Fluorescent Calcium Imaging and Subsequent In Situ Hybridization for Neuronal Precursor Characterization in Xenopus laevis
Summary
We present a two-part protocol that combines fluorescent calcium imaging with in situ hybridization, allowing the experimenter to correlate patterns of calcium activity with gene expression profiles on a single-cell level.
Abstract
Spontaneous intracellular calcium activity can be observed in a variety of cell types and is proposed to play critical roles in a variety of physiological processes. In particular, appropriate regulation of calcium activity patterns during embryogenesis is necessary for many aspects of vertebrate neural development, including proper neural tube closure, synaptogenesis, and neurotransmitter phenotype specification. While the observation that calcium activity patterns can differ in both frequency and amplitude suggests a compelling mechanism by which these fluxes might transmit encoded signals to downstream effectors and regulate gene expression, existing population-level approaches have lacked the precision necessary to further explore this possibility. Furthermore, these approaches limit studies of the role of cell-cell interactions by precluding the ability to assay the state of neuronal determination in the absence of cell-cell contact. Therefore, we have established an experimental workflow that pairs time-lapse calcium imaging of dissociated neuronal explants with a fluorescence in situ hybridization assay, allowing the unambiguous correlation of calcium activity pattern with molecular phenotype on a single-cell level. We were successfully able to use this approach to distinguish and characterize specific calcium activity patterns associated with differentiating neural cells and neural progenitor cells, respectively; beyond this, however, the experimental framework described in this article could be readily adapted to investigate correlations between any time-series activity profile and expression of a gene or genes of interest.
Introduction
Free cytosolic calcium is critical to a variety of biological processes, ranging from cell proliferation and migration to apoptosis and autophagy1,2,3. Within these pathways, calcium can exert downstream effects on gene expression by interacting with calcium-binding domains to induce conformational changes that modulate protein activity and interactions. For example, a neuronal calcium sensor known as the Downstream Regulatory Element Antagonist Modulator (DREAM) is held in an unfolded intermediate conformation when bound by calcium, preventing it from interacting with its protein and DNA targets4. Beyond serving as a simple signaling molecule, however, the dynamic nature of intracellular calcium transients allows these activity patterns to encode more complex amplitude- or frequency-based signals5,6. Nuclear translocation of the transcription factor nuclear factor of activated T-cells (NFAT) is enhanced by high-frequency calcium oscillations but inhibited by low-frequency oscillations7. Compellingly, recent work has suggested that NFAT may actually responsive to cumulative calcium exposure8. Both calcineurin and Ca2+/calmodulin-dependent protein kinase II (CaMKII) also exhibit distinct responses to calcium transients of a specific frequency, duration, or amplitude9. To add an additional level of regulatory complexity, computational models suggest that many downstream calcium-binding proteins become more or less frequency-dependent in response to the presence or absence of binding competitors10,11.
Within the developing nervous system, two main classes of calcium activity behaviors have been defined and associated with specific biological processes. Calcium influxes are classified as "spikes" if they occur within individual cells, reach a peak intensity of ~400% of baseline within five seconds, and exhibit double exponential decay12. This type of signal is associated primarily with neurotransmitter phenotype specification13. In contrast, "waves" are defined as slower, less extreme calcium transients in which a cell's intracellular calcium concentration rises to ~200% of baseline over a period of thirty seconds or more, then decays over several minutes12. These signals often propagate across multiple neighboring cells, and their presence has been associated with neurite outgrowth and cell proliferation14,15. However, although these two classes have been defined based on characteristic kinetic profiles, it remains unclear exactly which characteristics of these patterns are actually being detected by cells and translated by downstream effectors.
Understanding the relationship between intracellular calcium oscillations and gene expression would provide crucial insight into one of the regulatory mechanisms that ensures appropriate development and patterning of the nervous system. To this end, studies of the embryonic spinal cord have demonstrated that increased calcium spike activity during development is associated with higher levels of inhibitory neurons, while decreased calcium spike activity is associated with higher levels of excitatory neurons13. However, these population-level assays have not been used to associate calcium activity with gene expression on a single-cell level.
Approaching these questions on the level of the single cell offers several distinct advantages over previous work. For one, the ability to assess calcium activity and gene expression in many cells individually allows the full repertoire of distinct activity patterns to be observed without being obfuscated by a bulk-level measurement. Additionally, studying these relationships in single-cell primary culture means that cell-autonomous links between calcium activity and gene expression will be maintained, while interactions requiring cell-cell communication will be abrogated. Therefore, this approach allows these cell-autonomous mechanisms to be studied in isolation. However, it also allows the role of non-cell-autonomous calcium activity to be elucidated and interrogated. For example, cells can be dissected from an embryo at the neural plate stage, cultured until sibling controls reach the neural tube stage, and then compared to cells that have been freshly dissected from a neural-tube-stage embryo. This allows direct comparison of cells that retained cell-cell communication across a key developmental period to those in which cell-cell communication was abolished.
In seeking to address the limitations of previous experimental approaches, we developed a protocol that would enable the assessment of both calcium activity and gene expression in individual neural progenitor cells, facilitating the correlation of specific activity patterns with subsequent differentiation programs. Neural tissue was dissected from Xenopus laevis at various stages of neural development, dissociated into single cells, and imaged via confocal microscopy in the presence of a fluorescent calcium indicator. Following live-cell imaging, samples were fixed and assayed via fluorescence in situ hybridization (FISH) to detect expression of a gene or isoform of interest. Importantly, individual cells can be tracked across both imaging experiments, meaning that a cell's calcium activity profile and its gene expression level can be associated with one another (Figure 1). The protocol reported here is intended to probe relationships between calcium activity patterns and gene expression across embryonic neurodevelopment in Xenopus laevis. However, the broader experimental framework (single-cell time-course imaging followed by FISH and image coregistration) can be modified and applied to virtually any cell type, fluorescent reporter, and gene of interest.
Protocol
All work involving animals was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the College of William and Mary.
1. Animal Care and Embryo Handling
- Induce natural mating by administering a subcutaneous injection of human chorionic gonadotropin (HCG) into the dorsal lymph sac of adult Xenopus laevis at a dose of 600 U for females and 400 U for males.
- After injection, place at least one male and one female frog in a room-temperature holding tank overnight. Egg laying typically begins 9-12 h after HCG administration.
- Collect embryos. Dejelly by gently washing with 2% cysteine (pH 8.0) for 2-4 min.
- Rinse the embryos 3x in 0.1x Mark's Modified Ringer's solution (MMR) (100 mM NaCl, 2 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 5 mM HEPES, pH adjusted to 7.4–7.6).
- Transfer the embryos to 100 mm glass Petri dishes containing 0.1x MMR and 50 µg/mL gentamycin. A density of 50-100 embryos per plate is appropriate.
- Incubate the dishes at 14 °C–22°C and allow the embryos to develop until they reach the desired developmental stage(s). Periodically remove unfertilized cells and necrotic or abnormally developing embryos with a plastic transfer pipette.
NOTE: To ensure consistency, developmental staging is performed according to morphological criteria defined by Nieuwkoop and Faber16. Stages of interest will vary based on experimental focus. For example, key neurodevelopmental landmarks are associated with Stage 14 (onset of neurulation), Stage 18 (onset of neural tube closure), and Stage 22 (onset of tailbud elongation).
2. Embryo Dissection and Sample Preparation
- Solution preparation
- Prepare 2 mM Ca2+ solution containing 116 mM NaCl, 0.67 mM KCl, 2 mM CaCl2·2H2O, 1.31 mM MgSO4, and 4.6 mM Tris. For every 100 mL solution, add 1 mL of penicillin/streptomycin (10,000 U/mL penicillin; 10,000 µg/mL streptomycin). Adjust pH to 7.8 and filter-sterilize.
- Prepare calcium- and magnesium-free (CMF) solution by combining 116 mM NaCl, 0.67 mM KCl, 4.6 mM Tris and 0.4 mM EDTA. Adjust pH to 7.8 and autoclave to sterilize. For every 100 mL solution, add 1 mL of penicillin/streptomycin (10,000 U/mL penicillin; 10,000 µg/mL streptomycin). Adjust pH to 7.8 and filter-sterilize.
- Preparation of plates for dissection and imaging
- UV-sterilize two 35 mm plastic Petri dishes and one 35 mm Cell Culture Dish (see Table of Materials).
- While working in a laminar flow hood, prepare two 50 mL plastic conical tubes containing 10 mL of 2 mM Ca2+ solution each.
- Remain working in the laminar flow hood and add 2 mL of 2 mM Ca2+ solution to one 35 mm plastic Petri dish, 2 mL of 2 mM Ca2+ solution to one 35 mm Cell Culture Dish, and 2 mL of CMF solution to one 35 mm plastic Petri dish.
- Outside of the laminar flow hood, fill two 100 mm plastic Petri dishes and one 35 mm plastic Petri dish with 0.1x MMR with gentamycin (50 µg/mL). Fill a 60 mm plastic Petri dish with 70% ethanol.
- Immediately before dissection, add 0.01 g of Collagenase B to one of the 50 mL tubes containing Ca2+ solution. Mix well and transfer the solution to a fresh 60 mm plastic Petri dish.
- With the help of a dissecting microscope, identify embryos of the desired developmental stage. Use a sterile transfer pipette to transfer at least six appropriate embryos to one of the 100 mm plates containing 0.1x MMR + gentamycin prepared in step 2.2.4. This will serve as the holding plate.
- Use a sterile transfer pipette to transfer one embryo to the second 100 mm plate containing 0.1x MMR + gentamycin. This will serve as the dissection plate.
- If dissecting embryos old enough to move (approximately stage 23 or older), anesthetize each embryo prior to dissection by transferring it to a dish containing 0.1% 3-aminobenzoic acid ethyl ester diluted in 0.1x MMR with gentamycin (50 µg/mL). Once the embryo is immobilized, transfer it back to the dish containing 0.1x MMR with gentamycin (50 µg/mL) and continue with the dissection.
- Carefully remove the vitelline membrane that surrounds the embryo. This can most easily be done by using a pair of blunt forceps to stabilize the embryo while using a pair of fine forceps to grasp the membrane. Carefully pull with the fine forceps to peel the vitelline membrane apart.
- Use fine forceps to separate the dorsal and ventral regions of the embryo. This can be done by using the forceps to 'pinch' the embryo along the anterior-posterior axis, cutting it in half. With a sterile transfer pipette, transfer the dorsal portion to the 60 mm plate with collagenase solution prepared in step 2.2.5. Discard the ventral portion.
- Allow the dorsal explant to incubate in the collagenase solution for 1–2 min at room temperature. Gently transfer it back to the dissection plate.
- Complete the dissection by carefully removing all residual endodermal and mesodermal contamination from the presumptive neural tissue of the ectoderm. For embryos at Stage 22 or older, the neural tube should also be removed and discarded.
NOTE: If necessary during the dissection, the dish of 70% ethanol prepared in step 2.2.4 can be used to clear or re-sterilize the forceps. - Once the dissection is complete, gently transfer the explant to the 35 mm plate of Ca2+ solution prepared in step 2.2.3.
- Repeat steps 2.4-2.9 until four explants have been collected.
- Use a P1000 micropipette to transfer all four explants to the 35 mm plate containing CMF solution, taking care to avoid any contact between the explants and the air-water interface. Gently swirl the dish so that all of the explants cluster in the center of the plate.
- Incubate 1 h at room temperature to allow explants to dissociate.
NOTE: To aid in dissociation, 0.025%–0.01% trypsin can be added to the CMF solution. This may be necessary for efficient dissociation of older embryos (Stage 22 and older). - At this point, at least two appropriately staged embryos should remain on the holding plate. Transfer these embryos to a fresh dish filled with 0.1x MMR with gentamycin prepared in step 2.2.4 and allow them to develop undisturbed with the dish covered to match the explant dish. These embryos will serve as sibling controls.
- Use superglue to attach a micro-ruled coverslip (see Table of Materials) to the bottom of the 35 mm Cell Culture Dish prepared in step 2.2.3.
NOTE: Place small dabs of superglue around the edges of the coverslip, then press it firmly against the underside of the Cell Culture Dish. The positional markings will be obscured anywhere the glue contacts the grid, so it is important to keep the central gridded portion of the coverslip free from adhesive. - After the explants have dissociated for 1 h, use a P100 micropipette to transfer them to the Cell Culture Dish. In order to plate as many cells as possible on the gridded portion of the dish, hold the pipette at a shallow angle close to the surface of the dish, position the pipette tip in the corner of the grid facing inwards, and firmly expel the cell suspension across the gridded area. Ideally, cells will settle in a tight dense cluster.
- Incubate for 1 h at room temperature to allow cells to adhere to the plate. Determine and record the developmental stage of the sibling control embryos when this incubation begins.
- Combine 5 µL 1 mM Fluo-4 AM (see Table of Materials) with 2 µL of 10% Pluronic F-127 acid.
NOTE: Fluo-4 AM is light sensitive and should be kept in a light-safe or foil-covered tube at all times. - After the incubation is complete, move the sample dish to a darkroom or other light-protected location. Use a micropipette to remove 100 µL of solution from the edge of the dish. Add this solution to the aliquot of Fluo-4 AM/Pluronic F-127 acid, pipette up and down to mix, and return the full volume to the sample dish. Swirl gently to mix.
- Cover the plate with aluminum foil and allow to incubate for 1 h at room temperature. Determine and record the developmental stage of the sibling control embryos when this incubation begins.
- At the end of the incubation, use the remaining conical tube of 2 mM Ca2+ to perform three media washes in the following way: 1) remove 1 mL of solution from the dish, add 3 mL of fresh solution, 2) remove 3 mL of solution from the dish, add 3 mL of fresh solution, 3) remove 3 mL of solution from the dish, add 3 mL of fresh solution.
3. Calcium Imaging
NOTE: Calcium imaging was performed using an inverted confocal microscope (Table of Materials).
- Place the sample plate on the microscope stage, taking care to protect it from ambient light exposure. Once the plate is secured, use a marker to label the front point of the plate so that the same field of view can be found in subsequent imaging.
- Locate the sample under the microscope — first at 10X and then at 20X magnification — and select an appropriate field of view for imaging. An ideal field of view is cell-dense, but not so dense that cells are clumped or difficult to distinguish individually.
- Adjust microscope focus so that the grid-ruled coverslip is visible. The numbers marked on the coverslip serve as unique identifiers for particular grid locus and can be used to locate the same field of view for additional imaging. If the originally selected field of view does not overlap with any numbers, readjust until an identifiable number is in frame.
- Take a bright-field image of the selected field of view with the grid-ruled coverslip in focus.
- Adjust the focus settings and take a bright-field image of the selected field of view with the cells in focus.
- With the cell layer in focus, illuminate the samples with a 488 nm laser. HV and Offset values can be optimized for each experiment to ensure that a dynamic range of fluorescence is detected on the FITC channel.
- For a two-hour image, modify the imaging configuration to record 901 frames with a scan time of 3.93 s and an interval of 8 s. Run configuration to acquire image.
- Once imaging is complete, remove the plate from the microscope stage. Remove 1 mL of solution from the plate and replace it with 1 mL of 2x MEMFA (200 mM MOPS, 2 mM EGTA, and 2 mM MgSO4 in 7.4% formaldehyde).
- Incubate the plate for 2 h at room temperature or overnight at 4 °C. Determine and record the developmental stage of the sibling control embryos when this incubation begins.
- After fixation is complete, remove all solution from the plate and replace it with 2 mL of 1x PBS. Store plates at 4 °C for further processing.
4. Gene Expression Analysis: Probe Synthesis
- As described below, generate an antisense RNA probe for in situ hybridization. Additionally, generate a sense probe for the same gene for use as a negative control.
- In order to purify plasmid DNA containing the probe template sequence, inoculate 150 mL of LB broth with bacterial glycerol stocks containing the template plasmid. Incubate at 37 °C with shaking overnight or until culture is turbid.
- Purify plasmid DNA from the bacterial culture using your method of choice.
NOTE: We use the McNary-Nagel midi-prep kit to obtain high yields of plasmid DNA. - To confirm that the plasmid contains the expected insert, perform a restriction digest and analyze the products on an agarose gel. Uncut plasmid can also be analyzed on an agarose gel to check for genomic DNA contamination.
- To linearize the template DNA, set up a 100 µL restriction digest reaction containing 20 µg of plasmid DNA, 2 µL of appropriate restriction enzyme, and 1x appropriate buffer. Incubate at 37 °C for at least 2 h.
- Extract linearized DNA by performing a phenol/chloroform extraction followed by a chloroform extraction.
- Precipitate DNA with 100% ethanol. This can be done quickly by adding two volumes of cold ethanol to the sample and incubating it at -80 °C until it solidifies (15-30 min).
- Use a refrigerated centrifuge to pellet DNA by spinning for 20 min at 12,000 x g/4 °C.
- Remove the supernatant and wash the pellet with 200 µL of 70% ethanol. Spin for 5 min at 12,000 x g/4 °C.
- Remove the supernatant and air-dry pellet for approximately 5 min. Resuspend in 20 µL of 1x TE and store at 4 °C until further use.
- To synthesize and purify antisense RNA probe, create a 2.5 mM rNTP mix by combining 15 µL of 10 mM rCTP, 15 µL of 10 mM rGTP, 15 µL of 10 mM rATP, 9.75 µL of 10 mM rUTP, and 5.25 µL of 10 mM dig-11 UTP (See Table of Materials).
- Set up a 50 µL in vitro transcription reaction containing 4 µg of linearized template DNA from step 4.2-4.10, 15 µL of 2.5 mM rNTP mix from step 4.11, 10 µL of 5x transcription buffer, 5 µL of 0.1 M DTT, 0.5 µL of RNAse inhibitor (20 U/µL), and 1.5 µL of appropriate RNA polymerase (T3, T7, or SP6). Incubate 1 h at 37 °C.
- Add an additional 1.5 µL of RNA polymerase to the reaction and return to 37 °C for an additional hour.
- Add 1 µL of RQ1 DNAse to reaction and incubate at 37 °C for 10 min to degrade DNA template.
- Add 30 µL of 7.5 M LiCl solution to sample. Pipette to mix and incubate at -20 °C for at least 1 h.
- Using a refrigerated centrifuge, spin the sample 25 min at 14,000 x g/4 °C.
- Remove the supernatant and rinse the pellet with 500 µL of 70% ethanol. Spin for 5 min at 14,000 x g/4 °C.
- Remove the supernatant and air-dry pellet for approximately 5 min. Resuspend in 20 µL of nuclease-free water.
- Create at 10x probe stock by diluting the sample to a concentration of 10 ng/µL in Hybridization Buffer (50% formamide, 5x SSC (saline-sodium citrate; 750 mM NaCl, 75 mM sodium citrate, pH 7.0), 1 mg/mL torula RNA, 0.1 % Tween-20, 1x Denhardt's Solution, 0.1% CHAPS, 10 mM EDTA, and 100 µg/mL heparin). Store at -20 °C until further use.
5. Gene Expression Analysis: Fluorescence In Situ Hybridization
NOTE: All washes should be performed with approximately 1 mL of solution using a sterile, individually wrapped transfer pipette. The pipette should be positioned at the edge of the plate when removing or adding solution, and washes should be performed as gently as possible to ensure that cells are not dislodged from the plate surface and lost.
- Remove 1x PBS from plate (from step 3.10). Replace with fresh 1x PBS and incubate 5 min at room temperature.
- Combine 25 mL of 0.1 M triethanolamine (pH 8.0) with 62.5 µL of acetic anhydride. Mix well. Wash the plate with this solution for 10 min.
- Wash the plate with 1x SSC for 5 min.
- Wash the plate with 0.02 M HCl for 10 min to permeabilize cells.
- Wash 2x with 1x PBS for 5 min each.
- Remove the solution and add 1 mL of Hybridization Buffer (50% formamide, 5x SSC (750 mM NaCl, 75 mM sodium citrate, pH 7.0), 1 mg/mL torula RNA, 0.1% Tween-20, 1x Denhardt's Solution, 0.1% CHAPS, 10 mM EDTA, and 100 µg/mL heparin) to plate. Incubate with shaking for at least 6 h at 60 °C.
- Remove Hybridization Buffer and replace with 750 µL of 1x RNA Probe solution (diluted form the 10x stock made in step 4.19. Sense RNA probes can be used as a negative control.
- Incubate with shaking for 8-14 h at 60 °C.
- Remove the probe and store at -20 °C.
NOTE: 1x probe dilution can be reused up to three times before being discarded. - Rinse the plate with 0.2x SSC.
- Wash with fresh 0.2x SSC for 1 h at 60 °C.
- Move the plates to room temperature and equilibrate for 5 min.
- Wash the plate with 0.2x SSC for 5 min.
- Wash the plate with 1x PBT for 15 min.
- Wash the plate with 2% H2O2 in 1x PBT (0.1% Triton-x-100) for 1 h.
NOTE: This solution is light-sensitive, so it should be made fresh for each experiment and shielded from light. Plates should also be shielded from light or foiled during this incubation. - Wash the plate with 1x TBST (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 0.1%Tween-20) for 15 min.
- Dilute Blocking Reagent to 2% in Maleic Acid Buffer (100 mM maleic acid, 150 mM NaCl, pH 7.5). Block the cells in this mixture for at least 1 h at room temperature.
- Replace blocking solution with anti-digoxygenin-POD antibody diluted 1:1,000 in 2% Blocking Reagent in Maleic Acid Buffer. Incubate overnight at 4 °C.
- Rinse the plate 3x with 1x TBST.
- Wash 4x with 2mL of 1x TBST for at least 15 min per wash with continuous rocking.
- Wash 2x with 1x PBT for at least 10 min per wash with continuous rocking.
- Dilute Cy3-conjugated tyramide 1:25 in 1x PBT. Wash the plate with 750 µL of this dilution for 5 min.
NOTE: This solution is extremely light sensitive, and plates should be kept foiled or shielded from light for the remainder of the experiment to avoid signal deterioration. - Add 2.5 µL of 0.3% H2O2 to this solution and incubate with continuous rocking for an additional 40 min at room temperature.
- Wash 4x with 1x TBST for at least 15 min per wash with continuous rocking.
- Rinse with 1x PBT.
- Fix the cells by incubating for 1 h at room temperature in 1x MEMFA (100 mM MOPS, 1 mM EGTA, and 1 mM MgSO4 in 3.7% formaldehyde).
- Remove the solution and replace with 1x PBS. Store the plates in a foiled container at 4 °C until further processing.
6. Imaging Cells
NOTE: Imaging was performed using an inverted confocal microscope.
- Place the sample plate on the microscope stage, aligning the mark made in step 3.1 to the front of the stage.
- Focus the image so that the grid-lined coverslip is visible and, using the grid image taken in step 3.4 as a reference, adjust the field of view to match the field of view captured in the calcium image (Section 3).
- Acquire bright-field images of both the grid and the cells.
- Illuminate the samples with a 595 nm TRITC laser. Adjust gain values to appropriately distinguish signal from background using the images from the negative control cells and acquire a still image.
NOTE: Ideally, background fluorescence levels are determined based on a negative control plate processed in parallel with a non-targeting sense RNA probe. Gain settings are adjusted so that this plate appears completely black (corresponding to background levels), then held constant for other plates imaged from that experimental batch.
7. Data Processing
NOTE: Data processing was performed using Nikon Elements software.
- Open the 2 h calcium image from step 3.7. Identify the pixels corresponding to each individual cell by selecting Binary > Spot Detection > Bright Spots. Make sure the FITC channel is selected.
- Colored circles will appear over individual cells after this layer has been generated. Adjust the cell distribution and size parameters so that as many cells as possible are recognized and associated with a unique identifier.
- Track the cells across all frames of the image by navigating to View > Analysis Controls > Tracking Options. Set 5 frames as the maximum gap between tracks, delete objects associated with fewer than 600 frames, and select the Close Gaps option. Select Track Binaries to apply cell tracking to the image.
- After the cells have been tracked, manually delete any object that does not correspond to an individual cell (for example a clump of cells). However, data points should not be excluded from further analysis based on the morphology of the calcium activity trace.
- On the Image pane, select View Overlay > Show Binary Object ID. Scroll to the end of the image (Frame 901) and select Edit > Create View Snapshot (8bit RGB)> Current Frame > OK. This will create a snapshot of the last frame of the image with each cell's associated binary ID visible. Save this image.
- Export time-series data by selecting all objects followed by Export Data to Excel. Save output as a CSV file.
- Open the FISH image. Optimize spot detection as in steps 7.1–7.2, using the TRITC channel instead of the FITC channel. Manually delete any incorrectly assigned binaries, then select Automated Measurement Results > Update Measurement to calculate the signal intensity of each cell. Export an image snapshot and data table by repeating steps 7.5 and 7.6.
- Create a spreadsheet where Column A is labeled FISH Binary ID and Column B is labeled Calcium Binary ID. Open the images exported in steps 7.5 and 7.7. For each object identified in the FISH image (step 7.7), record the binary ID in Column A. Then, locate the corresponding cell in the calcium image (step 7.5) and record that binary ID in Column B. Cells that cannot be confidently identified in both images should not be added to the spreadsheet.
NOTE: It can be helpful to use a photo editing program such as Adobe Photoshop or GIMP image editor to open both images, make one semi-transparent, and overlay it onto its partner image to more easily identify and link the two binary IDs associated with each cell. - For each identified cell, collate (either manually or with a script) its time-series calcium data (associated with the Calcium Binary ID and exported in step 7.6) and its gene expression data (associated with the FISH Binary ID and exported in step 7.7).
NOTE: Downstream data processing and analysis may involve collating these data into a single data table and applying an array of analytical techniques including spike counting, fractal analysis, and Markovian entropy that allow the investigator to discern novel patterns of calcium activity 17,18.
Representative Results
A successful example of dissociated cells prepared for calcium imaging can be seen in Figure 2A. Cells are densely plated, allowing the maximum amount of information to be collected from each image, but not so densely plated that individual cells cannot be confidently distinguished form one another. Fluorescence is detected for each defined cell over the 2 h imaging period. Visualization of a composite plot containing the traces for all cells recorded in an experiment reveals the degree to which bulk or population measurements can obscure more nuanced patterns of spiking behavior (Figure 2B). When the recorded profiles of individual cells are isolated, examples of the irregular spiking activity characteristic of neural progenitor cells can be clearly identified. Unlike mature neurons, embryonic neuronal cells exhibit irregular, highly variable and complex nature of calcium activity (Figure 2B). In order to quantify this complexity, application of different data analysis methods have been applied17,18, including diverse parameters to define a spike (Figure 2C).
Successful fluorescence in situ hybridization, including successful design and synthesis of an antisense mRNA probe, can be assessed by comparing the experimental plate against a background control incubated with a non-binding sense RNA control (Figure 3A,B). A positive probe control can also be performed by processing a cell type known to express the target mRNA at detectable levels.
Identification of the same cell across calcium and FISH imaging requires that cells retain roughly the same position during probe hybridization and processing. If plates are handled roughly or washes are performed too forcefully, cells can be dislodged from the plate surface and either lost when solution is discarded or deposited on a different location on the plate, making it impossible for them to be matched across images (Figure 4A). If this disruption affects only some of the cells in the field of view, it may still be possible to detect and assign some cells within the image (Figure 4B). However, the maximum amount of data is gained from an experiment in which FISH is performed carefully and few cells are lost or repositioned between images (Figure 4C).
Once data has been collected to describe both the calcium activity and gene expression of a reasonable number of cells at the developmental stage(s) of interest, further analyses can be performed to assess correlations between these two features (Figure 1). A number of metrics have been applied to quantify calcium activity patterns, including spike counting/frequency, average power, Hurst exponent estimation, and Markovian entropy measurement17,18. Gene expression can be defined quantitatively by absolute fluorescence level or graded on a binary (yes/no) scale, depending on the experimental questions being addressed.
Results from experiments collating calcium activity with the expression of neural progenitor marker genes revealed numerous associations between specific patterns of calcium activity and neurotransmitter phenotypes. At the neural plate stage (Stage 14), GABAergic cells expressing the inhibitory neuron marker gad1.1 exhibit calcium activity that is more regular and higher-amplitude than that of cells that lack gad1.1 expression (Figure 5A). Furthermore, while these gad1.1-expressing cells are associated with higher levels of high-amplitude spiking, low-amplitude spiking is more frequent in glutamatergic cells expressing the excitatory neuron marker slc17a7.
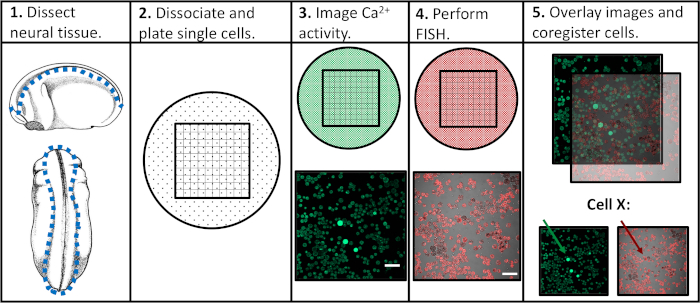
Figure 1: Schematic of experimental workflow. Scale bar = 100 μm. Images in panels 3-5 were taken from Paudel et al. (2019)17. Please click here to view a larger version of this figure.
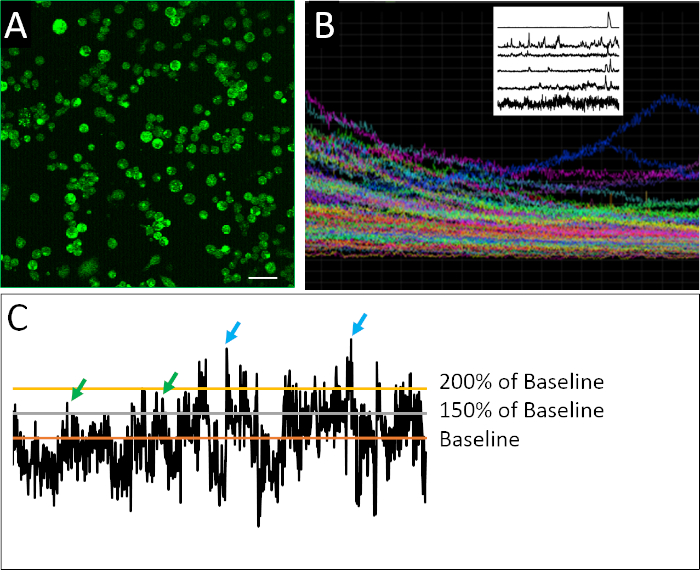
Figure 2: Calcium imaging and example activity profiles. (A) Intracellular calcium activity as reported by Fluo4-AM. Each 2 h image is composed of 901 frames, with one representative frame show here. (B) Composite plot of fluorescence intensity over time in all cells within the imaged field of view. The traces clearly indicate photobleaching of indicator dye (Fluo4) overtime. Raster plot on top left shows representative traces of calcium activity after application of a de-trending algorithm developed by Eilers and Boelen19, where cells shown here exhibit diverse pattern of spiking behavior. (C) Application of different thresholds (150% and 200% of baseline, where the baseline is the average of de-trended fluorescent intensity) to define a spike (green and blue arrows). Scale bar = 100 μm. Please click here to view a larger version of this figure.
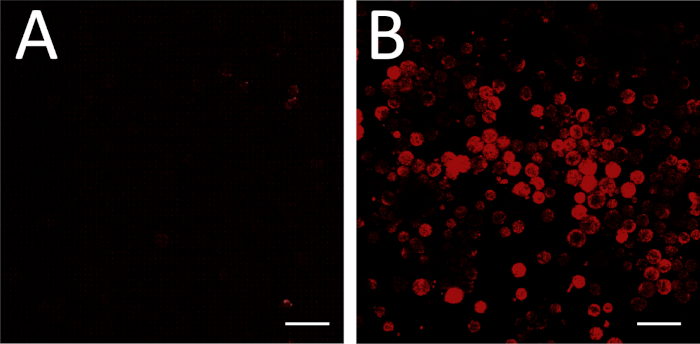
Figure 3: FISH imaging. (A) FISH performed with a non-binding sense RNA probe as a negative control. Imaging settings have been adjusted so that no cells appear fluorescent. Some fields of view may include non-cell debris with some fluorescence, such as seen in the top right and bottom right corners of (A); these can be ignored for the purpose of background setting. (B) The same imaging settings are then used to image an experimental plate (antisense RNA probe). Fluorescence under these conditions corresponds to gene expression above background. Scale bar = 100 μm. Please click here to view a larger version of this figure.
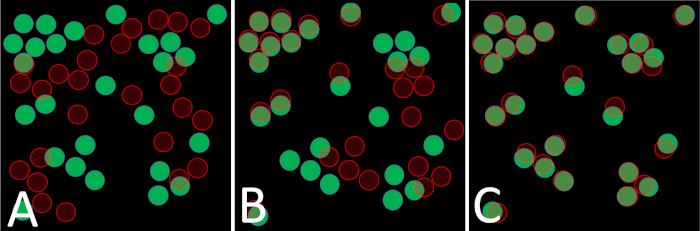
Figure 4: Image overlay and coregistration. Schematic representations of a sample imaged for calcium activity (cells represented by filled green circles) and after FISH (cells represented by shaded red circles). (A) Cells that have moved significantly during sample handling and processing cannot be reliably identified across the two images. (B) Cell disruption may affect only some cells in the field of view. Some cells can be clearly identified in both images, while others cannot be confidently matched. (C) If samples are handled carefully, most cells will remain undisturbed and can be identified in both images. Please click here to view a larger version of this figure.
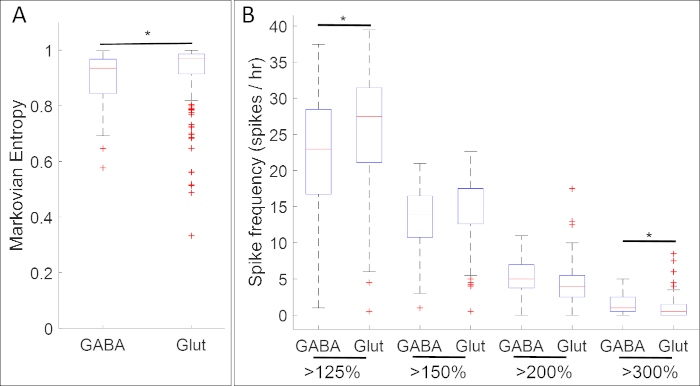
Figure 5: An example of application of this method, boxplots showing associations between calcium activity and gene expression (GABA and Glut for genes gad1.1 and slc17a7 respectively) in neural plate stage Xenopus laevis. At stage 14, gad1.1-postive cells (GABA) exhibit higher-amplitude and more regular calcium activity as defined by (A) Markovian entropy18 and (B) spike counts using thresholds 125%, 150%, 200% and 300% of the average of the de-trended fluorescent intensity (baseline)17 than slc17a7 positive cells (Glut). Stars indicate statistically significant differences according to both Bonferroni-corrected two-sample Kolmogorov–Smirnov Test (p < 0.05) and Cohen's d statistics for effect size (n = 5 cultures and >100 cells; * 0.2 ≤ |d| < 0.5). The figure was redrawn and adapted from data set obtained from Paudel et al.17. Please click here to view a larger version of this figure.
Discussion
Characteristic patterns of calcium activity have been observed in the cells that make up the developing nervous system, with specific types of activity associated with distinct neurodevelopmental processes. However, further understanding of the mechanisms by which these information-dense activity patterns are translated into transcriptional responses requires information about calcium activity and gene expression to be collected with single-cell resolution. While systems that exhibit more stereotypical calcium activity, such as mature neurons, can be reasonably assayed on a bulk level, the irregular patterns that characterize the embryonic nervous system are easily masked by less precise recordings.
The experimental framework established in this protocol is easily adaptable to a wide variety of cell types and fluorescent reporters. Tissue containing virtually any cell type or combination of cell types can be dissected from a model organism of interest and plated for single-cell imaging. In addition to allowing cell identification and isolating the effect of cell-autonomous processes, a primary cell culture approach allows the experimenter to define media components as desired. For example, experiments comparing the activity of neuronal precursors in 2 mM Ca2+ solution have been performed to investigate whether the relationships between spike frequency and neurotransmitter phenotype in the embryonic spinal cord can be recapitulated without the influence of cell-cell interactions13,20.
While this protocol leverages the fluorescent marker Fluo4-AM to detect intracellular calcium activity, depending upon the selection criteria, users can select other commercially available markers21, including genetically encoded calcium indicators. Similarly, alternative markers could be used to monitor dynamic changes to concentration of an ion of interest (including K+, Na+, and Zn2+), membrane potential, or cellular pH. Imaging settings and image duration can be modified as necessary.
Although we correlated calcium activity and neuronal phenotype as a specific application, this method is also applicable for a variety of other cellular properties. For example, fluorescence in situ hybridization can be performed with probes against any gene of interest, including the neuronal marker ChAT or the transcription factor Engrailed, allowing sensitive detection of a customizable panel of mRNA species. These probes can be designed to be isoform-specific, supporting additional target specificity if desired. Double FISH can be performed using probes conjugated to several two different fluorophores, allowing the simultaneous assessment of the expression of multiple genes. However, the additional washes required by this type of experiment are associated with an increased chance of cell loss or movement and require experience and delicacy to be performed successfully.
Regardless of any experiment-specific modifications made to this protocol, there are several key steps that require careful attention. Dissections should be performed with care to remove all contaminating tissues or cell populations; because spatial patterning is lost when the explants are dissociated, any remaining cells from neighboring tissues will become interspersed with and indistinguishable from the cells of interest. After cells are plated, samples should be handled as gently as possible to prevent cells from being dislodged. Most importantly, this means that all solution changes should be performed slowly and carefully, with the pipette placed at the edge of plate when solution is being removed and added. This will ensure that cells can be confidently identified in both calcium and FISH images. If cells are disrupted during processing, it may be impossible to identify some or all of the corresponding cells between the two images. We advise erring on the side of caution with these assignments, such that only unambiguously corresponding cells are used for further analysis.
Depending on the biological question being addressed, a variety of analysis approaches may be appropriate. Time-series calcium activity can be processed and quantified in a variety of ways, with experimenter flexibility in choosing de-trending parameters, analysis metrics, and analysis parameters (for example, the % of baseline threshold used to define a calcium spike). Correlations between calcium activity and level of gene expression can be drawn by analyzing gene expression as an absolute or relative fluorescence value extracted from the FISH image. Alternatively, correlations between calcium activity and gene expression (presence/absence) can be drawn by defining a fluorescence threshold for positive gene expression signal and assigning 'yes' or 'no' identifiers to individual cells. As a whole, this experimental schema provides an incredibly flexible pipeline for the collection and preliminary analysis of time-series data in conjunction with cell-matched gene expression data. Such experiments will be critical for better understanding the complex relationships between cellular dynamics and transcriptional changes, as exemplified by the identification of calcium activity patterns characteristic of inhibitory-fated and excitatory-fated neuronal precursors in embryonic Xenopus laevis.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank Wendy Herbst and Lindsay Schleifer for their contributions to the development of these protocols. This work was supported by grants from the National Institutes of Health (1R15NS067566-01, 1R15HD077624-01 and 1R15HD096415-01) to MSS.
Materials
| For Animal Husbandry & Cell Culture | |||
| CHORULON (chorionic gonodotropin) | Merck Animal Health | ||
| Gentamycin sulfate salt | Millipore Sigma | G1264 | |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | 15140122 | |
| Pyrex petri dishes, 100 mm x 20 mm | Millipore Sigma | CLS3160102 | |
| Corning Falcon Easy-Grip Tissue Culture Dishes, 35mm | Fisher Scientific | 08-772A | |
| Corning Falcon Easy-Grip Tissue Culture Dishes, 60mm | Fisher Scientific | 08-772F | |
| Falcon Standard Tissue Culture Dishes | Fisher Scientific | 08-772E | |
| Thermo Scientifc Nunc Cell Culture / Petri Dishes, 35x10mm Dish, Nunclon Delta | Fisher Scientific | 12-565-90 | |
| Fisherbrand Standard Disposable Transfer Pipettes, Nongraduated; Length: 5.875 in.; Capacity: 7.7 mL | Fisher Scientific | 13-711-7M | |
| Ethyl 3-aminobenzoate methanesulfonate | Millipore Sigma | E10521 | |
| Collagenase B | Millipore Sigma | 11088807001 | |
| Dumont #55 Forceps, Dumostar | Fine Science Tools | 11295-51 | |
| Dumont #5 Forceps, Dumostar | Fine Science Tools | 11295-00 | |
| Cellattice Micro-Ruled Cell Culture Surface | Nexcelom Bioscience | CLS5-25D-050 | |
| For Calcium Imaging | |||
| Fluo-4, AM, cell permeant | Thermo Fisher Scientific | F14201 | |
| Pluronic F-127, 0.2 µm filtered (10% Solution in Water) | Thermo Fisher Scientific | P6866 | |
| For RNA Probe Generation | |||
| PureYield Plasmid Miniprep System | Promega | A1222 | |
| rATP | Promega | P1132 | |
| rCTP | Promega | P1142 | |
| rGTP | Promega | P1152 | |
| rUTP | Promega | P1162 | |
| Digoxigenin-11-UTP | Millipore Sigma | 3359247910 | |
| Rnase Inhibitor | Thermo Fisher Scientific | N8080119 | |
| T3 RNA Polymerase | Promega | P2083 | |
| T7 RNA Polymerase | Promega | P2075 | |
| SP6 RNA Polymerase | Promega | P1085 | |
| RQ1 Rnase-Free Dnase | Promega | M6101 | |
| LiCl Precipitation Solution (7.5 M) | Thermo Fisher Scientific | AM9480 | |
| For Fluorescence In Situ Hybridization | |||
| Acetic Anhydride | Thermo Fisher Scientific | 320102 | |
| Blocking Reagent | Millipore Sigma | 11096176001 | |
| Anti-Digoxigenin-POD, Fab fragments | Millipore Sigma | 11207733910 | |
| Cy3 Mono-Reactive NHS Ester | Millipore Sigma | GEPA13105 | |
| Solution Components | |||
| Calcium chloride, 96% extra pure, powder, anhydrous, ACROS Organixs | Fisher Scientific | AC349610 | |
| Calcium chloride dihydrate | Millipore Sigma | C3306 | |
| CHAPS hydrate | Millipore Sigma | C3023 | |
| Denhardt's Solution (50X) | Thermo Fisher Scientific | 750018 | |
| DTT, Molecular Grade (DL-Dithiothreitol) | Promega | P1171 | |
| Ethylenediaminetetraacetic Acid, Disodium Salt Dihydrate | Fisher Scientific | S311 | |
| Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid | Millipore Sigma | E3889 | |
| Formamide (Deionized) | Thermo Fisher Scientific | AM9342 | |
| Herparin sodium salt from porcine intestinal mucosa | Millipore Sigma | H3393 | |
| HEPES (Ultra Pure) | Thermo Fisher Scientific | 11344041 | |
| Hydrogen peroxide solution | Millipore Sigma | H1109 | |
| L-Cysteine | Millipore Sigma | 168149 | |
| Magnesium chloride, pure, ACROS Organics | Fisher Scientific | AC223211000 | |
| Magnesium sulfate, 97% pure, ACROS Organixs, anhydrous | Fisher Scientific | AC413480050 | |
| Maleic Acid, 99%, ACROS Organics | Fisher Scientific | ACS125231000 | |
| MOPS (Fine White Crystals/Molecular Biology), Fisher BioReagents | Fisher Scientific | BP308 | |
| Potassium chloride | Millipore Sigma | P9541 | |
| Ribonucleic acid from torula yeast, Type IX | Millipore Sigma | R3629 | |
| Sodium chloride | Millipore Sigma | S7653 | |
| Triethanolamine | Millipore Sigma | 90279 | |
| Tris | Millipore Sigma | GE17-1321-01 | |
| TWEEN 20 | Millipore Sigma | P9416 | |
| Equipment | |||
| Laminar Flow Hood | model of choice | ||
| Dissecting Microscope | model of choice | ||
| Inverted Fluorescence Microscope | Nikon | TE200 | |
| NIS-Elements Imaging Software | Nikon | ||
| Shaking Incubator | model of choice | ||
| Refrigerated Centrifuge | model of choice | ||
| Miscellaneous | |||
| Corning bottle-top vaccum filter system, 0.22 μm pore, 500 mL bottle capacity | Millipore Sigma | CLS430769 | |
| Falcon 50mL Conical Centrifuge Tubes | Fisher Scientific | 14-432-22 |
Referenzen
- Humeau, J., et al. Calcium signaling and cell cycle: Progression or death. Cell Calcium. 70, 3-15 (2017).
- Kim, J. M., Lee, M., Kim, N., Heo, W. D. Optogenetic toolkit reveals the role of Ca2+ sparklets in coordinated cell migration. PNAS. 112 (21), 5951-5957 (2016).
- Orrenius, S., Zhivotovsky, B., Nicotera, P. Regulation of cell death: the calcium-apoptosis link. Nature Reviews Molecular Cell Biology. 4 (7), 552-565 (2003).
- Pham, K., et al. Ca2+ and Mg2+ module conformational dynamics and stability of downstream regulatory element antagonist modulator. Protein Science. 24 (5), 741-751 (2015).
- Smedler, E., Uhlén, P. Frequency decoding of calcium oscillations. Biochimica et Biophysica Acta. 1840 (3), 964-969 (2014).
- Moreau, M., Néant, I., Webb, S. E., Miller, A. L., Riou, J. F., Leclerc, C. Ca(2+) coding and decoding strategies for the specification of neural and renal precursor cells during development. Cell Calcium. 59 (2-3), 75-83 (2016).
- Tomida, T., Hirose, K., Takizawa, A., Shibasaki, F., Iino, M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO. 22 (15), 3825-3832 (2003).
- Hannanta-Anan, P., Chow, B. Y. Optogenetic Control of Calcium Oscillation Waveform Defines NFAT as an Integrator of Calcium Load. Cell Systems. 2 (4), 283-288 (2016).
- Li, L., Stefan, M. I. Le Novère N. Calcium input frequency, duration and amplitude differentially module the relative activation of calcineurin and CaMKII. PLoS One. 7 (9), 43810 (2012).
- Romano, D. R., Pharris, M. C., Patel, N. M., Kinzer-Ursem, T. L. Competitive tuning: Competition’s role in setting the frequency-dependence of Ca2+-dependent proteins. PLoS Computational Biology. 13 (11), 1005820 (2017).
- Pharris, M. C., Patel, N. M., Kinzer-Ursen, T. L. Competitive Tuning Among Ca2+/Calmodulin-Dependent Proteins: Analysis of in silico Model Robustness and Parameter Variability. Cellular and Molecular Bioengineering. 11 (5), 353-365 (2018).
- Gu, X., Olson, E. C., Spitzer, N. C. Spontaneous neuronal calcium spikes during early differentiation. Journal of Neuroscience. 14 (11), 6325-6335 (1994).
- Borodinsky, L. N., Root, C. M., Cronin, J. A., Sann, S. B., Gu, X., Spitzer, N. C. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 429 (6991), 523-530 (2004).
- Ciccolini, F., Collins, T. J., Sudhoelter, J., Lipp, P., Berridge, M. J., Bootman, M. D. Local and Global Spontaneous Calcium Events Regulate Neurite Outgrowth and Onset of GABAergic Phenotype during Neural Precursor Differentiation. Journal of Neuroscience. 23 (1), 103-111 (2003).
- Weissman, T. A., Riquelme, P. A., Ivic, L., Flint, A. C., Kriegstein, A. R. Calcium Waves Propagate through Radial Glial Cells and Modulate Proliferation in the Developing Neocortex. Neuron. 43 (5), 647-661 (2004).
- Nieuewkoop, P. D., Faber, J. . The stages of Xenopus embryonic development. Normal Table of Xenopus laevis. , (1994).
- Paudel, S., et al. Calcium Activity Dynamics Correlate with Neuronal Phenotype at a Single Cell Level and in a Threshold-Dependent Manner. International Journal of Molecular Science. 20 (8), 1880 (2019).
- Marken, J. P., et al. A Markovian Entropy Measure for the Analysis of Calcium Activity Time Series. PLoS One. 11 (12), 0168342 (2016).
- Eilers, P. H. C., Boelens, H. F. M. Baseline Correction with Asymmetric Least Squares Smoothing. Leiden University Medical Centre Report. , (2005).
- Guemez-Gamboa, A., et al. Non-cell-autonomous mechanism of activity-dependent neurotransmitter switching. Neuron. 82 (5), 1004-1016 (2014).
- Paredes, R., Madelaine, , et al. Chemical calcium indicators. Methods. 46 (3), 143-151 (2008).

