In Vitro Culture Strategy for Oocytes from Early Antral Follicle in Cattle
Summary
We describe the procedures for isolation of growing oocytes from ovarian follicles at early stages of development, as well as the setup of an in vitro culture system which can support the growth and differentiation up to the fully-grown stage.
Abstract
The limited reserve of mature, fertilizable oocytes represents a major barrier for the success of assisted reproduction in mammals. Considering that during the reproductive life span only about 1% of the oocytes in an ovary mature and ovulate, several techniques have been developed to increase the exploitation of the ovarian reserve to the growing population of non-ovulatory follicles. Such technologies have allowed interventions of fertility preservation, selection programs in livestock, and conservation of endangered species. However, the vast potential of the ovarian reserve is still largely unexploited. In cows, for instance, some attempts have been made to support in vitro culture of oocytes at specific developmental stages, but efficient and reliable protocols have not yet been developed. Here we describe a culture system that reproduce the physiological conditions of the corresponding follicular stage, defined to develop in vitro growing oocytes collected from bovine early antral follicles to the fully-grown stage, corresponding to the medium antral follicle in vivo. A combination of hormones and a phosphodiesterase 3 inhibitor was used to prevent untimely meiotic resumption and to guide oocyte's differentiation.
Introduction
During the reproductive life span, only a minimal fraction of the oocytes that are present in an ovary mature, are released in the fallopian tubes upon ovulation, and are available for being fertilized and develop into a viable embryo1. On the other hand, most of the oocytes within an ovary undergo atresia and are never ovulated. In vitro embryo production (IVP) technologies have attempted to increase the exploitation of the ovarian reserve2,3. Thus far, such technologies have allowed interventions of fertility preservation, selection programs in livestock, and conservation of endangered species. Nevertheless, most protocols use oocytes that have basically completed the growth phase within the antral ovarian follicle, and hence are referred to as fully-grown oocytes. In cattle, where IVP technologies are widely used, fully grown oocytes reach a final diameter of approximately 120 µm and are collected from follicles that span from 2 to 8 mm in diameter (medium antral follicles)1. Upon isolation from the follicles, such oocytes are in vitro matured and fertilized. The zygotes are then cultured up to the blastocyst stage and either transferred into a recipient or cryopreserved. In cattle, as well as in many other species, despite the potential offered by IVP, the number of in vitro produced embryo per cow did not largely improve for the last 40 years. This is in part due to the limited number of fully grown oocytes that populate an ovary at a given time which can be retrieved and subjected to standard IVP techniques4,5,6.
The oocytes enclosed within early antral follicles, i.e., those follicles that are less than 2 mm in diameter, represent a potential source to be used in fertility preservation programs7 , as an ovary roughly contains 10 times more early antral follicles than medium antral8. However, these oocytes are still in the growth phase and have not yet reached the fully-grown stage9. As such, they are still transcriptionally active, producing mRNAs that will be stored for later developmental steps, and have not yet undergone all the differentiation process required to confer the oocytes with the ability of spontaneously resuming and completing meiosis I once isolated from the follicular compartment10,11. Therefore, they cannot be directly submitted to standard in vitro maturation (IVM) protocols, but they require an additional period of culture that would allow them to complete the growth phase and properly differentiate.
The transition from the growing to the fully-grown stage, which in cattle occurs when the follicle develops from the early antral to the medium antral stage, is one of the critical steps during oocyte development. In cattle, several studies attempted to recapitulate these events in vitro2,12,13,14,15,16,17,18,19. However, to date no reliable protocols have been developed and only limited success has been reported. According to previous studies20, these growing oocytes constitute a homogeneous population. Besides being transcriptionally active, their chromatin is dispersed in the germinal vesicle (GV), in a configuration that is named GV02,21. Conversely, the population of fully-grown oocytes obtained from medium antral follicles is more heterogeneous, a condition that is mirrored by the various degrees of chromatin compaction (GV1, GV2 and GV3) that can be observed20. Among these, previous data have shown that GV2 and GV3 oocytes are overall characterized by a better quality and higher embryonic developmental competence20,21,22,23,24.
Starting from the above observations, here we describe a 5-days long culture system of oocytes (L-IVCO) that allows the differentiation of oocytes isolated as cumulus-oocyte complexes (COCs) from early antral follicles. This culture strategy has evolved from 10 years long studies conducted in our lab and roots its ground on the previously developed 24-48 hours in vitro oocyte culture (IVCO)2, prematuration systems23,25 and zinc supplementation during oocyte culture . A combination of follicle stimulating hormone (FSH) and a phosphodiesterase-3 (PDE3) inhibitor, able to enhance cumulus-oocyte communication2, prevent untimely meiotic resumption2, and support oocyte growth2 was used.
Protocol
Ovaries were collected from 4 to 8 years old Holstein dairy cows recovered at the local abattoir (INALCA S.p.A., Ospedaletto Lodigiano, LO, IT 2270M CE, Italy).
1. Media preparation
NOTE: All media must be prepared at least four hours before use. Sodium bicarbonate buffered media are incubated at 38.5 °C and 5% CO2 in air, maximum humidity. HEPES-buffered media are maintained at 38.5 °C in thermostatic oven.
- Long in vitro culture of oocytes (L-IVCO) medium
- Prepare 15 mL of the basic culture medium (M199-B): Supplement M199 with 2 mM glutamine, 0.4% fatty acid free bovine serum albumin (BSA), 0.2 mM sodium pyruvate, 25 mM sodium bicarbonate, 0.1 mM cysteamine, 21.3 µg/mL of phenol red, 75 μg/mL of kanamycin and 4% Polyvinylpyrrolidone (PVP; 360 k molecular weight).
- Prepare 3 mL of the holding medium (M199-H): To M199-B add 5 µM cilostamide and pour it in a 35 mm Petri dish.
- Prepare the L-IVCO medium (M199-L): Supplement M199-B with 0.15 μg/mL Zn sulphate, 10-4 IU/mL FSH, 10 ng/mL estradiol, 50 ng/mL testosterone, 50 ng/mL progesterone and 5 µM Cilostamide.
- Place 200 µL of M199-L medium in each well of the 96 well coated plate. Fill the wells in the four edges of the plate with sterile culture water to compensate for evaporation and to maintain appropriate humidity during culture.
- Incubate the 96 well plate and the M199-H medium in the incubator at 38.5 °C and 5% CO2 in air, maximum humidity.
- Dissection medium
- Prepare the dissection medium (M199-D): Supplement M199 with 0.4% BSA fraction V, 0.164 mM penicillin, 0.048 mM streptomycin, 1790 units/L heparin. M199-D can be prepared in bulk, dispensed in 20 mL aliquots and stored at 4 °C for 6 months. When needed, warm and supplement 1 aliquot.
- Prepare 20 mL of M199-D supplemented with 5 µM cilostamide (M199-D cilostamide).
2. Ovary collection and processing
NOTE: All procedures are conducted at room temperature (26 °C) unless otherwise indicated.
- Recover the ovaries at the abattoir from cows.
- Place the ovaries in sterile saline (NaCl, 9 g/L) at 26 – 28 °C added with penicillin 100 U/mL and streptomycin 0.1 mg/mL.
- Transport the organs to the laboratory in warm sterile saline within 4 h.
- Wash the ovaries 4x in sterile saline maintained at 26 °C.
- Remove all mid-to-large antral follicles by aspirating all follicles more than 2 mm in diameter using a 18 G needle connected to an aspiration pump with vacuum pressure set at -28 mmHg and place the aspirated ovaries in a beaker with sterile saline at 26 °C.
NOTE: Removal of the content of follicles > 2 mm is a critical step to remove as much as possible the source of fully-grown oocytes that would ‘contaminate’ the experiment. - Under a horizontal laminar flow hood, place one ovary at the time on a sterile polytetrafluoroethylene cutting board. Using a surgical blade No. 22 mounted on a scalpel handle, cut slices of ovarian cortex (the outer portion of the ovary, which contains the follicles), 1.5 – 2 mm thick and parallel to the major axis of the organ.
- Place the slices of ovarian cortex in a sterile glass Petri dish covered with dissecting medium on a warm plate at 38.5 °C.
NOTE: From now on all the procedures are performed at 38.5 °C using a warm plate.
3. Selection and isolation of the follicles and retrieval of the COCs
- Place one ovarian cortex slice in a 60 mm glass Petri dish with 2-3 mL of M199-D.
- Using a dissection microscope, select the follicles between 0.5 – 2 mm using a micrometer-equipped eyepiece.
- Identify the healthy, non-atretic follicles under the stereomicroscope. Assess follicle atresia by observing morphological parameters, such as very clear translucent appearance, with a dark COC inside. Discard the atretic follicles and process all the others.
- Using a surgical blade No. 22 mounted on a scalpel handle remove the ovarian tissue surrounding the follicle on one side until the follicle is exposed on one edge.
- Using a 26G needle mounted on a syringe, carefully make a slit in the exposed follicle wall. This action will release the follicular content, comprising the COC, follicular fluid and clumps of cells.
- Identify the COC under the microscope and examine for cumulus integrity, zona pellucida integrity and homogeneity of the cytoplasm. If these criteria are fulfilled, aspirate the COC using a P20 pipette.
- Place the isolated COC in M199-D cilostamide.
- Continue the isolation procedure for 30 min.
4. Selection of COCs to be subjected to in vitro culture
- Under the dissection microscope, select healthy COCs based on the criteria in step 3.6.
- In a 60 mm Petri dish, prepare 16 drops of 20 µL of M199-D cilostamide and place one healthy COC per drop (Figure 1A).
- Using an inverted microscope attached to a camera measure the oocyte diameter, excluding the zona pellucida, using the software provided with the camera.
- With a clear visualization of the oocyte, make two perpendicular measurements excluding the zona pellucida (Figure 1B).
- Assure whether the mean of the two oocyte’s measurement, excluding the zona pellucida, is within a range of 100 – 110 µm. Discard COCs with not-rounded shaped oocyte or with oocytes that are not measurable.
- Transfer the selected COCs in a 35 mm dish containing M199-H medium and keep them in the incubator at 38.5 °C and 5% CO2 in air, maximum humidity until step 5.1.
- Repeat steps 3 and 4 up to 4x. The overall working time must not exceed 2 h.
5. Long in vitro culture of the oocytes (L-IVCO)
- Transfer one COC per well in the center of a well of the 96 well plate to be prepared in step 1.1.5.
- Incubate the plate for 5 days at 38.5 °C and 5% CO2 in air, maximum humidity.
- Every other day (day 2 and day 4) prepare fresh M199-L as described in step 1.
- Renew half of the medium by removing 100 µL of medium and replacing with 100 µL of freshly prepared M199-L. Perform the medium renewal under the stereomicroscope and avoid moving the COCs into the well.
6. COC classification after the culture
- At the end of the L-IVCO, analyze the COCs’ morphology under the dissection microscope.
- Classify as depicted in Figure 2.
- Classify as Class 1 if COCs show a compact cumulus cell investment with no sign of cumulus expansion and cell degeneration.
- Classify as Class 2 if COCs show a compact cumulus cell investment with no sign of cumulus expansion and cell degeneration and with antrum-like formation in the cumulus mass.
- Classify as Class 3 if COCs show several layers of cumulus cell with no sign of cumulus expansion and some disaggregated cells in the outer layer of cumulus cells and no antrum-like formation.
- Classify as Class 4 if COCs shows abundant loss of cumulus cells extending for more than 50% of the oocyte surface, and signs of cell degeneration and cell debris.
7. Evaluation of meiotic progression after culture
- Oocyte denudation
- Place each COC in a single well of a four-well plate containing 400 µL of 199D per well.
- Under a dissection microscope gently remove the cumulus cells mechanically by repeated pipetting using a pipette set at 130-140 µL.
- Once the oocytes are free of the cumulus investment, transfer them to another well containing 199D.
- Repeat the process until all the oocytes are completely denuded.
- Oocyte nuclear staining
NOTE: From now on all the procedures are performed at room temperature. Reagents are at room temperature.- Fix the oocytes in paraformaldehyde 4% in phosphate buffer saline (PBS) for 1 h.
CAUTION: Wear personal protective equipment when handling paraformaldehyde and dispose of contaminated materials in accordance with hazardous waste disposal guidelines. - Wash the oocytes 3x for 5 min each in PBS containing 1% polyvinylalcohol (PVA).
NOTE: The samples can be processed right away or stored at 4 °C for maximum one week. - Place the oocytes in PBS containing 0.1% Triton X for 10 min.
- Wash the oocytes 3x for 5 min each in PBS containing 1% PVA.
- Place the oocytes singularly in drops of 5 µL of antifade medium supplemented with 4’,6-diamidino-2-phenylindole (DAPI) dilactate (1 µg/mL) over a slide.
- Place two strips of double-sided tape along the long sides of the slide, to avoid excessive flattening of the oocytes when putting the cover slip on top.
- Place the cover slip on top, make it adhere to the tape and keep in the dark while processing all the samples.
- Analyze the oocytes using a conventional epifluorescence microscope equipped with DAPI filters (Excitation/Emission: 358⁄461) to assess the meiotic progression of the oocytes.
- Classify the oocytes according to their meiotic progression: GV – oocytes with different degrees of chromatin condensation within the GV; MI – oocytes from GV breaking down to metaphase I; and degenerate – oocytes that could be not identified as being at any of the previous stages.
- Fix the oocytes in paraformaldehyde 4% in phosphate buffer saline (PBS) for 1 h.
Representative Results
At the end of the L-IVCO, the gross morphology of the COCs changed and 4 classes were identified based on the appearance of the cumulus cells, as shown in Figure 2. Based on the morphological criteria commonly adopted to select healthy COCs11,26,27, the class 1, 2 and 3 were judged healthy, while the class 4, which showed clear signs of degeneration such as the absence of complete layers of cumulus cells surrounding the oocytes, were considered severely compromised and unsuitable to undergo downstream procedures in a prospective IVP setting. Overall, 74 oocytes in 5 biological replicates were analyzed, of which 9.45% were in class 4 and were discarded from further evaluation.
As shown in Figure 3 and Figure 4, assessment of the meiotic stage at the end of the L-IVCO showed that a significantly higher percentage of the oocytes (78.57 ± 4.43%) remained arrested at the immature stage, with the chromatin still enclosed within the GV (therefore, also referred to as GV stage), without degenerating. Among them 59.43% were in a GV2/3 configuration. A small percentage resumed meiosis reaching the metaphase I stage (13.76 ± 5.85%) or degenerate (7.67 ± 4.61%). Overall, 67 oocytes in 5 biological replicates were analyzed. Altogether these data indicate that the L-IVCO culture supports the oocyte viability while preventing meiotic resumption for 5 days.
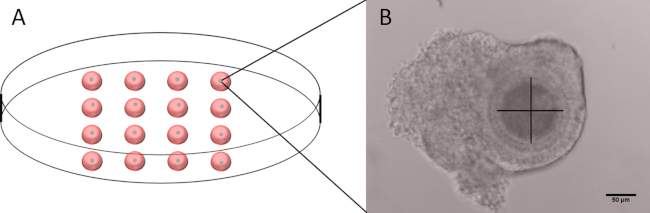
Figure 1: Outline of the dish used for measuring the oocyte diameter and representative image of a COC. (A) Schematic representation of a 60 mm Petri dish with 16 drops of 20 µL of M199-D, each one containing a single COC. (B) Representative image of a COC with the axis used for measuring the diameter. Note that the zona pellucida is not included. Scale bar 50 µm. Please click here to view a larger version of this figure.
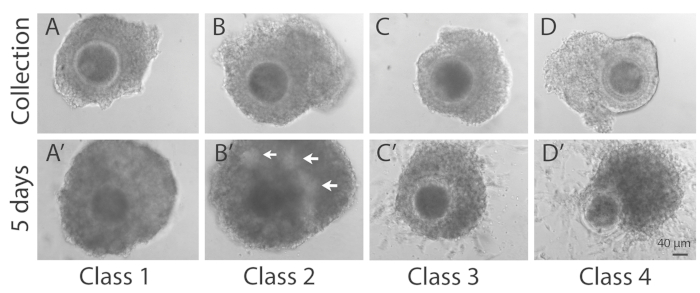
Figure 2: Representative images of COCs at the time of collection and after L-IVCO. (A, B, C, D) The upper row (Collection) represents COCs at the time of retrieval. (A’, B’, C’, D’) The same COC is pictured 5 days later, at the end of L-IVCO and classified as reported in step 6.1. The lower row (5 days) represents COCs classified as: Class 1, showing a compact cumulus cell investment with no sign of expansion and cell degeneration (A’); Class 2, showing a compact cumulus cell investment with no sign of expansion and cell degeneration and with antrum-like formation (arrows) in the cumulus mass (B’); Class 3, showing several layers of cumulus cell with no sign of cumulus expansion and some disaggregated cells in the outer layer of cumulus cells (C’); Class 4, showing abundant loss of cumulus cells on more than 50% of the oocyte surface and signs of cell degeneration and cell debris (D’). Scale bar 40 µm. Please click here to view a larger version of this figure.
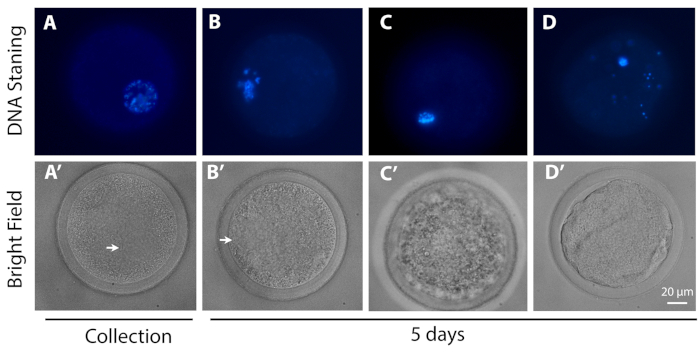
Figure 3: Representative images of the meiotic progression. The upper row (DNA staining) shows the DNA (blue) of representative oocytes at (A) the GV0 stage and (B) GV2-like configuration, (C) MI stage and (D) degenerated oocytes, (A) at the time of collection and (B, C, D) after 5 days of L-IVCO. The lower row is the corresponding image in bright field of the oocyte in the upper row. The arrow indicates the GV. Scale bar 20 µm. Please click here to view a larger version of this figure.
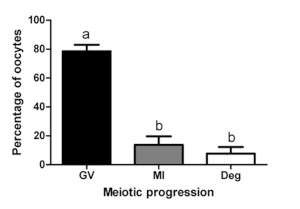
Figure 4: Meiotic progression of the oocytes at the end of culture. The bar graph represents the distribution of oocytes at GV and MI stage and degenerated oocytes at the end of the L-IVCO. The oocytes previously classified in Class 4 were excluded. Data were analyzed by 1-way ANOVA followed by Tukey’s multiple comparison test and values are means ± SEM (N=5; P<0.05). Please click here to view a larger version of this figure.
Discussion
Here we describe a culture system for growing oocytes that promotes oocyte development for 5 days by supporting their viability and preventing meiotic resumption. This latter aspect is of the outmost importance to allow the continued growth and differentiation necessary to confer the oocyte with meiotic and embryonic developmental competence2,20, that would be otherwise blocked by a premature resumption of the meiotic division.
When developing this culture system, we took into consideration several characteristics of the physiological growth and differentiation that occurs in the follicle. In this section we provide an overview of the main aspects that we considered when developing this strategy.
First, growing oocytes in bovine early antral follicles take approximately 5 days to undergo the transition from the growing to the fully grown stage in vivo8,19. Therefore, the length of the culture was increased to 5 days as opposed to previous attempts made in our lab where the oocytes were cultured for up to 24 h2.
Another factor that we included in the L-IVCO was the increased viscosity of the medium in which the COCs are cultured to mimic the physiological viscosity of the follicular fluid. This was recreated by adding 4% PVP and, together with the use of Collagen I coated culture surface, it promoted the formation of a 3D like culture, as reported by previous studies13.
Cilostamide, a PDE3 inhibitor, was added to maintain oocytes meiotically arrested at the GV stage, preventing precocious meiotic resumption by keeping high levels of cyclic nucleotides within the oocytes2,19,25,28,29. Our results indicate that a 5-day-long treatment with cilostamide does not have a gross impact on COCs health, as only a small fraction of complexes degenerated, also in agreement with the results obtained by Alam et al.19.
The inclusion of Zn sulfate, and its concentration, is substantiated by recent results showing that this trace element has a role in supporting the differentiation and transcriptional activity of bovine growing oocytes in culture30.
Finally, a combination of hormones was introduced to closely mimic the physiological hormonal milieu typical of the early antral follicle31,32,33. For instance estradiol has known activities in supporting the oocyte growth16,17,19 and the connections among granulosa cells17, while also promoting the acquisition of meiotic competence34. Similarly, testosterone, besides being a precursor of estradiol, also stimulates follicular growth and development35, while progesterone was mainly added for its antiapoptotic activity36.
Importantly and in agreement with our previous study2, the concentration of FSH was kept at a concentration that is physiological for the growing phase. Indeed, a low FSH concentration promotes oocyte development by sustaining gap-junction mediated communication between the oocyte and the companion cumulus cells and promotes transcriptional activity and oocyte differentiation without inducing meiotic resumption2.
In our experience, one of the keys for the success of the L-IVCO is the selection of a homogeneous population of healthy COCs coming from early antral follicles. According to data in the literature, 80% of the oocytes collected from early antral follicles are characterized by chromatin organized in a configuration termed GV020. This homogeneity represents an advantage for in vitro culture, as in principle it ensures that the cells will behave similarly when exposed to the culture environment. With this in mind, COCs collection must be performed trying to minimize the ‘contamination’ with COCs coming from less or more advanced stages of differentiation. However, due to the fact, that processing of the cortical slices is quite time consuming and should be carried out in a relatively short time, the collection/selection step probably represents the most critical passage of the L-IVCO. To achieve that, some key considerations should be beard in mind.
For instance, the researcher/technician needs to be trained to recognize and discard follicles with signs of follicular atresia. At this stage, only morphological parameters can be used to recognize atretic follicles, such as very clear translucent appearance, and the presence of a dark COC inside. All the other follicles, in which atretic signs cannot be clearly distinguished, should be opened and further selection based on the morphology of the isolated COCs should be carried out to identify the healthy ones2,3,37,38,39. This is achieved again by morphological observations such as the presence of at least four layers of cumulus cells, grossly spherical shape, intact oolemma and homogeneous and finely granulated ooplasm11,26,27.
COCs isolation and manipulation represent an additional technical challenge, which requires skilled personnel and proper equipment for micro-dissection under the stereomicroscope and accurate determination of oocyte diameter. This last step is essential to select a uniform population of oocytes, thus excluding any possible source of contamination with COCs coming from other follicular stages. For this reason, it is important to make sure that the oocytes enclosed in the retrieved COCs have a diameter between 100 and 110 µm2,40.
Besides supporting oocyte viability and preventing meiotic resumption, the L-IVCO promoted the transition of the chromatin configuration from GV0 to the progressively more condensed GV2 and GV3 in 59% of the oocytes. Notably chromatin condensation within the GV is a marker of ‘gain’ of meiotic and developmental competence in basically all the mammalian oocytes studied thus far20. This result is very promising, especially when compared to our previous 24 hours IVCO system. In that study, the highest degree of chromatin compaction within the GV were not reached and 22% of oocytes were found with a GV1 configuration2, a stage associated with full meiotic competence but still scarce developmental competence20. Even in those conditions, the otherwise incompetent growing oocytes were able to mature and produce embryos, although in limited amount. The consistent increase in GV2/3 stages observed in the L-IVCO is therefore compatible with a higher potential to produce viable embryos. We are in the process of testing this hypothesis experimentally by submitting COCs derived from L-IVCO to the following steps of IVP (in vitro maturation, fertilization, and embryo culture up to the blastocyst stage). If confirmed, the L-IVCO will unleash some of the yet unexploited potential of the ovarian reserve, with important implications on several areas of interest for female fertility preservation. For instance, it will increase the source of fertilizable gametes to be used in preservation programs of high genetic merit breeders. Another application that we foresee is for the genetic salvage of threatened species of the bovid family as well as of local breeds that are endangered or at risk of genetic erosion due to the widespread diffusion of cosmopolite breeds. Last but not the least, L-IVCO represents a tool for all the scientists that are interested in dissecting the cellular and molecular processes that regulate the formation of a competent gamete.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by Regione Lombardia PSR INNOVA n.201801061529 and UNIMI n.PSR 2019_DIP_027_ALUCI_01
Materials
| 4-well dishes | Nunclon | 179830 | |
| 96-well dish | Becton Dickinson Biosciences | 356649 | BioCoat™ Collagen I |
| Bovine Serum Albumin (Fatty acid free) | Sigma | A8806 | |
| Bovine Serum Albumin (Fraction V) | Sigma | A3311 | |
| Cell culture water | Sigma | W3500 | |
| Cilostamide | Sigma | C7971 | |
| Cysteamine | Sigma | M9768 | |
| Digital camera | Nikon Corp | Camera DS-5M | |
| Disodium phosphate | Sigma | S5136 | |
| Estradiol | Sigma | E2758 | |
| Glutamax Supplement | Thermo Fisher Scientific | 35050061 | |
| Gonal F | Merck Serono | ||
| Heparin | Sigma | H3149 | |
| Hepes | Sigma | H3784 | |
| Vacuum pump | Cook-IVF | ||
| Incubator | Sanyo | ||
| Kanamycin sulfate from Streptomyces kanamyceticus | Sigma | K1377 | |
| Medium 199 | Sigma | M3769 | Powder for hepes-buffered TCM199 |
| Medium 199 | Sigma | M2520 | Powder for M199-D |
| Microscope | Nikon Corp | Nikon Diaphot | |
| Microscope | Nikon Corp | Eclipse E 600 | |
| Monopotassium phosphate | Sigma | P5655 | |
| Paraformaldehyde | Sigma | 158127 | |
| Penicilin | Sigma | P3032 | |
| Phenol Red | Sigma | P5530 | |
| Polyvinyl alcohol | Sigma | P8137 | |
| Polyvinylpyrrolidone | Sigma | P5288 | 360k molecular weight |
| Potassium chloride | Sigma | P5405 | |
| Progesterone | Sigma | P8783 | |
| Sodium bicarbonate | Sigma | S5761 | |
| Sodium choride | Sigma | P5886 | |
| Sodium pyruvate | Sigma | P4562 | |
| Streptomycin | Sigma | S9137 | |
| Testosterone | Sigma | 86500 | |
| Triton X | Sigma | T9284 | |
| Vectashield with DAPI | Vector Laboratories | H1200 | |
| Water | Sigma | W3500 | |
| Zinc sulfate heptahydrate | Sigma | Z0251 |
Referenzen
- Lonergan, P., Fair, T. Maturation of Oocytes in Vitro. Annual Review of Animal Biosciences. 4, 255-268 (2016).
- Luciano, A. M., Franciosi, F., Modina, S. C., Lodde, V. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s). Biology of Reproduction. 85 (6), 1252-1259 (2011).
- McLaughlin, M., Telfer, E. E. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 139 (6), 971-978 (2010).
- Galli, C. Achievements and unmet promises of assisted reproduction technologies in large animals: a per-sonal perspective. Animal Reproduction. 14 (3), 614-621 (2017).
- Luciano, A. M., Sirard, M. A. Successful in vitro maturation of oocytes: a matter of follicular differentiation. Biology of Reproduction. 98 (2), 162-169 (2018).
- Lonergan, P., Fair, T. In vitro-produced bovine embryos: dealing with the warts. Theriogenology. 69 (1), 17-22 (2008).
- Clement, M. D. F., Dalbies-Tran, R., Estienne, A., Fabre, S., Mansanet, C., Monget, P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link. Biology of Reproduction. 90 (4), 85 (2014).
- Lussier, J. G., Matton, P., Dufour, J. J. Growth rates of follicles in the ovary of the cow. Journal of Reproduction and Fertility. 81 (2), 301-307 (1987).
- Fair, T., Hulshof, S. C., Hyttel, P., Greve, T., Boland, M. Oocyte ultrastructure in bovine primordial to early tertiary follicles. Anatomy and Embryology (Berlin). 195 (4), 327-336 (1997).
- Pavlok, A., Lucas-Hahn, A., Niemann, H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Molecular Reproduction and Development. 31 (1), 63-67 (1992).
- Blondin, P., Sirard, M. A. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Molecular Reproduction and Development. 41 (1), 54-62 (1995).
- Harada, M., et al. Bovine oocytes from early antral follicles grow to meiotic competence in vitro: effect of FSH and hypoxanthine. Theriogenology. 48 (5), 743-755 (1997).
- Hirao, Y., et al. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biology of Reproduction. 70 (1), 83-91 (2004).
- Alm, H., Katska-Ksiazkiewicz, L., Rynska, B., Tuchscherer, A. Survival and meiotic competence of bovine oocytes originating from early antral ovarian follicles. Theriogenology. 65 (7), 1422-1434 (2006).
- Taketsuru, H., et al. Bovine oocytes in secondary follicles grow in medium containing bovine plasma after vitrification. Journal of Reproduction and Development. 57 (1), 99-106 (2011).
- Endo, M., et al. Estradiol supports in vitro development of bovine early antral follicles. Reproduction. 145 (1), 85-96 (2013).
- Makita, M., Miyano, T. Steroid hormones promote bovine oocyte growth and connection with granulosa cells. Theriogenology. 82 (4), 605-612 (2014).
- Yamamoto, K., et al. Development to live young from bovine small oocytes after growth, maturation and fertilization in vitro. Theriogenology. 52 (1), 81-89 (1999).
- Alam, M. H., Lee, J., Miyano, T. Inhibition of PDE3A sustains meiotic arrest and gap junction of bovine growing oocytes in in vitro growth culture. Theriogenology. 118, 110-118 (2018).
- Lodde, V., Modina, S., Galbusera, C., Franciosi, F., Luciano, A. M. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence. Molecular Reproduction and Development. 74 (6), 740-749 (2007).
- Lodde, V., et al. Oocyte morphology and transcriptional silencing in relation to chromatin remodeling during the final phases of bovine oocyte growth. Molecular Reproduction and Development. 75 (5), 915-924 (2008).
- Dieci, C., et al. Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production. Molecular Human Reproduction. 22 (12), 882-897 (2016).
- Soares, A. C. S., et al. Steroid hormones interact with natriuretic peptide C to delay nuclear maturation, to maintain oocyte-cumulus communication and to improve the quality of in vitro-produced embryos in cattle. Reproduction, Fertililty and Development. 29 (11), 2217-2224 (2017).
- Soares, A. C. S., et al. Characterization and control of oocyte large-scale chromatin configuration in different cattle breeds. Theriogenology. 141, 146-152 (2020).
- Franciosi, F., et al. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biology of Reproduction. 91 (3), 61 (2014).
- Luciano, A. M., et al. Effect of different levels of intracellular cAMP on the in vitro maturation of cattle oocytes and their subsequent development following in vitro fertilization. Molecular Reproduction and Development. 54 (1), 86-91 (1999).
- Bilodeau-Goeseels, S., Panich, P. Effects of oocyte quality on development and transcriptional activity in early bovine embryos. Animal Reproduction Science. 71 (3-4), 143-155 (2002).
- Dieci, C., et al. The effect of cilostamide on gap junction communication dynamics, chromatin remodeling, and competence acquisition in pig oocytes following parthenogenetic activation and nuclear transfer. Biology of Reproduction. 89 (3), 68 (2013).
- Shu, Y. M., et al. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Human Reproduction. 23 (3), 504-513 (2008).
- Lodde, V., et al. Zinc supports transcription and improves meiotic competence of growing bovine oocytes. Reproduction. 159 (6), 679-691 (2020).
- Henderson, K. M., McNeilly, A. S., Swanston, I. A. Gonadotrophin and steroid concentrations in bovine follicular fluid and their relationship to follicle size. Journal of Reproduction and Fertility. 65 (2), 467-473 (1982).
- Kruip, T. A., Dieleman, S. J. Steroid hormone concentrations in the fluid of bovine follicles relative to size, quality and stage of the oestrus cycle. Theriogenology. 24 (4), 395-408 (1985).
- Sakaguchi, K., et al. Relationships between the antral follicle count, steroidogenesis, and secretion of follicle-stimulating hormone and anti-Mullerian hormone during follicular growth in cattle. Reproductive Biology and Endocrinology. 17 (1), 88 (2019).
- Makita, M., Miyano, T. Androgens promote the acquisition of maturation competence in bovine oocytes. Journal of Reproduction and Development. 61 (3), 211-217 (2015).
- Walters, K. A., Allan, C. M., Handelsman, D. J. Androgen actions and the ovary. Biology of Reproduction. 78 (3), 380-389 (2008).
- Luciano, A. M., Pappalardo, A., Ray, C., Peluso, J. J. Epidermal growth factor inhibits large granulosa cell apoptosis by stimulating progesterone synthesis and regulating the distribution of intracellular free calcium. Biology of Reproduction. 51 (4), 646-654 (1994).
- Gordon, I. . Laboratory Production of Cattle Embryos, 2nd edn. , (2003).
- Telfer, E. E., McLaughlin, M., Ding, C., Thong, K. J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Human Reproduction. 23 (5), 1151-1158 (2008).
- McLaughlin, M., Albertini, D. F., Wallace, W. H. B., Anderson, R. A., Telfer, E. E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Molecular Human Reproduction. 24 (3), 135-142 (2018).
- Fair, T., Hyttel, P., Greve, T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Molecular Reproduction and Development. 42 (4), 437-442 (1995).

