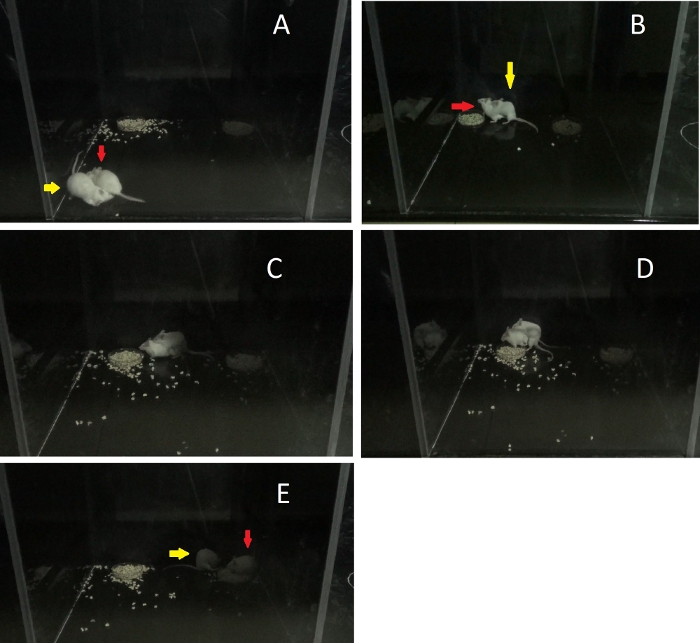
Figure 2: Process of sexual behavior of mice. The red arrow indicates the female and the yellow arrow indicates the male. (A) The male's sniffing of anal-genital areas at the beginning of sexual behavior. (B) The male mounting the female. (C) The intromission posture of the male. (D) The ejaculation of the male. (E) The male grooming the genital areas after ejaculation. Please click here to view a larger version of this figure.
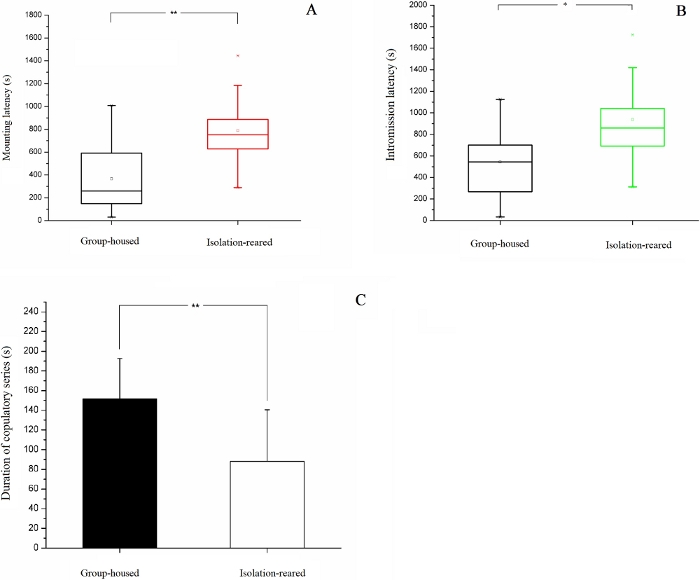
Figure 3: Results of sexual behavior test. (A) Box plot of the mounting latency of IS and GH mice. (B) Box plot of the intromission latency of IS and GH mice. (C) The total mating duration of IS and GH mice. *p < 0.05, **p < 0.01. This figure has been modified from Liu et al.19. Please click here to view a larger version of this figure.
Discussion
There are a few critical steps in the presented protocol. Regarding the ovariectomy of females, the surgery incision opening from the back is less detrimental than that from the abdomen. Given that the position of the ovary is deep, pulling other organs when the incision is cut open from the abdomen often leads to bleeding and results in unclear surgical vision20. We performed the incision on the back to reach the ovary easily and shorten the surgical time, as well as to ensure the safety of the surgery.
The maintenance of sterile conditions during surgery is important for survival. Four main variables are considered during a surgical procedure: the surgical space, the instruments, the surgeon, and the animal. For the surgical space, including workspaces and surgical rooms, all necessary surfaces are cleaned and disinfected with appropriate disinfectants (e.g., diluted bleach, hydrogen peroxide products) prior to the surgery. Additionally, pressurized steam with an autoclave is recommended. During a surgical procedure, traffic flow is limited in the surgical room. In a surgical procedure, newly sterilized instruments and materials should be used for every animal. When instruments fall outside the sterile field or become contaminated, they should be immediately replaced. Meanwhile, after washing/scrubbing hands and arms thoroughly, surgeons need to don all sterile attire, including a sterile gown and sterile surgical gloves. If any material is contaminated, the affected article needs to be changed immediately prior to surgery (e.g., new gown or surgical glove). Finally, hair from the surgical site and surrounding area should be removed for the prevention of contamination. The skin must be scrubbed with 75% ethanol after hair removal. Electric clippers or depilatory cream can be used to remove the hair. Prior to surgery, a sterile drape is placed over the animal allowing access to the surgical site for the prevention of contamination.
Three aspects of the post-surgical treatment of the animals require attention: anesthetic recovery, analgesia and surgical site monitoring, and suture removal. Upon the completion of the surgical procedure, animals must be monitored during recovery from the anesthesia. The animal should not be left unattended until it has regained sufficient consciousness to maintain sternal recumbency and should not be returned to the company of other animals until it has fully recovered. In addition, appropriate recovery conditions must be provided, including a warm environment free from objects that could cause harm. For example, paper towels instead of corn cob bedding are used in the recovery of the mice and large toys or water bowls are removed from large animal pens. According to the guidelines of the University of Minnesota, the ovariectomy of female causes moderate to severe pain. Thus, analgesia must be administered directly post-operatively by parenteral injection or oral gavage21. In this study, a lidocaine injection beneath the skin was performed after surgery and water containing ibuprofen was administered for at least 1–2 days for pain treatment. However, a veterinarian must be consulted in the development of the analgesic plan. Finally, the animal's post-surgical health and the surgical site must be observed and recorded for a minimum of 3 days. An operating line or wound clips are used for suturing the incision, which should be removed from the skin 7–14 days after surgery21. In this study, an absorbable line to suture the incision to avoid suture removal was used.
The estrus of the female was artificially controlled with ovariectomy and hormone usage, instead of using a female with natural estrus. This step was taken to ensure the consistency of the sexual receptivity of the female in the test and guarantee the reliability of measurements when monitoring male mating behavior. Furthermore, the hormone-induced estrus female can be reused in a set of experiments, and the influence of pregnancy is prevented. Estrus in the female is induced by injecting estradiol benzoate and progesterone before the experiment. This method is easy to manage, has a high success rate, and multiple estrus females can be obtained at the same time, thus greatly improving the efficiency of the test.
Sexually naïve and experienced mice show different behavior patterns. Attention needs to be paid to the test-retest reliability of the experiment in various stages of mice development. The dynamic change in sexual behavior needs to be considered before conducting the test and in the experimental design stage. In this study, sexually naïve males were used for the sexual behavior test and only the first occurrence of sexual behavior was measured without any training prior to the test. Copulation is the final outcome of a series of pheromone detection, mounting, intromission, and ejaculation. There is a limitation to the present experimental protocol when applied to mutant mice. For example, male mice with targeted mutations of Adcy3, Cnga2, and Gnao exhibit reduced ability to detect pheromones14,15,16, whereas the Trpc2 knockout mice show altered partner preference22. The present protocol may not be able to exhibit the sexual behavior of mutant mice because of its reduced ability to detect pheromones.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank Lu Cong, Zhang Hongxia, Zhang Beiyue, and Hu Mi for their suggestions for the experiments.
Materials
| Choral hydrate | Sinopharm Chenmical Reagent Co., Ltd. | 20160225 | |
| Coated VICRYL Plus Sutures | Ethicon, Inc. | missing | |
| Estradiol benzoate | J&K Scientific, Ltd. | L930Q170 | |
| Ethanol absolute | Beijing Chemical Works Co., Ltd. | 20160715 | |
| Ibuprofen (Children's Motrin) | Shanghai Johnson & Johnson Co., Ltd. | 160629478 | |
| Isoflurane | RWD Life Science Co., Ltd. | 217180501 | |
| Lidocaine | HebeI Tiancheng Pharmacreutical Co., Ltd. | 1170506107 | |
| Male and female CD-1 mice | Vital River Beijing | SCXK( )2013-0023 )2013-0023 |
|
| Olive oil | |||
| Penicillin sodium | North China Pharmaceutical Co., Ltd. | F5126420 | |
| Progesterone | J&K Scientific, Ltd. | LR50Q07 | |
| Sony digital camera | Sony Corporation | HDR-CX290E | |
| Test box | DIY | ||
| ThinkStation Computer | Lenovo | S/N PCOGLQKG | |
| Vaporizer for Isoflurane | RWD Life Science Co., Ltd. | E05904-009M |
Referenzen
- The staff of the Jackson laboratory. . Biology of the Laboratory Mouse. , (2007).
- Burns-Cusato, M., Scordalakes, E. M., Rissman, E. F. Of mice and missing data: what we know (and need to learn) about male sexual behavior. Physiology & Behavior. 83 (2), 217-232 (2004).
- Crawley, J. N. . What’s Wrong With My Mouse?. , (2006).
- Whishaw, I. Q., Haun, F., Kolb, B., Windhorst, U., Johansson, H. Analysis of Behavior in Laboratory Rodents. Modern Techniques in Neuroscience Research. , (1999).
- Sachs, B., Barfield, R. . Functional Analysis of Masculine Copulatory Behavior in the Rat. 7, (1976).
- Hull, E. M., Dominguez, J. M. Sexual behavior in male rodents. Hormones and Behavior. 52 (1), 45-55 (2007).
- Mosig, D. W., Dewsbury, D. A. Studies of the copulatory behavior of house mice (Mus musculus). Behavioral Biology. 16 (4), 463-473 (1976).
- Park, J. H., Gould, T. Assessment of Male Sexual Behavior in Mice. Mood and Anxiety Related Phenotypes in Mice. 63, (2011).
- Bonthuis, P. J., et al. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Frontiers in Neuroendocrinology. 31 (3), 341-358 (2010).
- Levine, L., Barsel, G. E., Diakow, C. A. Mating behaviour of two inbred strains of mice. Animal Behavior. 14 (1), 1-6 (1966).
- McGill, T. E. Sexual Behavior in Three Inbred Strains of Mice. Behaviour. 19 (4), 341 (1962).
- James, P. J., Nyby, J. G. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus). Physiology & Behavior. 75 (3), 287-294 (2002).
- Arteaga-Silva, M., Rodriguez-Dorantes, M., Baig, S., Morales-Montor, J. Effects of castration and hormone replacement on male sexual behavior and pattern of expression in the brain of sex-steroid receptors in BALB/c AnN mice. Comparative Biochemistry and Physiology – Part A: Molecular & Integrative Physiology. 147 (3), 607-615 (2007).
- Zhang, Z., et al. Deletion of Type 3 Adenylyl Cyclase Perturbs the Postnatal Maturation of Olfactory Sensory Neurons and Olfactory Cilium Ultrastructure in Mice. Frontiers in Cellular Neuroscience. 11, 1 (2017).
- Mandiyan, V. S., Coats, J. K., Shah, N. M. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature Neurosciemce. 8 (12), 1660-1662 (2005).
- Choi, C. I., et al. Simultaneous deletion of floxed genes mediated by CaMKIIalpha-Cre in the brain and in male germ cells: application to conditional and conventional disruption of Goalpha. Experimental & Molecular Medicine. 46, 93 (2014).
- Pelch, K. E., Sharpe-Timms, K. L., Nagel, S. C. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. Journal of Visualized Experiments. (59), e3396 (2012).
- McLean, A. C., Valenzuela, N., Fai, S., Bennett, S. A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of Visualized Experiments. (67), e4389 (2012).
- Liu, Z. W., et al. Postweaning Isolation Rearing Alters the Adult Social, Sexual Preference and Mating Behaviors of Male CD-1 Mice. Frontiers in Behavioral Neuroscience. 13, 21 (2019).
- TIan, E. P., Long, T., Qin, D. N. Establishment and applications of mating model in male rat. Chinese Journal of Andrology. 22 (1), 7-10 (2008).
- . Anesthesia Guidelines: Mice Available from: https://www.researchservices.umn.edu/services-name/research-animal-resources/research-support/guidelines/anesthesia-mice (2019)
- Leypold, B. G., et al. Altered sexual and social behaviors in trp2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 99 (9), 6376-6381 (2002).

