Tomato Root Transformation Followed by Inoculation with Ralstonia Solanacearum for Straightforward Genetic Analysis of Bacterial Wilt Disease
Summary
Here, we present a versatile method for tomato root transformation followed by inoculation with Ralstonia solanacearum to perform straightforward genetic analysis for the study of bacterial wilt disease.
Abstract
Ralstonia solanacearum is a devastating soil borne vascular pathogen that can infect a large range of plant species, causing an important threat to agriculture. However, the Ralstonia model is considerably underexplored in comparison to other models involving bacterial plant pathogens, such as Pseudomonas syringae in Arabidopsis. Research targeted to understanding the interaction between Ralstonia and crop plants is essential to develop sustainable solutions to fight against bacterial wilt disease but is currently hindered by the lack of straightforward experimental assays to characterize the different components of the interaction in native host plants. In this scenario, we have developed a method to perform genetic analysis of Ralstonia infection of tomato, a natural host of Ralstonia. This method is based on Agrobacterium rhizogenes-mediated transformation of tomato roots, followed by Ralstonia soil-drenching inoculation of the resulting plants, containing transformed roots expressing the construct of interest. The versatility of the root transformation assay allows performing either gene overexpression or gene silencing mediated by RNAi. As a proof of concept, we used this method to show that RNAi-mediated silencing of SlCESA6 in tomato roots conferred resistance to Ralstonia. Here, we describe this method in detail, enabling genetic approaches to understand bacterial wilt disease in a relatively short time and with small requirements of equipment and plant growth space.
Introduction
Ralstonia solanacearum, the causal agent of bacterial wilt disease, is a devastating soil borne vascular pathogen with a worldwide distribution that can infect a large range of plant species, including potato, tomato, tobacco, banana, pepper and eggplant, among others1,2. Yield losses caused by Ralstonia can reach 80-90% of production in tomato, potato or banana, depending on cultivar, climate, soil and other factors3. However, the Ralstonia model is considerably underexplored in comparison to other models involving bacterial plant pathogens, such as Pseudomonas syringae or Xanthomonas spp. Additionally, most studies in plant-microbe interactions are focused on the model plant Arabidopsis thaliana. Although research using these models has largely contributed to our understanding of plant-bacteria interactions, they do not address the current necessity to understand these interactions in crop plants. Research targeted to understanding the interaction between Ralstonia and crop plants is essential to develop sustainable solutions to fight against bacterial wilt disease but is currently hindered by the lack of straightforward experimental assays to characterize the different components of the interaction. Particularly, tomato, a natural host for Ralstonia, is the second most important vegetable crop worldwide and is affected by a plethora of diseases4, including bacterial wilt disease. In this work, we have developed an easy method to perform genetic analysis of Ralstonia infection of tomato. This method is based on Agrobacterium rhizogenes-mediated transformation of tomato roots, using DsRed fluorescence as selection marker5, followed by Ralstonia soil-drenching inoculation of the resulting plants, containing transformed roots expressing the construct of interest. The versatility of the root transformation assay allows performing either gene overexpression or gene silencing mediated by RNAi.
A potential limitation of this method consists on the residual growth of non-transformed roots. This is particularly important in the cases where the plasmid used lacks a reporter gene that allows the selection of transformed roots. To solve this problem, we have developed an alternative method based on antibiotic selection, which inhibits the growth of non-transformed roots while allowing the growth of healthy antibiotic-resistant transformed roots. Since A. rhizogenes does not induce the transformation of shoots, they are susceptible to the antibiotic, and, therefore, they should be kept separated from the antibiotic-containing medium.
Although plant resistance against Ralstonia is not well understood, several reports have associated cell wall alterations to enhanced resistance to bacterial wilt6,7,8,9. It has been suggested that these cell wall alterations affect vascular development, an essential aspect for the lifestyle of Ralstonia inside the plant10. Mutations in genes encoding the cellulose synthases CESA4, CESA7 and CESA8 in Arabidopsis thaliana have been shown to impair secondary cell wall integrity, causing enhanced resistance to Ralstonia, which appears to be linked to ABA signalling8. Therefore, as a proof of concept for our method, we performed RNAi-mediated gene silencing of SlCESA6 (Solyc02g072240), a secondary cell-wall cellulose synthase, and ortholog of AtCESA8 (At4g18780). Subsequent soil-drenching inoculation with Ralstonia showed that silencing SlCESA6 enhanced resistance to bacterial wilt symptoms, suggesting that cell wall-mediated resistance to Ralstonia is likely conserved in tomato, and validating our method to carry out genetic analysis of bacterial wilt resistance in tomato roots. Here, we describe this method in detail, enabling genetic approaches to understand bacterial wilt disease in a relatively short time and with small requirements of equipment and plant growth space.
Protocol
NOTE: Important parts of this method involve handling plant materials in vitro, and therefore it is important to keep sterile conditions during all these procedures, including the visualization of DsRed fluorescence. During all the transformation process, tomato seedlings grow at 25−28 °C and 16 h/8 h light/dark (130 µmol photons m-2s-1 light). Plates are sealed with micropore tape in order to facilitate gas exchange and transpiration.
1. Preparation of tomato plants and Agrobacterium rhizogenes
- Sterilize tomato seeds (Solanum lycopersicum cv. Moneymaker, LA2706, Tomato Genetics Resource Center, TGRC) with 5% (v/v) sodium hypochlorite for 5 min. Wash 4-5 times with distilled sterile water and keep the seeds shaking slowly in sterile water over-night to facilitate germination.
- Transfer the tomato seeds to half-strength Murashige and Skoog (1/2 MS) medium without sucrose (2.21 g/L MS, 8% w/v agar). Keep the seeds in the dark at 25−28 °C for three days (Figure 1A).
NOTE: Around 40 seeds are usually needed for each construct. - Autoclave 8.5 cm2 square filter papers. Place the square filter papers inside 9 cm2 square petri dishes containing 1/2 MS medium (place the paper on top of the agar) and place six germinated tomato seeds on each plate (Figure 1B). Seal the plate with micropore tape and incubate the germinated seeds at 25−28 °C for 3−4 days.
- Grow Agrobacterium rhizogenes MSU440 in solid LB medium (with appropriate antibiotics) at 28 °C two days before plant transformation.
NOTE: A. rhizogenes is provided by Dr. Juan Antonio López Ráez, EEZ-CSIC, Granada, Spain. In the experiment described in this article, A. rhizogenes containing pK7GWIWG2_II-RedRoot::CESA6 or an empty vector, as control, were used. pK7GWIWG2_II-RedRoot contains a reporter gene, DsRed, driven by the Arabidopsis thaliana ubiquitin promoter (pAtUBQ10), and confers resistance to spectinomycin (50 µg/mL). It is possible to use other vectors for gene overexpression, as reported before5. In this work, the amplification of the specific fragment for SlCESA6 silencing was obtained by reverse transcription (RT) PCR using RNA isolated from tomato (S. lycopersicum cv. Moneymaker) and ligated into p-ENTR/D-TOPO vector (Table 1). Subsequently, the SlCESA6-RNAi fragment was cloned into the pK7GWIWG2_II-RedRoot binary vector.
2. Plant transformation and selection
- Using a sterile scalpel, cut the radicle and the bottom of the hypocotyl of tomato seedlings (Figure 1C,D).
- Harvest A. rhizogenes biomass from the surface of the LB medium using plastic tips or a scalpel blade and carefully dip the cut tomato seedlings in the bacterial biomass (Figure 1E).
- After inoculation with A. rhizogenes, cover the tomato seedlings with a 2 cm x 4 cm semi-circular filter paper (Figure 1F), in order to keep a high humidity and facilitate survival and new root development.
- Store the transformed tomato seedlings for 6-7 days. Then, use a sterile scalpel to cut the new emerging hairy roots (Figure 1G,H; at this step, the roots are not transformed yet) and allow the seedlings to produce new hairy roots.
- Once the second generation of new hairy roots appear (Figure 1I), remove the filter paper on top of the seedlings, and seal the plate with micropore tape again.
NOTE: At this point, the presence of the DsRed fluorescent marker in the transformation vector allows to visualize the transformation efficiency in the new roots. - To visualize DsRed fluorescence, use a stereomicroscope or any other equipment for plant in vivo imaging (Figure 1J). Mark the positive (red fluorescence) transformed roots and remove the negative non-transformed roots (no red fluorescence) using a sterile scalpel.
- Transfer the seedlings showing red fluorescence to a new plate containing 1/2 MS medium, in order to facilitate the development of the transformed root as the main root (Figure 1K). Keep the seedlings that do not show red fluorescence in the same plate to check the emergence of fluorescent roots (Figure 1L) in later time points.
- Alternative method based on antibiotic selection
- Alternative to step 2.5, prepare half-filled 9 cm2 square plates containing 1/2 MS medium with the appropriate antibiotic. Incline the plates approximately 5° during the preparation process to generate an empty space without medium (Figure 2A), allowing the shoots to grow avoiding contact with the antibiotic.
- Alternative to step 2.6, after cutting the first non-transformed hairy roots emerged (step 2.4), transfer the seedlings without roots to the half-filled plates using filter papers with the appropriate size (Figure 2B).
NOTE: As positive control, it is recommended to transform several seedlings with a plasmid containing the same antibiotic resistance and a reporter gene (in this protocol, pK7GWIWG2_II-RedRoot; kanamycin 50 µg/mL). - Alternative to step 2.7, let the seedlings develop new hairy roots and cut those roots that are not in direct contact with the surface of the filter sandwich papers, since these roots may avoid antibiotic selection.
NOTE: Due to the antibiotic effect, root development may be slower than in plates without antibiotics. New transformed hairy roots appear within 14−18 days after transferring the seedlings without roots to the half-filled plates (step 2.8.2) (Figure 2C-E).
- Cover the seedlings with a 2 cm x 4 cm semi-circle filter paper, seal the plate and incubate the seedlings to let them develop new hairy roots.
NOTE: It is not necessary to eliminate the A. rhizogenes using antibiotics. The filter paper on top of the MS medium and the lack of sucrose inhibit the spread of the A. rhizogenes on the plate5. - Five-to-seven days after the first root selection, repeat the selection process to select new transformed roots and remove non-transformed roots. Transfer the seedlings containing transformed roots to a new plate containing 1/2 MS medium.
3. Transfer to inoculation pots
- Prepare inoculation pots where the surface of the roots will be exposed to the bacterial inoculum: soak the inoculation pots with water, pour off any excess water, and place them in a plastic planting tray. Transfer the selected seedlings with transformed roots to inoculation pots using tweezers (Figure 3A).
- Cover the tray with plastic wrap or a transparent lid and keep them at 25−28 °C and 65% humidity (16 h/8 h light/dark; 130 µmol photons m-2s-1 light), to maintain a high level of humidity Figure 3B). Remove the cover after five or six days.
NOTE: The transformed tomato plants are ready for inoculation two-to-three weeks after transferring them to soil (Figure 3C). During the growth on soil, tomato plants may produce new roots that are not transformed. To determine the extent of this phenomenon, red fluorescence was visualized in roots of 3-week-old transformed tomato plants. Most roots (80%-100%) showed red fluorescence, indicating that they are indeed transformed (Figure 3D,E).
4. Soil-drenching inoculation
- Grow R. solancearum (strain GMI1000 in this protocol) in phi liquid medium (Table 211) in an orbital shaker (200 rpm) at 28 °C until stationary phase.
- Determine bacterial numbers by measuring the optical density of the bacterial culture at 600 nm (OD600). Dilute the bacterial culture with water to an OD600 of 0.1 (in conditions used here, this corresponds to approximately 108 colony-forming units [CFU]/mL).
- Place 16−20 inoculation pots containing transformed tomato plants in an inoculation tray (29 cm x 20 cm; Figure 4A).
- Pour 300 mL (~15 mL per plant) of bacterial inoculum (OD600 of 0.1) into the tray containing the inoculation pots. Let them soak in the inoculum for 20 min (Figure 4B).
- Prepare a new tray with a layer of potting soil. Move the inoculated pots into the new tray (Figure 4C) and place the trays in a growth chamber with 75% humidity, 26−28 °C, and a photoperiod of 12 h light and 12 h darkness (130 µmol photons m-2s-1 light).
5. Determination of infection parameters and statistical analysis
- Score disease symptoms as previously described12,13, using a scale ranging from 0 (no symptoms) to 4 (complete wilting) (Figure 4D-H), each day after Ralstonia inoculation for two weeks.
NOTE: The disease index data are collected from the same experimental unit (each plant) over time.
6. Gene expression analysis
NOTE: The expression of the transgene or the silencing of the target gene can be determined by RT-PCR or by quantitative RT-PCR (qRT-PCR).
- Collect samples and extract RNA from a representative part of the transformed root system (to evaluate the effect on the target gene) and leaves (as internal control).
- Synthesize cDNA using 1 µg of total DNase-treated RNA.
- Analyze gene expression of the target genes by qRT-PCR. Calculate the relative transcription levels using the 2-ΔΔCT method14, using SlEFα-1 as housekeeping gene15.
NOTE: Primer sequences are listed in Table 1.
Representative Results
Figure 5 shows the development of disease symptoms of tomato plants with roots transformed with an empty vector (EV), and plants with roots transformed with an RNAi construct targeting SlCESA6 (Solyc02g072240). The disease index data (Figure 5A) are collected from the same experimental unit (each plant) over time according to an arbitrary scale from 0 to 4, and do not follow a Gaussian distribution, ruling out the use of standard tests for parametric data. Moreover, there is an intrinsic plant-to-plant variation in this kind of experiments, due to the colonization and infection dynamics. Below we considered different methods for the representation and statistical analysis of symptoms associated to Ralstonia infection.
As a standard approach, a U Mann-Whitney two-tailed non-parametric test to compare both control (EV) and SlCESA6-RNAi infection curves was used. According with this analysis, the difference between the medians of both curves appears to be non-significant (P = 0.16).
It is also possible to quantify the area under the disease progress curve (AUDPC), which allows combining multiple observations of disease progress into a single value. AUDPC showed a higher value for control plants (EV; 28.75 ± 4.75) compared to SlCESA6-RNAi (21.01 ± 4.74) plants at the end of the infection process, indicating that SlCES6-silenced plants are more resistant to Ralstonia infection than control plants (Figure 5B).
Confidence intervals (CI) offer a way of estimating, with high probability, a range of values in which the population value (or parameter) of a given variable is found. As it is shown in Figure 5C, the area of 95% CI for control (EV) and SlCESA6-RNAi infection curves estimate a higher chance of resistance when SlCESA6 is silenced.
Disease index values can be transformed into binary data, considering a disease index lower than 2 corresponding to "0", and a disease index equal or higher than 2 corresponding to "1"12. This allows the representation of a survival curve after Ralstonia inoculation. This transformation is based on the observation that, once the plants start developing clear symptoms (disease index of 2), they are considered as "infected" and will die as a consequence of this infection. The differences in the survival rate between EV and SlCESA6-RNAi plants were not statistically significant according to a Gehan-Breslow-Wilcoxon Statistical Test (P = 0.13) (Figure 5D).
In addition to performing statistical analysis, and regardless of the obtained P values, it is worth interpreting these data based on the reproducibility of the observed tendencies within different replicates (Supplementary Figure 1). In keeping with this notion, an increasing number of scientists have recently commented on the risks of overusing strict statistical thresholds (such as the standard P ≤ 0.05)16.
The expression of SlCESA6 was analyzed in two randomly selected transformed roots before the inoculation step, showing that the enhanced resistance to Ralstonia correlates with the reduced expression of SlCESA6 (Figure 5E). Using this method, a transformation rate of 35−40% after two rounds of selection can be obtained (Figure 5F). This value can be increased by performing additional selection rounds5.
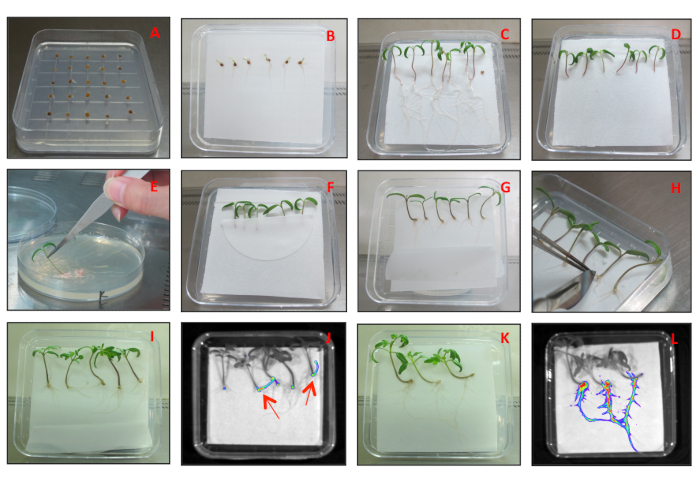
Figure 1: Plant preparation, transformation and selection. (A) Germination of tomato seeds in half-strength MS (1/2) medium. (B) Germinated tomato seeds placed on filter paper lying on a plate containing 1/2 MS medium. Tomato seedlings before (C) and after (D) cutting the radicle and the bottom of the hypocotyl. (E) Tomato seedling transformation by dipping in bacterial biomass. (F) Transformed tomato seedlings covered with filter paper to maintain humidity. Emergence (G) and removal (H) of new (non-transformed) hairy roots. (I) Transformed roots. (J) Selection of transformed tomato hairy roots by visualization of DsRed fluorescence. Red arrows indicate positive transformed roots expressing DsRed fluorescence. (K) Development of the transformed roots as the main roots. (L) Visualization of DsRed fluorescence in mature transformed roots. Please click here to view a larger version of this figure.

Figure 2: Alternative method based on antibiotic selection. (A) Half-filled square plate containing 1/2 MS medium. (B) Cut seedlings disposed on filter paper lying on 1/2 MS medium with antibiotic, after removal of first non-transformed hairy roots. (C) Roots covered with additional filter paper. (D) Tomato transformed roots grown on 1/2 MS medium with antibiotic. (E) Seedlings transformed with pK7GWIWG2_II-RedRoot (kanamycin 50 µg/mL), expressing DsRed, as positive control. Please click here to view a larger version of this figure.

Figure 3: Transfer of transformed tomato to inoculation pots. (A) Root-transformed tomatoes transferred to full-soaked inoculation pots. (B) Transformed tomatoes in inoculation pots covered with a plastic lid to maintain high level of humidity. (C) Two-to-three-week-old root-transformed tomatoes, after transference to inoculation pots, ready to be inoculated. (D) Three-week-old tomato plants after removing the soil. (E) DsRed fluorescence in roots of 3-week-old transformed tomato plants. Please click here to view a larger version of this figure.
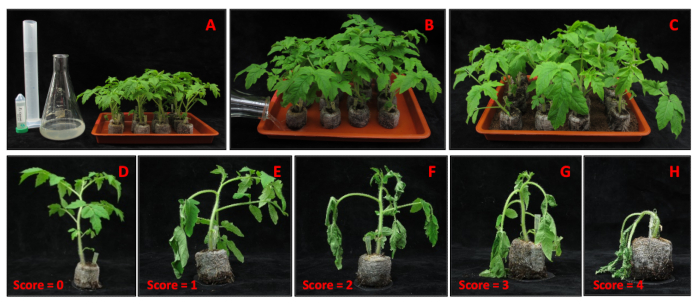
Figure 4: Ralstonia soil-drenching inoculation. (A) Materials needed for R. solanacearum GMI1000 inoculation. (B) Soil-drenching of transformed tomatoes in inoculation pots with R. solanacearum GMI1000 inoculum. (C) Inoculated tomatoes placed on a layer of potting soil. (D-H) Ralstonia disease symptoms scale ranging from 0 (no symptoms) to 4 (complete wilting). Please click here to view a larger version of this figure.
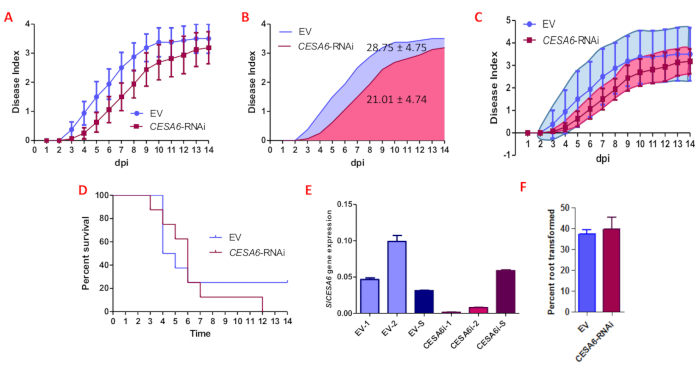
Figure 5: SlCESA6 silencing enhances resistance to R. solanacearum. (A) Disease symptoms of RNAi-mediated SlCESA6-silenced (CESA6-RNAi) and empty vector (EV) root-transformed tomato plants upon inoculation with R. solanacearum. Values correspond to the means ± SE of eight plants. (B) Area under the disease progress curve of plants shown in panel A. (C) 95% confidence Interval of plants shown in panel A. (D) Percent of surviving plants shown in panel A. (E) Gene expression analysis by qRT-PCR of RNAi-mediated SlCESA6-silenced (CESA6i) and EV root-transformed tomato plants in roots (1,2) and shoot (S). Values correspond to the means ± SE of three technical replicates. (F) Transformation rate of EV and CESA6-RNAi root-transformed tomato plants of two independent experiments. Values correspond to the means ± SE, after two rounds of selection. Please click here to view a larger version of this figure.
| Primer name | Primer sequence (5'-3') |
| EFα-1-F | GGTGGCGAGCATGATTTTGA |
| EFα-1-R | CGAGCCAACCATGGAAAACAA |
| qCESA6-F | GATCTGGTTCGCTTTCTCGT |
| qCESA6-R | TCCCTCCCTTTCATACCTTG |
| CESA6-RNAi-F | CACCGGCGAACAAGTGGGGTTAG |
| CESA6-RNAi-R | TTTGAGACTTTGGCACTGGA |
Table 1: Primer sequences.
| Ingredients | For 1 L |
| Bacto peptone | 10 g |
| Yeast extract | 1 g |
| Casamino acids | 1 g |
Table 2: Phi (Ø) medium composition.
Supplementary Figure 1: Reproducibility of the result shown in Figure 5A. Disease symptoms of RNAi-mediated SlCESA6-silenced (CESA6-RNAi) and EV root-transformed tomato plants upon inoculation with R. solanacearum. Values correspond to the means ± SE of eight plants. Gene expression analysis was performed by qRT-PCR of RNAi-mediated SlCESA6-silenced (CESA6i) and Empty Vector (EV) root-transformed tomato plants in roots (1,2) and shoot (S). Values correspond to the means ± SE of three technical replicates. Please click here to download this figure.
Discussion
Ralstonia solanacearum poses an important threat to agriculture; however, its interaction with natural hosts of agricultural importance is still poorly understood compared with other bacterial pathogens, especially in crop plant species. In most cases, genetic analysis is hindered by the time and expenses required to genetically modify host plants. To address this problem and facilitate genetic analysis of R. solanacearum infection in tomato, we have developed an easy method based on Agrobacterium rhizogenes-mediated transformation of tomato roots (Figure 1), followed by soil-drenching inoculation (Figure 3). The transformed roots are selected using a fluorescent reporter (DsRed in this protocol) (Figure 1). Additionally, we have also developed an alternative method based on antibiotic selection that does not damage the non-transformed aerial part (Figure 2).
The transformation protocol is based on that described by Ho-Plágaro et al.5, with several modifications. The versatility of this method allows multiple additional assays in transformed roots, such as those to analyze plant physiology, response to chemical treatments, and/or responses to different biotic and abiotic stresses.
After transferring the transformed plants to soil, we considered the possibility that plants may develop new roots that may not be transformed. We explored this possibility by observing the fluorescence from DsRed in the root system of transformed plants two weeks after transferring them to soil (before Ralstonia inoculation). The results showed red fluorescence in the majority of the roots (Figure 3D).
T-DNA transfer by Agrobacterium is integrated in the host genome in a random manner, and, therefore, this method generates a heterogeneous population of tomato plants with different expression level of the target gene. R. solanacearum infection normally causes the death of the plants, which, subsequently, hinders the collection of root samples after the experiment to analyze the gene expression of each plant. To analyze the expression level of the target gene during the experiments, we select two to three root-transformed plants before the inoculation step, as representative sample of the population that will be inoculated. Figure 5 shows that the reduction of disease symptoms in tomato plants correlates with the efficiency of SlCESA6 silencing before the inoculation step. Therefore, and despite of the limitations of the protocol, our representative results clearly demonstrate that this method is a powerful tool to study candidate genes involved in resistance or susceptibility to R. solanacearum in tomato. The example shown in this article allowed us to determine that knocking-down SlCESA6, a secondary cell wall-related cellulose synthase, enhances resistance to infection by R. solanacearum, resembling previous observations using its ortholog AtCESA8 (At4g18780) from Arabidopsis thaliana8.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank all lab members of the Macho laboratory for helpful discussions, Alvaro López-García for statistical advice, and Xinyu Jian for technical and administrative assistance during this work. We thank the PSC Cell Biology core facility for assistance with fluorescence imaging This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant XDB27040204), the Shanghai Center for Plant Stress Biology (Chinese Academy of Sciences) and the Chinese 1000 Talents program.
Materials
| 90 mm square Petri-dishes | |||
| Agar powder | Sigma-Aldrich | ||
| Bacto peptone | BD (Becton and Dickinson) | ||
| Casamino acids | Sigma-Aldrich | ||
| Filter paper | |||
| In Vivo Plant Imaging System NightShade LB 985 | Berthold Technologies | ||
| Jiffy pots | Jiffy Products International A.S. | ||
| Micropore tape | 3M | ||
| Murashige and Skoog medium (M519) | Phytotechlab | ||
| Pindstrup substrate | Pindstrup Mosebrug A/S | ||
| Scalpel and blade | |||
| Sodium hypochlorite | Sigma-Aldrich | ||
| Sterile clean bench | |||
| Tweezers | |||
| Wahtman paper | Wahtman International Ltd. Maldstone | ||
| Yeast extract | OXOID |
Referenzen
- Jiang, G., et al. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Frontiers in Plant Science. 11 (8), 1549 (2017).
- Mansfield, J., et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular plant pathology. 13 (6), 614-629 (2012).
- Elphinstone, J. G., Allen, C., Prior, P., Hayward, A. C. . The current bacterial wilt situation: a global overview. In: Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex. , 9-28 (2005).
- Jones, J. B., Jones, J. P., Stall, R. E., Zitter, T. A. . Compendium of Tomato 1094 Diseases. , (1991).
- Ho-Plágaro, T., Huertas, R., Tamayo-Navarrete, M. I., Ocampo, J. A., García-Garrido, J. M. An improved method for Agrobacterium rhizogenes-mediated transformation of tomato suitable for the study of arbuscular mycorrhizal symbiosis. Plant Methods. 14, 34 (2018).
- Wydra, K., Beri, H. Structural changes of homogalacturonan, rhamnogalacturonan I and arabiogalactan protein in xylem cell walls of tomato gentoypes in reaction to Ralstonia solanacearum. Physiological and Molecular Plant Pathology. 68, 41-50 (2006).
- Wydra, K., Beri, H. Immunohistochemical changes in methyl-ester distribution of homogalacturonan and side chain composition of rhamnogalacturonan I as possible components of basal resistance in tomato inoculated with Ralstonia solanacearum. Physiological and Molecular Plant Pathology. 70, 13-24 (2007).
- Hernández-Blanco, C., et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell. 19 (3), 890-903 (2007).
- Denancé, N., et al. Arabidopsis wat1 (walls are thin1)-mediated resistance to the bacterial vascular pathogen, Ralstonia solanacearum, is accompanied by cross-regulation of salicylic acid and tryptophan metabolism. Plant Journal. 73 (2), 225-239 (2013).
- Digonnet, C., et al. Deciphering the route of Ralstonia solanacearum colonization in Arabidopsis thaliana roots during a compatible interaction: focus at the plant cell wall. Planta. 236 (5), 1419-1431 (2012).
- Sang, Y., et al. The Ralstonia solanacearum type III effector RipAY targets plant redox regulators to suppress immune responses. Molecular Plant Pathology. 19 (1), 129-142 (2018).
- Remigi, P., Anisimova, M., Guidot, A., Genin, S., Peeters, N. Functional diversification of the GALA type III effector family contributes to Ralstonia solanacearum adaptation on different plant hosts. New Phytologist. 192, 976-987 (2011).
- Wang, K., et al. Functional assignment to positively selected sites in the core type III effector RipG7 from Ralstonia solanacearum. Molecular Plant Pathology. 17, 553-564 (2016).
- Livak, K. J., Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 25 (4), 402-408 (2001).
- León-Morcillo, R. J., Martín-Rodríguez, J. A., Vierheilig, H., Ocampo, J. A., García-Garrido, J. M. Late activation of the 9-oxylipin pathway during arbuscular mycorrhiza formation in tomato and its regulation by jasmonate signalling. Journal of Experimental Botany. 63 (10), 3545-3558 (2012).
- Amrhein, V., Greenland, S., McShane, B. Retire statistical significance. Nature. 567, 305-307 (2019).

