Characterization of Amyloid Structures in Aging C. Elegans Using Fluorescence Lifetime Imaging
Summary
Fluorescence lifetime imaging monitors, quantifies and distinguishes the aggregation tendencies of proteins in living, aging, and stressed C. elegans disease models.
Abstract
Amyloid fibrils are associated with a number of neurodegenerative diseases such as Huntington's, Parkinson's, or Alzheimer's disease. These amyloid fibrils can sequester endogenous metastable proteins as well as components of the proteostasis network (PN) and thereby exacerbate protein misfolding in the cell. There are a limited number of tools available to assess the aggregation process of amyloid proteins within an animal. We present a protocol for fluorescence lifetime microscopy (FLIM) that allows monitoring as well as quantification of the amyloid fibrilization in specific cells, such as neurons, in a noninvasive manner and with the progression of aging and upon perturbation of the PN. FLIM is independent of the expression levels of the fluorophore and enables an analysis of the aggregation process without any further staining or bleaching. Fluorophores are quenched when they are in close vicinity of amyloid structures, which results in a decrease of the fluorescence lifetime. The quenching directly correlates with the aggregation of the amyloid protein. FLIM is a versatile technique that can be applied to compare the fibrilization process of different amyloid proteins, environmental stimuli, or genetic backgrounds in vivo in a non-invasive manner.
Introduction
Protein aggregation occurs both in aging and disease. The pathways that lead to the formation and deposition of large amyloids or amorphous inclusions are difficult to follow and their kinetics are similarly challenging to unravel. Proteins can misfold due to intrinsic mutations within their coding sequences, as in the case of genetic diseases. Proteins also misfold because the proteostasis network (PN) that keeps them soluble and properly folded is impaired, as happens during aging. The PN includes molecular chaperones and degradation machineries and is responsible for the biogenesis, folding, trafficking, and degradation of proteins1.
C. elegans has emerged as a model to study aging and disease due to its short lifespan, isogenic nature, and ease of genetic manipulation. Several C. elegans transgenic strains that express human disease-causing proteins in vulnerable tissues have been created. Importantly, many of the strains containing aggregation-prone proteins recapitulate the hallmark of amyloid disorders, the formation of large inclusions. Thanks to C. elegans' transparent body, these aggregates can be visualized in vivo, noninvasively and nondestructively2. Generating any protein of interest (POI) in fusion with a fluorophore allows to investigate its locations, trafficking, interaction network, and general fate.
We present a protocol to monitor the aggregation of disease-causing proteins in living and aging C. elegans via fluorescence lifetime imaging microscopy (FLIM). FLIM is a powerful technique based on the lifetime of a fluorophore, rather than its emission spectra. The lifetime (tau, τ) is defined as the average time required by a photon to decay from its excited state back to its ground state. The lifetime of a given molecule is calculated with the time-domain technique of time-correlated single photon counting (TCSPC). In TCSPC-FLIM, the fluorescent decay function is obtained by exciting the fluorophore with short, high-frequency laser pulses and measuring the emitted photon's arrival times to a detector in respect to the pulses. When scanning a sample, a three-dimensional data array is created for each pixel: the array includes information on the distribution of the photons in their x,y spatial coordinates and their temporal decay curve. A given sample therefore becomes a map of lifetimes revealing information on the protein's structure, binding, and environment3,4. Each fluorescent protein possesses an intrinsic and precisely defined lifetime, usually of a few nanoseconds (ns), dependent on its physiochemical properties. Importantly, the lifetime of a fluorophore is independent of its concentration, fluorescent intensity, and of the imaging methodology. However, within a biological system, it can be affected by environmental factors such as pH, temperature, ion concentrations, oxygen saturation, and its interaction partners. Lifetimes are also sensitive to internal structural changes and orientation. Fusing a fluorophore to a POI results in a change in its lifetime and consequently information on the behavior of the fused protein. When a fluorophore is surrounded or encapsulated in a tightly bound environment, such as the antiparallel beta sheets of an amyloid structure, it loses energy non-radiatively, a process known as quenching5. Quenching of the fluorophore results in a shortening of its apparent lifetime. When soluble, a protein's lifetime will stay closer to its original, higher value. In contrast, when a protein starts to aggregate, its lifetime will inevitably shift to a lower value6,7. Therefore, it becomes possible to monitor the aggregation propensity of any amyloid-forming protein at different ages in living C. elegans.
Here we describe a protocol to analyze the aggregation of a fusion protein comprising different polyglutamine (CAG, Q) stretches (Q40, Q44, and Q85). We illustrate how the technique can be applied equally to different fluorophores, such as cyan fluorescent protein (CFP), yellow fluorescent protein (YFP) and monomeric red fluorescent protein (mRFP); and in all tissues of C. elegans, including the neurons, muscles, and the intestine. Moreover, in the context of proteostasis, FLIM is a very useful tool to observe changes upon depletion of molecular chaperones. Knocking down one of the key molecular chaperones, heat shock protein 1 (hsp-1), via RNA interference produces premature misfolding of proteins. The increase in aggregation load as a result of aging, disease, or deficient chaperones, is then measured as a decrease in fluorescence lifetime.
Protocol
1. Synchronization of C. elegans
- Synchronize C. elegans either via alkaline hypochlorite solution treatment or via simple egg laying for 4 h at 20 °C8.
- Grow and maintain nematodes at 20 °C on nematode growth medium (NGM) plates seeded with OP50 E. coli according to standard procedures9. Age the nematodes until the desired developmental stage or day.
NOTE: In this protocol, young adults are imaged on day 4 and old nematodes are imaged on day 8 of life.
2. RNAi-mediated knockdown of chaperone machinery via feeding
NOTE: Perform knockdown of heat shock protein 1 (hsp-1) chaperone by feeding the corresponding RNAi vector to the nematodes10. The hsp-1 RNAi plasmid was obtained from the Ahringer library (clone ID: F26D10.3).
- Grow the HT115 (DE3) E. coli expressing the hsp-1 RNAi plasmid for 6 h to overnight in Luria Bertani (LB) medium containing 50 µg/mL ampicillin.
- Prepare fresh NGM agar plates containing isopropyl β-D-1-thiogalactopyranoside (IPTG; 1 mM) and ampicillin (25 µg/mL) and seed with the hsp-1 RNAi bacteria. Leave plates to dry and induce at room temperature for 1-3 days.
- Place synchronized eggs on an siRNA plate and leave to hatch, or place gravid nematodes and allow to lay eggs for 4 h at 20 °C before removing. Grow the nematodes until the desired age or stage.
NOTE: The second generation of nematodes might present a stronger phenotype of the knockdown. The siRNA protocol and conditions described here are general and adapted to hsp1. RNA interference via siRNA of a specific clone/gene needs to be established and optimized by the end user. It is important to note that not all siRNA have the same efficiency, and it is therefore recommended to test the efficacy of the knockdown by quantification by either quantitative reverse transcription polymerase chain reaction (RT-qPCR) or Western blot.
3. Preparation of microscopy slides
- On the day of imaging, start by preparing the imaging slides. Melt agarose in ddH2O at a concentration of 3% (w/v) and let cool slightly.
- Cut the tip of a 1 mL pipette tip and take roughly 200 µL of melted agarose. Pipette the agarose onto a clean glass slide and immediately place a second one on top, avoiding the formation of any bubbles. Leave to dry and gently remove the top glass slide. The result is a glass slide with an even agarose surface where the nematodes will be positioned.
NOTE: Each slide will be used to image between 5-10 nematodes. Slides can be prepared and stored for a few hours in a humified box to prevent the agarose from drying out.
4. Mounting nematodes onto microscopy slides
NOTE: FLIM requires the nematodes to be immobilized. Perform this step once the imaging setup (e.g., microscopes, lasers, detectors) is ready to use.
- Using a platinum wire pick, place nematodes of the desired age onto a fresh unseeded plate and let them crawl to remove the excess OP50 bacteria from their bodies.
- Prepare the anesthetic compound (sodium azide or levamisole) to immobilize the nematode. Keep a 500 mM NaN3 stock in the dark at 4 °C and dilute in fresh ddH2O to a final concentration of 250 mM. If using levamisole, dilute a 20 mM stock to 2 mM working solution in ddH2O.
CAUTION: Sodium azide (NaN3) is highly toxic. Use gloves and protective eyewear and work under a ventilated hood. - Working under a stereomicroscope, place a 10 µL drop of anesthetic compound onto an agarose pad and gently transfer 5-10 nematodes into it. Use an eyelash tip to separate the nematodes. Keep them close together but not touching to allow for easier localization of the nematodes during image acquisition.
- Carefully overlay the nematodes with a coverslip. Take measurements within 1 h after mounting.
NOTE: Both anesthetics will eventually kill C. elegans. The nematodes must be completely immobile during imaging, because the map of the lifetime is recorded from each pixel. Any movement of the x,y parameters prevents the reading of the lifetime in the same excited pixel.
5. Acquisition of FLIM data
NOTE: In this protocol, the lifetime of the fluorophore is acquired via the time-domain TCSPC method. FLIM requires a pulse of light to be generated by the laser at a set and constant repetition rate. The repetition rate varies according to the laser type and needs to be known by the user. Lifetime measurements are achieved by detectors and electronic equipment installed alongside a conventional microscope. In this protocol, measurements are performed on three different laser scanning confocal microscopes with detectors and software provided by two different companies (Table of Materials) for acquisition of mRFP, CFP, and YFP lifetimes, respectively. Check that the correct filters of emission/excitation are in place and minimize any background or monitor backlight before starting. Before starting any experiment, establish the photostability of the chosen fluorophore. If the fluorophore bleaches within a short time within the nematode tissues, it is not suitable for FLIM measurements in C. elegans.
- Open the FLIM acquisition software. The FLIM software also allows control of the confocal microscope. Locate the tab/button to allow for the detector's outputs to be enabled and press Enable Outputs.
- Acquire the instrument response function (IRF), which describes the timing precision of the instrumental setup.
NOTE: This step should be performed preferably before mounting the nematodes.- If available, remove the excitation/emission filters.
- Place an empty coverslip above the objective and find its surface. Record the scatter signal obtained from the coverslip for a minimum of 30 s.
NOTE: For lifetimes of several nanoseconds, the acquisition software can automatically estimate the IRF shift. Acquiring an IRF is always recommended.
- Place the slide with the mounted C. elegans on the stage. Using a 10x magnification lens in transmission mode and localize the position of the nematodes on the slide.
- Remove the slide, switch the objective to a 63x magnification lens, and apply the required immersion medium (e.g., oil). Replace the slide on the stage and localize the nematodes.
- Locate the Pinhole Manager on the acquisition software and open it to the Maximum. Start scanning the sample, select a region of interest (e.g., head, upper body), and focus on its maximum projection plane.
- Monitor the laser pulse rate and the three other values present on the interface of the software: The Constant Fraction Discriminator (CFD), the Time-to-Amplitude (TAC), and the Analogue-to-Digital Converter (ADC).
NOTE: The laser should have a maximum gate of 1 x 108 single photon counts. This number represents the maximum number of photons supplied by the laser. The CFD provides information on the receipt of the single photon pulse in reference to the laser pulse by the detector. This value should be roughly 1 x 105. The TAC discriminates between the time one photon was detected and the next laser pulse. Finally, the ADC converts the TAC voltage into a storable memory signal11. The CFD, TAC, and ADC should all have similar values to ensure that photons emitted by the fluorophore are not lost. Correct evaluation of these parameters ensures that enough photons are being collected to create an accurate lifetime map. - On the interface of the FLIM software, preview the number of photons detected: the ADC value should be between 1 x 104 and 1 x 105. If necessary, shift the focus on a different plane or increase the laser power to collect more photons.
NOTE: In general, the number of recorded photons per second should not exceed 1% of the laser's repetition rate. - In the menu bar, select the tab to set the acquisition parameters. Select scan sync in to allow for single photon detection.
- Set the acquisition to a fixed amount of time or a fixed number of photons. For example, acquire a lifetime decay curve for 2 min or until a single pixel reaches a photon count of 2,000 single events. Press Start to begin acquisition.
NOTE: Different fluorophores will require different excitation and emission lasers and filters. According to the brightness of the sample, the laser power can also be adjusted, which will not interfere with the lifetime. These protocols use the following excitation/emission settings: YFP ex500/em520-50 nm, mRFP ex561/em580-620 nm. A pulsed two photon laser was employed for CFP measurements using ex800/em440 nm. The amount of time and photon count required for acquisition of a FLIM map will need to be empirically established for each setup and each experimental purpose.
6. Analysis of FLIM data using FLIMfit software
NOTE: Perform data analysis using the FLIMfit software tool developed at Imperial College London12 (see Figure 1).
- Open the software and import FLIM data files via File | Load FLIM Data. Load all samples from one condition, even if obtained in different sessions and from different biological repeats.
- If necessary, segment a single nematode from any FLIM picture via Segmentation | Segmentation Manager. Drag the cropping tool around the area of interest until it is highlighted. Once completed, press OK.
NOTE: Segmentation must be done for all images. - Select a small region where the intensity-based image of a C. elegans appears (Figure 1, Arrows 1). The decay curve of that region will appear in the large decay window on the right side of the interface (Figure 1, Arrow 2).
NOTE: The decay can be displayed linearly or logarithmically. - Set the correct parameters to extrapolate the lifetime via the software's algorithm as described in steps 6.5-6.8.
- On the Data tab (Figure 1, Arrow 3):
- Set an arbitrary Integrated Minimum value to exclude any pixels that are too dim to produce a good fit. Depending on the C. elegans sample this value varies from 40-300. Input different values until a satisfactory preview is achieved.
- Select a Time Min and a Time Max number to limit the FLIM signal to these values. All events that appear before and after this threshold will be excluded.
NOTE: For example, for the analysis of mRFP, the events prior to 800 ps and after 4,000 ps were excluded. These values depend on the lifetime of the fluorophore and need to be determined by the end user. - Do not change the preset Counts/Photon of 1.
- Input the Repetition Rate, in MHz, of the laser utilized during acquisition.
NOTE: For the current protocol, different lasers were utilized with various repetition rates. The two photon laser used for acquisition of CFP lifetimes possesses a repetition rate of 80 MHz, for YFP the laser repeats at 40 MHz, and for mRFP the value is 78.01 MHz. These values were inputted into FLIMfit according to the sample analyzed. - Input a Gate Max value to exclude all saturated pixels.
NOTE: For lifetime measurements in C. elegans, this value is set to any large number (e.g., 1 x 108).
- On the Lifetime tab, select a global fitting to be used (e.g., a pixel-wise fitting). See Figure 1, Arrow 4.
NOTE: A Pixel-wise fitting will produce a decay fitted to each individual pixel. An Image-wise fitting will produce a global fitting of each individual image and display a single lifetime value per image. A Global-wise fitting will produce a single fitting across the whole dataset. A single lifetime value is provided for all images. - Do not change any other parameter except for the No. Exp selection if it is known that the chosen fluorescence decay is multiexponential and exhibits more than a single lifetime.
NOTE: In the present protocol, this function was utilized to calculate the lifetimes of the biexponential CFP fluorophore. - Upload the IRF via the IRF menu: IRF | Load IRF. To estimate the IRF shift, select IRF | Estimate IRF Shift. A set of values will automatically appear on the IRF tab. Once this is established, do not change any other parameters of this tab.
- Once all parameters are set, press Fit Dataset (Figure 1, Arrow 5). The algorithm will produce a fit for the decay curve and establish a lifetime value for each image.
NOTE: The resulting fit, highlighted in a blue line, should overlap with all the events. A good fit is obtained when all events are aligned along the fit. - Click the Parameters tab (Figure 1, Arrow 6), located within the top right menus of the software's interface, and select Statistic: w_mean (weighted mean) and check that the chi2 value is as close as possible to 1.
NOTE: A chi2 close to one ensures the accuracy of the fit. The lifetime value of the selected image is thus revealed as tau_1. - Export any information of interest: File | Export Intensity Images/Fit Result Table/Images/Histograms. Save the data settings used to calculate the lifetime: File | Save Data Settings.
NOTE: The parameters employed will be saved for future analysis of the selected samples.
7. Graphical representations of FLIM data
NOTE: The lifetimes collected from different samples can be visually represented in various ways. Select to denote the lifetime values either in nanoseconds or picoseconds.
- Show the quality of the fit and the accuracy of the curve by exporting the decay curve directly from FLIMfit.
- Represent the distribution of the photons by plotting the frequency of the photon count versus the lifetime value in a histogram.
- Finally, for statistical comparison, if comparing two or more samples, place lifetime values plus standard deviation of the mean in a scatter plot bar graph. Perform any desired statistical analysis.
Representative Results
The protocol shows how to accurately monitor the formation of aggregated species in living C. elegans, both during its natural aging and when subjected to stress. We selected four different strains of transgenic nematodes expressing polyglutamine proteins of either 40Q, 44Q, or 85Q repeats. These proteins are synthesized in different tissues and were fused to different fluorophores. The C. elegans strains either expressed Q40-mRFP in the body wall muscles (mQ40-RFP), Q40-CFP in the nervous system (nQ40-CFP), and either Q44-YFP or Q85-YFP in the intestine (iQ44-YFP and iQ85-YFP)13. To illustrate how aging promotes aggregation, we collected the lifetime of these polyQ strains in young nematodes, at day 4 of life, and old nematodes, at day 8. To show the effects of a deficiency in the PN, we performed a knockdown of hsp-1 in the mQ40-RFP and the nQ40 strains.
Once the lifetime values were extrapolated via the FLIMfit software, the obtained data showed a clear reduction in the lifetime of any of the polyQ constructs when aggregated due to either glutamine load, aging, or stress. FLIM distinguished between the soluble protein fraction and aggregated species, and their transition, by recording a shift in their lifetimes.
At day 4, mQ40-RFP displayed an average fluorescence lifetime of 1.69 ns (Figure 2). Upon aging, more aggregated species arose, appearing as low lifetime foci in the lifetime images and shifting the histogram to reduced lifetimes (Figure 2A). By plotting the mean fluorescence lifetime of every acquired image over the age of the nematodes a significant reduction of fluorescence lifetime, and therefore accumulation of aggregated species, became visible (Figure 2B). The protein folding capacity of the PN declined after day 4 of life in C. elegans14 and aggregation-prone proteins further misfolded to cluster into amyloid and amorphous aggregates. Apart from the PN, the intrinsic aggregation propensities of a certain protein played an important role in the progression of aggregate formation. This was analyzed by comparing the behavior of iQ44-YFP and iQ85-YFP. The longer Q-stretch of the iQ85 was more prone to aggregation and exhibited a fluorescence lifetime shift in the histogram already at day 4 of life (Figure 3A). In fact, at day 4, foci formation was observed for iQ85, while still absent in iQ44. Upon aging, however, iQ44 also exhibited foci formation and thus a reduced fluorescence lifetime. Because iQ85 already exhibited aggregates in early adulthood the progression of aggregation upon aging was less pronounced, yet significant (Figure 3B). Finally, we did not detect foci formation nor decreased fluorescence lifetime in the nQ40-CFP strain (Figure 4A). For this strain, there were only subtle, nonsignificant changes to the mean fluorescence lifetime upon aging (Figure 4B), potentially due to the neurons being less susceptible for yet unknown reasons.
Knocking down hsp-1 poses a challenge to the PN of mQ40 and nQ40 expressing nematodes. RNAi-mediated depletion of hsp-1 led to a significant increase in aggregation (Figure 5 and Figure 6). Q40 expressed in body wall muscles tended to form a small number of large foci surrounded by nonaggregated material. This resulted in two distinguishable peaks in the histograms (around 1.7 ns and 1.4 ns, see Figure 5A). The aged and RNAi treated nematodes showed a strong increase in the low lifetime peak ultimately decreasing the average fluorescence lifetime (Figure 5B). Compared to this biphasic behavior of Q40 in muscles, the neuronal Q40 displayed a more diverse aggregation behavior. We could not directly correlate foci formation with aggregation as in the muscular expression strain (Figure 6A). Because FLIM offers an opportunity to assess the degree of aggregation, the histograms revealed that there was no distinct peak but a widespread distribution of fluorescence lifetimes, pointing to a complex composition of different oligomers and higher order aggregates. Still, the overall degree of aggregation could be evaluated by plotting the mean fluorescence lifetime (Figure 6B), showing that hsp-1 knockdown led to a boost in aggregation.
It is important to note that the lifetime of the fluorophores, free from a fusion partner and outside of a biological system, was higher. Because the lifetime is affected primarily by its environment, a slight reduction of the lifetime of YFP and RFP was already noticeable within C. elegans' tissues. It is therefore important to obtain the lifetime of the soluble POI within the nematode as a suitable control. A comparison between the soluble fraction with a higher lifetime and aggregated fraction with a lower lifetime can then be made. Here, the decrease in lifetime correlated with the formation of visible foci within the muscle and intestinal cells. Still, a fraction of foci exhibited no decrease of fluorescence lifetime (see Figure 2 and Figure 3, white arrows). This feature highlights how only part of the fusion construct might be aggregated at a particular spatiotemporal point, and the presence and availability of unbound protein. A more complex scenario arose from investigation of the neuronal Q40-CFP strain. CFP intrinsically possesses two distinct fluorescence lifetimes. While CFP is an ideal fluorophore for Förster resonance energy transfer (FRET)15 measurements, in conjunction with YFP, it is not advisable to employ it to monitor formation of aggregates in C. elegans.
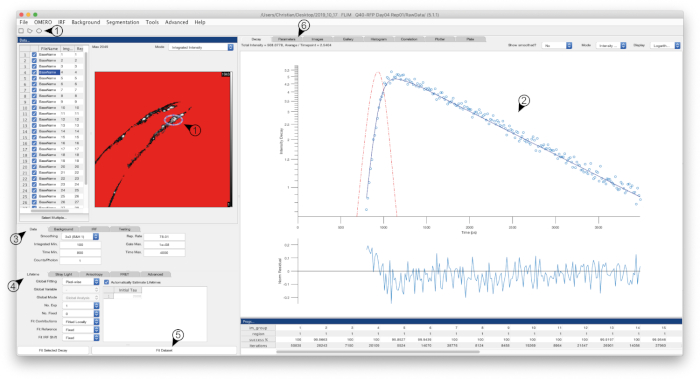
Figure 1: Screenshot of FLIMFit software interface. Screenshot of the software used to calculate the fluorescence lifetimes. The window depicts the interface after settings were defined as described in the text, and calculation of lifetimes was performed. Numbered arrows refer to specific steps within the protocol. Please click here to view a larger version of this figure.
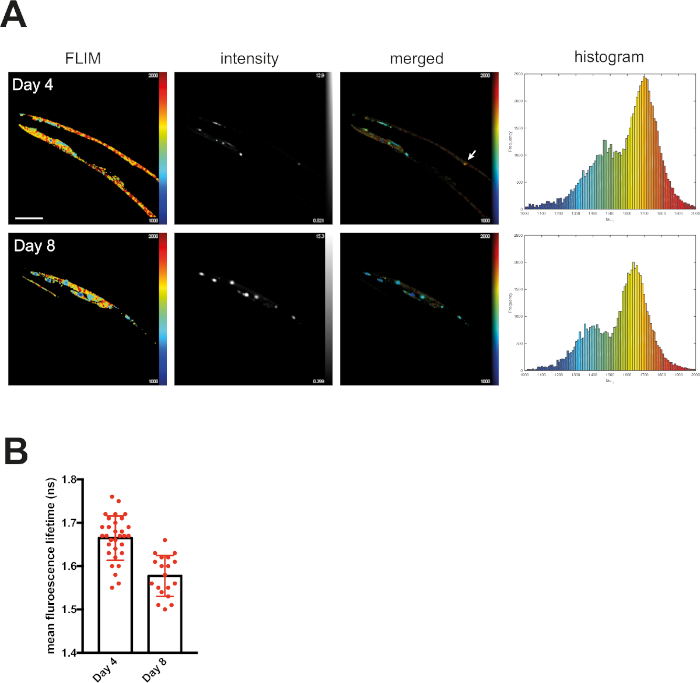
Figure 2: Fluorescence lifetimes of muscular Q40-RFP decreased with age. (A) Representative maps of C. elegans expressing muscular Q40-RFP on day 4 or day 8 of life generated by FLIMfit. Fluorescence lifetimes, fluorescence intensity, and a merged image of both are provided. Scale bars = 25 µm. Histograms show a distribution of measured lifetimes for all analyzed nematodes divided into 100 categories. (B) Bar plots showing the weighted mean fluorescence lifetimes of all analyzed animals on day 4 or day 8 of life, respectively. Please click here to view a larger version of this figure.
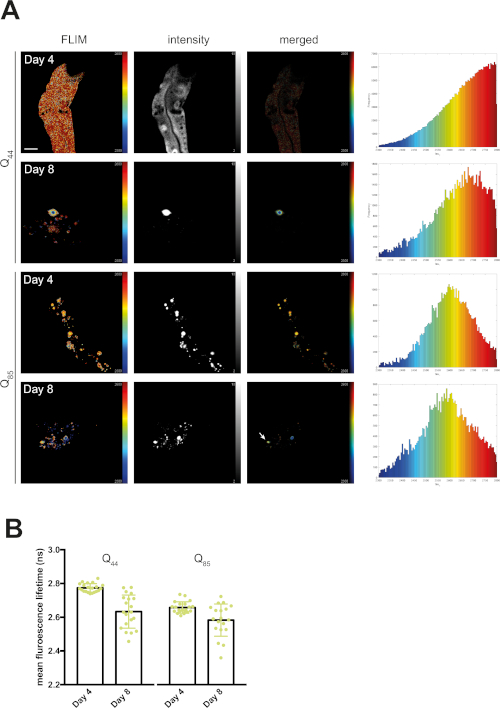
Figure 3: Fluorescence lifetimes of intestinal Q44-YFP and intestinal Q85-YFP decreased with age. (A) Representative maps of C. elegans expressing intestinal Q44-YFP or intestinal Q85-YFP on day 4 or day 8 of life generated by FLIMfit. Fluorescence lifetimes, fluorescence intensity, and a merged image of both are provided. Scale bars = 25 µm. Histograms show a distribution of measured lifetimes for all analyzed nematodes divided into 100 categories. (B) Bar plots showing the weighted mean fluorescence lifetimes of all analyzed animals on day 4 or day 8 of life, respectively. Please click here to view a larger version of this figure.
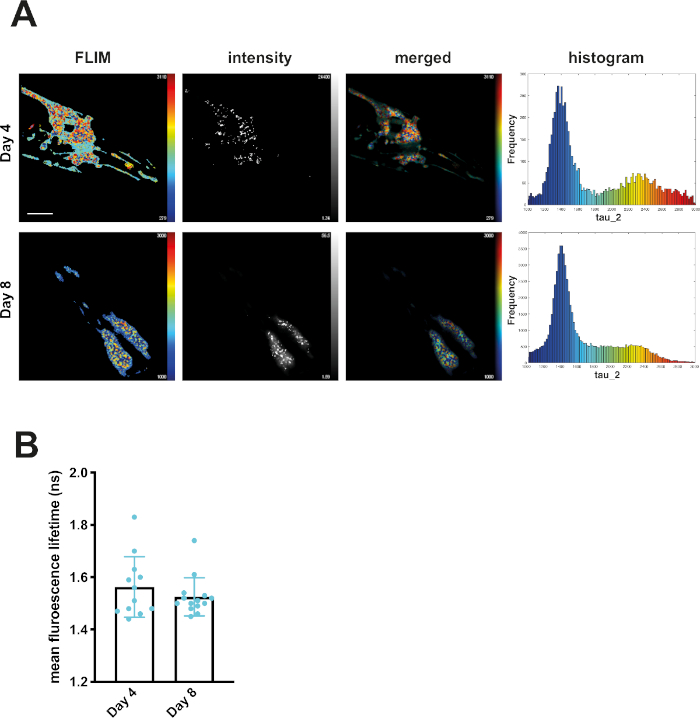
Figure 4: Fluorescence lifetimes of neuronal Q40-CFP did not change with age. (A) Representative maps of C. elegans expressing neuronal Q40-CFP on day 4 or day 8 of life generated by FLIMfit. Fluorescence lifetimes, fluorescence intensity, and a merged image of both are provided (the second lifetime, τ2, is indicated in all samples). Scale bars = 25 µm. Histograms show a distribution of measured lifetimes for all analyzed nematodes divided into 100 categories. (B) Bar plots showing the weighted mean fluorescence lifetimes of all analyzed animals on day 4 or day 8 of life, respectively. Please click here to view a larger version of this figure.
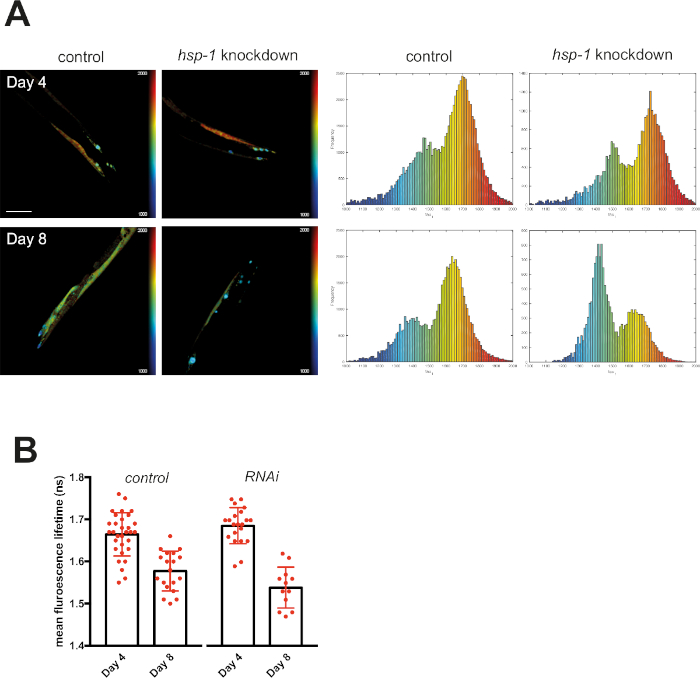
Figure 5: Fluorescence lifetimes of muscular Q40-RFP decreased upon knockdown of hsp-1. (A) Representative maps of C. elegans expressing muscular Q40-RFP on day 4 or day 8 of life generated by FLIMfit. A merge of the fluorescence lifetime and intensity map is displayed. For both time points, nematodes that grew on bacteria expressing an empty vector (control) or bacteria expressing the hsp-1 RNAi construct are shown. Scale bars = 25 µm. Histograms show a distribution of measured lifetimes for all analyzed nematodes divided into 100 categories. (B) Bar plots showing the weighted mean fluorescence lifetimes of all analyzed animals on day 4 or day 8 of life, with control or hsp-1 RNAi, respectively. Please click here to view a larger version of this figure.
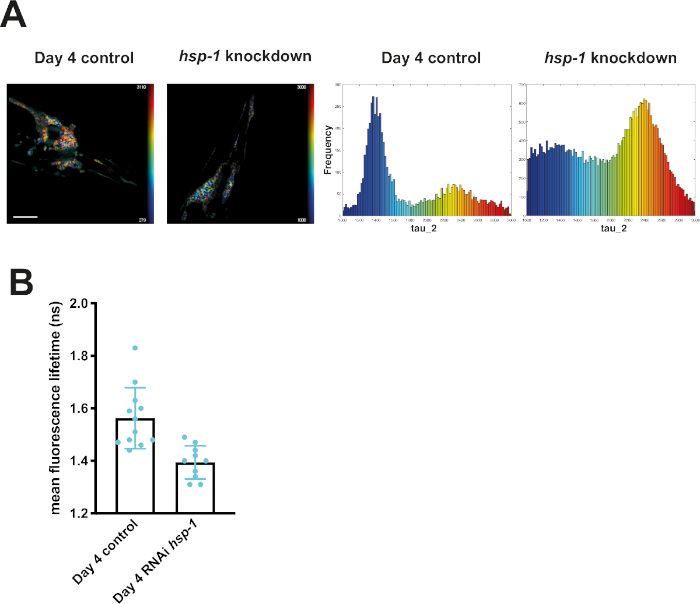
Figure 6: Fluorescence lifetimes of neuronal Q40-CFP decreased upon knockdown of hsp-1. (A) Representative maps of C. elegans expressing neuronal Q40-CFP on day 4 of life generated by FLIMfit. A merge of the fluorescence lifetime and intensity map is displayed. Nematodes shown were grown on bacteria expressing an empty vector (control) or bacteria expressing the hsp-1 RNAi construct. Scale bars = 25 µm. Histograms show a distribution of measured lifetimes for all analyzed nematodes divided into 100 categories. (B) Bar plots showing the weighted mean fluorescence lifetimes of all analyzed animals on day 4, with control RNAi or the hsp-1 RNAi. Please click here to view a larger version of this figure.
Discussion
The protocol presented here describes a microscopy-based technique to identify aggregated species in the C. elegans model system. FLIM can accurately characterize the presence of both aggregated and soluble species fused to a fluorophore via measurement of their fluorescence lifetime decays. When a fusion protein starts to aggregate its recorded average lifetime will shift from a higher to a lower value16. The propensity of aggregation can then be deduced by the drop in lifetime: the lower the lifetime, the higher the presence of aggregated protein species in the system. Thereby, it becomes possible to follow the effects of aging, disease, or impairment of PN on the aggregation propensity of any protein.
To highlight the versatility of the technique, our results clearly showed that FLIM could identify the changes in structure of the various polyQ constructs, regardless of the tissue or fluorophore. Importantly, FLIM has been already successfully applied in the characterization of other aggregation-prone proteins within C. elegans, such as α-synuclein17. Furthermore, it becomes possible to apply any stress factor to C. elegans and follow the unfolding and aggregation of any POI. Osmotic, metal-ion, redox, or chemical stress can all promote toxic imbalances that can successfully be monitored in C. elegans employing FLIM. The reverse could also be possible: a delay in aggregation or even disaggregation due to the presence of beneficial compounds or boosting of the PN, can result in a rescue of the protein and a consequent lifetime increase.
FLIM has been widely employed to monitor changes in lifetime of any substance in a wide array of disciplines from chemistry to cancer biology18 to medical diagnostics, but some limitations remain. A main problem is the photostability of fluorophores. Because FLIM requires the recording of a large number of photons, photobleaching reduces the number of photons collected and can change the resulting decay curve. Furthermore, especially within the nematode system, if the intensity of the fluorophore itself is not sufficiently high for enough photons to be collected, a higher excitation is required, leading to quicker photobleaching and an unreliable decay curve. Finally, the technique requires sophisticated and costly equipment as add-ons to preexisting microscopy systems, with one critical element being the sensitivity of the detectors19.
Conversely, one of the main advantages of calculating the lifetime of a fluorophore and its fusion protein is that it provides information on different fractions of the same fluorophore-protein complex in diverse states of interactions within its environment, irrespective of its largely unknown concentration. FLIM can be used also to measure lifetime in any phase, gas, liquid, or solid and in any medium or organism that can be normally imaged, from cells to organisms, and organelles to immobilized, purified proteins20. Microscopy techniques usually rely on the steady-state imaging of a fluorescently tagged protein. Unlike steady state techniques, FLIM can resolve changes in binding, composition, and conformation of a biological substrate. For investigating aggregation-prone proteins, the presence of large fluorescent inclusions or foci can be imaged easily, but these represent only a static, intensity-based snapshot3. In the case of aggregated species, the visualized foci may also be misleading, as a high concentration of fluorophore does not necessarily result in strong amyloid formation. Via FLIM it is instead possible to distinguish soluble from insoluble material. Finally, it becomes beneficial for any investigation to obtain both the intensity-based measurements and the parallel lifetime measurement. Within the complementarity of these microscopy techniques lies their strength.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The muscle-Q40-mRFP strain provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The neuronal-Q40-CFP was a kind gift of the Morimoto Lab. We acknowledge the DFG (KI-1988/5-1 to JK, NeuroCure PhD fellowship by the NeuroCure Cluster of Excellence to MLP), EMBO (Short term fellowship to MLP) and the Company of Biologists (travel grants to CG and MLP) for funding. We also acknowledge the Advanced Light Microscopy imaging facility at the Max Delbrück Centre for Molecular Medicine, Berlin, for providing the setup to image the YFP constructs.
Materials
| Agar-Agar Kobe I | Carl Roth GmbH + Co. KG | 5210.2 | NGM component |
| Ahringer Library hsp-1 siRNA | Source BioScience UK Limited | F26D10.3 | |
| Ampicillin | Carl Roth GmbH + Co. KG | K029.3 | Antibiotic |
| B&H DCS-120 SPC-150 | Becker & Hickl GmbH | FLIM Aquisition software | |
| B&H SPC830-SPC Image | Becker & Hickl GmbH | FLIM Aquisition software | |
| BD Bacto Peptone | BD-Bionsciences | 211677 | NGM component |
| C. elegans iQ44-YFP | CAENORHABDITIS GENETICS CENTER (CGC) | OG412 | |
| C. elegans iQ85-YFP | Kind gift from Morimoto Lab | ||
| C. elegans mQ40-RFP | Kind gift from Morimoto Lab | ||
| C. elegans nQ40-CFP | Kind gift from Morimoto Lab | ||
| Deckgläser-18x18mm | Carl Roth GmbH + Co. KG | 0657.2 | Cover slips |
| Isopropyl-β-D-thiogalactopyranosid (IPTG) | Carl Roth GmbH + Co. KG | 2316.4 | |
| Leica M165 FC | Leica Camera AG | Mounting Stereomicroscope | |
| Leica TCS SP5 | Leica Camera AG | Confocal Microscope | |
| Levamisole Hydrochloride | AppliChem GmbH | A4341 | Anesthetic |
| OP50 Escherichia coli | CAENORHABDITIS GENETICS CENTER (CGC) | OP50 | |
| PicoQuant PicoHarp300 | PicoQuant GmbH | FLIM Aquisition software | |
| Sodium Azide | Carl Roth GmbH + Co. KG | K305.1 | Anesthetic |
| Sodium Chloride | Carl Roth GmbH + Co. KG | 3957.2 | NGM component |
| Standard-Objektträger | Carl Roth GmbH + Co. KG | 0656.1 | Glass slides |
| Universal Agarose | Bio & Sell GmbH | BS20.46.500 | |
| Zeiss AxioObserver.Z1 | Carl Zeiss AG | Confocal Microscope | |
| Zeiss LSM510-Meta NLO | Carl Zeiss AG | Confocal Microscope |
Referenzen
- Klaips, C. L., Jayaraj, G. G., Hartl, F. U. Pathways of cellular proteostasis in aging and disease. Journal of Cell Biology. 217 (1), 51-63 (2018).
- Kikis, E. A. The struggle by Caenorhabditis elegans to maintain proteostasis during aging and disease. Biology Direct. 11, 58 (2016).
- Becker, W. Fluorescence lifetime imaging – techniques and applications. Journal of Microscopy. 247 (2), 119-136 (2012).
- Lakowicz, J. R. . Principles of Fluorescence Spectroscopy. , (2006).
- Berezin, M. Y., Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chemical Reviews. 110 (5), 2641-2684 (2010).
- Kaminski Schierle, G. S., et al. A FRET sensor for non-invasive imaging of amyloid formation in vivo. ChemPhysChem. 12 (3), 673-680 (2011).
- Sandhof, C. A., et al. Reducing INS-IGF1 signaling protects against non-cell autonomous vesicle rupture caused by SNCA spreading. Autophagy. , 1-22 (2019).
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., Cerón, J. Basic Caenorhabditis elegans methods: Synchronization and observation. Journal of Visualized Experiments. 64, e4019 (2012).
- Stiernagle, T. Maintenance of C. elegans. WormBook the online review of C. elegans biology. 1999, 1-11 (2006).
- Kamath, R. S., Martinez-Campos, M., Zipperlen, P., Fraser, A. G., Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biology. 2 (1), 1-10 (2001).
- Becker, W., et al. Fluorescence Lifetime Imaging by Time-Correlated Single-Photon Counting. Microscopy Research and Technique. 63 (1), 58-66 (2004).
- Warren, S. C., et al. Rapid global fitting of large fluorescence lifetime imaging microscopy datasets. PloS one. 8 (8), e70687 (2013).
- Moronetti Mazzeo, L. E., Dersh, D., Boccitto, M., Kalb, R. G., Lamitina, T. Stress and aging induce distinct polyQ protein aggregation states. Proceedings of the National Academy of Sciences of the United States of America. 109 (26), 10587-10592 (2012).
- Ben-Zvi, A., Miller, E. A., Morimoto, R. I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proceedings of the National Academy of Sciences of the United States of America. 106 (35), 14914-14919 (2009).
- Wallrabe, H., Periasamy, A. Imaging protein molecules using FRET and FLIM microscopy. Current Opinion in Biotechnology. 16 (1), 19-27 (2005).
- Chan, F. T. S., Pinotsi, D., Kaminski Schierle, G. S., Kaminski, C. F. Structure-Specific Intrinsic Fluorescence of Protein Amyloids Used to Study their Kinetics of Aggregation. Bio-nanoimaging: Protein Misfolding and Aggregation. , 147-155 (2013).
- Laine, R. F., et al. Fast Fluorescence Lifetime Imaging Reveals the Aggregation Processes of α-Synuclein and Polyglutamine in Aging Caenorhabditis elegans. ACS Chemical Biology. 14 (7), 1628-1636 (2019).
- Kelbauskas, L., Dietel, W. Internalization of Aggregated Photosensitizers by Tumor Cells: Subcellular Time-resolved Fluorescence Spectroscopy on Derivatives of Pyropheophorbide-a Ethers and Chlorin e6 under Femtosecond One- and Two-photon Excitation. Photochemistry and Photobiology. 76 (6), 686-694 (2002).
- Becker, W., Su, B., Holub, O., Weisshart, K. FLIM and FCS detection in laser-scanning microscopes: Increased efficiency by GaAsP hybrid detectors. Microscopy Research and Technique. 74 (9), 804-811 (2011).
- Suhling, K., French, M. W., Phillips, D. Time-resolved fluorescence microscopy. Photochemical and Photobiological Sciences. 4 (1), 13-22 (2005).

