Preparing Acute Brain Slices from the Dorsal Pole of the Hippocampus from Adult Rodents
Summary
The purpose of this protocol is to describe a method to produce slices of the dorsal hippocampus for electrophysiological examination. This procedure employs perfusion with chilled ACSF prior to slice preparation with a near-coronal slicing angle which allows for preservation of healthy principal neurons.
Abstract
Whole-cell patch-clamp recordings from acute rodent brain slices are a mainstay of modern neurophysiological research, allowing precise measurement of cellular and synaptic properties. Nevertheless, there is an ever increasing need to perform correlated analyses between different experimental modes in addition to slice electrophysiology, for example: immunohistochemistry, molecular biology, in vivo imaging or electrophysiological recording; to answer evermore complex questions of brain function. However, making meaningful conclusions from these various experimental approaches is not straightforward, as even within relatively well described brain structures, a high degree of sub-regional variation of cellular function exists. Nowhere is this better exemplified than in the CA1 of the hippocampus, which has well-defined dorso-ventral properties, based on cellular and molecular properties. Nevertheless, many published studies examine protein expression patterns or behaviorally correlated in vivo activity in the dorsal extent of the hippocampus; and explain findings mechanistically with cellular electrophysiology from the ventro-medial region. This is further confounded by the fact that many acute slice electrophysiological experiments are performed in juvenile animals, when other experimental modes are performed in more mature animals. To address these issues, this method incorporates transcardial perfusion of mature (>60 day old rodents) with artificial cerebrospinal fluid followed by preparation of modified coronal slices including the septal pole of the dorsal hippocampus to record from CA1 pyramidal cells. This process leads to the generation of healthy acute slices of dorsal hippocampus allowing for slice-based cellular electrophysiological interrogation matched to other measures.
Introduction
The hippocampus is arguably the most well studied structure in the mammalian brain, due to its relatively large size and prominent laminar structure. The hippocampus has been implicated in a number of behavioral processes: spatial navigation, contextual memory, and episode formation. This is, in part, due to the relative ease of access to the dorsal portions of the hippocampus in rodents for in vivo analysis. Indeed, the major output cells are typically less than 2 mm from the pial surface.
In rodents, the hippocampus is a relatively large structure, formed of an invagination of the telencephalon extending from the dorsal septum to the ventral neocortex. It is composed of 2 major regions: the dentate gyrus and the cornu ammonis (CA); the latter of which is divided into 3 well-described sub-regions (CA1-3) that extend into the dentate gyrus hilus (formerly known as CA4), based on connectivity, cellular anatomy, and genetic properties1. This structure is maintained along the dorso-ventral extent of the hippocampus, albeit with major variations in synaptic properties2,3,4, anatomy5, genetic diversity6,7,8, and behavioral function9,10. Of the CA regions, the CA1 subfield is composed largely of glutamatergic CA1 pyramidal cells (CA1 PCs), for which 3 subtypes have been defined11, and inhibitory interneurons that make up ~10% of neurons, but are highly diverse with over 30 subtypes defined12,13,14. In addition to regional specific differences, normal aging has been shown to have dramatic effects on synaptic transmission15,16,17, anatomy18, and genetic profile19. The current gold-standard method to assess the intricacies of cellular and synaptic properties in a controlled manner is through the use of whole-cell patch-clamp recordings from acute brain slices20.
The understanding of hippocampal function is based largely on dorsal manipulation due to the ease with which it is accessed surgically or anatomically for behavioral tasks, implantation of electrodes or imaging windows, or viral plasmid expression. In many studies additionally, these procedures are performed with late-juvenile or adult rodents to prevent variability in brain structure during development. Despite this, many approaches to examine cellular and subcellular electrophysiology are performed in early- to mid-juvenile rodents, from mostly the ventro-medial portion of the hippocampus in its transverse plane21,22,23,24,25. Where the whole dorso-ventral extent has been assessed, a tissue-chopper is used to maintain the transverse extent4,26, or the experiment has been performed in young rats27 or mice28. Furthermore, cooling of tissue prior to dissection of the brain is known to preserve hippocampal structure in rats29 and neocortical neurons in mice30,31. Nevertheless, there is a paucity of detail regarding the production of brain slices from the dorsal transverse axis of the hippocampus, as generated by modified coronal slices, in mature rats.
This protocol describes an approach by which whole-cell patch-clamp recordings can be obtained from single or pairs of neurons in modified coronal slices of dorsal hippocampus from aged rats, followed by post-hoc morphological identification. Healthy brain slices are obtained following transcardial perfusion of chilled artificial cerebrospinal fluid (ACSF), facilitating measurement of electrophysiological properties from CA1 PCs and local interneurons.
Protocol
All animals were generated and maintained according to the Home Office and Institutional guidelines (HO# P135148E). All rats were maintained on a 12 h light/dark cycle and given ad libitum access to food and water.
1. Transcardial perfusion of chilled ACSF
- Prior to all experiments, place ~200 mL of sucrose-ACSF (Table 1) in the freezer at -20 °C (until semi-frozen, for slicing) and a further ~100-200 mL of filtered sucrose-ACSF on ice (for perfusion), bubbling with carbogen (95% O2/5% CO2).
- Collect an adult rat from its home cage and place it for ~30 minutes in a holding cage in the procedure room to acclimatize to noise and light levels.
- Prepare the dissection tools (Figure 1A), the perfusion area and the injectable anesthetic (approx. 1 mL of 200 mg/mL sodium pentobarbital for a final concentration of 100 mg/kg).
- Prepare an appropriate anesthesia chamber by placing a small swab of tissue paper or cotton wool inside. Introduce 1-2 mL of volatile isoflurane anesthetic to the absorbent material in the chamber.
- Place the rat in an anesthesia chamber to sedate. Monitor breathing until the breathing rate drops to ~1 shallow breath per second.
- At this point, start bubbling semi-frozen sucrose-ACSF with carbogen on ice for use during slicing.
- Weigh the rat and note its weight.
- Terminally anesthetize the rat by injecting the prepared sodium pentobarbital into the intraperitoneal cavity. The dose of sodium pentobarbital should be 100 mg/kg, calculated from the previously taken weight and stock concentration of drug. Place the rat in a holding chamber and allow 0.5-5 minutes for the onset of terminal anesthesia.
- Confirm cessation of reflexes: test both corneal blink (touch the pupil) and hind-paw pinch (lift the leg and pinch the hind-paw) reflexes using a blunt probe (i.e., rounded forceps). Once reflexes have ceased, pin the rat to the polystyrene or cork surgical board using hypodermic needles.
- Open the chest cavity and place the cannula at the base of the left ventricle of the heart. Puncture the right atrium and immediately start perfusion with the ice-cold (0-1 °C) sucrose-ACSF (using a peristaltic pump at 50 mL/minute).
- Once full exchange of fluids and cooling of the body has occurred (<5 minutes), remove the cannula and pins, and then decapitate using a guillotine.
- Carefully and rapidly remove the skull using bone scissors and Rongeur bone tools (Figure 1A,1B).
- Start by making 2x bilateral cuts through the foramen magnum using the bone scissors and remove the skull to the lambda suture with the Rongeurs. Cut carefully along the midline suture with the bone scissors to just behind the eyes.
- Make 2x bilateral cuts through the skull, perpendicular to the midline. Using the Rongeurs, open the skull along the midline. Take extra care to remove pia mater using fine scissors or a hooked needle.
- Scoop the brain out of the skull using a blunt spatula, severing the cranial and optic nerves with side to side compression. Place the brain into carbogenated, semi frozen (0– 1 °C) sucrose-ACSF for 1-2 minutes prior to slicing.
2. Preparation of brain slices from dorsal hippocampus
- Remove the perfused brain from the semi-frozen (0–1 °C) sucrose-ACSF and place in a glass Petri dish lined with filter paper. Place the brain onto its ventral surface.
- Using a scalpel (No. 22 blade), remove the posterior portion of the brain at ~10° from vertical to create a flat surface to glue the brain to the stage (Figure 1C).
- Apply a small amount of cyanoacrylate glue to the stage of the vibratome. Spread the glue to make a thin film approximately 50% larger than the cross-sectional area of the cut surface of the brain.
- Lift the brain out of the glass dish onto a spatula, cut side down, using a paintbrush to guide the tissue. Blot the brain with a piece of tissue to remove excess ACSF and slide the brain, cut surface down, onto the center of the glue. Using a Pasteur pipette, add 1-2 mL of ice-cold sucrose-ACSF over the brain to remove glue away from brain block.
- Place the brain into the slicing chamber and flood with semi-frozen sucrose ACSF. Use a spoon or spatula to keep excess ice away from brain block, and then carbogenate (Figure 1D).
- Move the blade of the vibratome into position: ~1 mm from the dorsal surface of the brain and vertically ~1 mm anterior to bregma. Ensure that the blade is fully submerged, and remove bubbles using a paintbrush.
- Start slicing the brain. To trim down to the dorsal hippocampus, use a speed of 0.1-0.2 mm/s, with a horizontal blade movement of 1-1.5 mm and a reciprocal oscillatory rate of ~90 Hz. When slicing the dorsal hippocampus, reduce the speed to 0.05-0.1 mm/s.
- Collect slices of dorsal hippocampus (nominally 3-4 full slices or 6-8 hemisected slices) per brain. If longitudinal slices of ventro-medial hippocampus are required, continue slicing. Once the dorsal hippocampus has been sliced, there is no need cut extra tissue beyond approximately the position of the 3rd ventricle. Stop the vibratome, separate the slice with a bent hypodermic needle, and collect in the base of the slicing chamber.
NOTE: Slices can be 250-500 µm thick, depending on experimental requirements. For recordings from the dorsal hippocampus, typically use 400 µm thick slices to preserve as much of the local network, whilst allowing the suitable microscopy conditions. - Trim the slices to contain only the hippocampus and overlying cortex under a dissecting microscope. Transfer slices to the pre-warmed 35 °C chamber with the anterior surface facing up.
- Determine the storage chamber based on the experiment to be performed. To obtain high quality whole-cell patch-clamp or extracellular field recordings close to the slice surface in submerged recording chambers, store in submerged chambers. Alternatively, store in a liquid/gas interface chamber for recordings of oscillatory network activity or interface extracellular field recordings.
- For submerged storage conditions, allow slices to recover at 35 ºC for 30 minutes from the time of the last slice entering the storage chamber. This allows for reactivation of metabolic processes and re-sealing of cut neurites. After 30 minutes, transfer the storage chamber to room temperature.
3. Recording the dorsal hippocampal neurons
- Fabricate recording patch pipettes from capillary glass. This protocol uses 1.5 mm outer diameter, 0.86 mm inner diameter borosilicate glass with filament, which yields a tip resistance of 3-5 MΩ when filled with intracellular solution (Table 1). Keep intracellular solutions chilled on ice to prevent degradation of energetic components and filtered prior to use (syringe filter, pore size: 0.2 μm).
- Carbogenate recording ACSF and pre-warm in a water bath (35-40 ºC). Deliver the carbogenated and pre-warmed ACSF to the recording chamber via perfusion tubing assisted by a peristaltic pump. Start perfusion several minutes prior to transferring a slice into the chamber.
- Stop the perfusion and transfer a brain slice to the recording chamber with the anterior surface facing up. Hold the slice in place with a platinum ring with single fibers of silk attached to form a “harp” shape. Position the slices so that stratum pyramidale of CA1 runs perpendicular to the axis of the first recording pipette.
- Restart the flow of carbogenated and pre-warmed (35-40 ºC) recording ACSF (Table 1) at an optimal rate of 6-8 mL∙min-1.
NOTE: High flow rates (6-8 mL∙min-1) are optimal for maintaining network activity in slices32. Lower flow rates (i.e., 2-3 mL∙min-1) can be used to maintain slice stability for imaging experiments or where biologically relevant network activity is not required. - Assess slice quality using infrared differential inference contrast (IR-DIC) optics with 40x objective magnification (visualized with a CCD camera). Assume good slice quality if a large number of ovoid-shaped, moderately contrasted CA1 PCs can be seen in str. pyramidale at depths of 20-30 μm below a smooth and lightly dimpled surface (Figure 2A). Poor quality slices contain large numbers of highly contrasted, shrunken or swollen cells, with an uneven slice surface.
- Fill patch pipettes with an intracellular solution (e.g., based on an intracellular [Cl–] of 24 mM) to allow comparison with other published data.
- Perform whole-cell patch-clamp recordings as previously described22. Exclude cells from analysis if the membrane potential (VM) on break-through is more depolarized than -50 mV, the series resistance is >30 MΩ; or the series resistance changes by >20% over the course of the recording. Under these recording conditions, series resistance is typically in the range of 8 – 25 MΩ and stable for up to 1 hour.
- To examine CA1 intrinsic excitability from the dorsal hippocampus, test intrinsic physiological properties with whole-cell recordings with the following protocols in current-clamp configuration:
- From the resting membrane potential with no bias current applied, apply small (-10 pA, 500 ms) current steps repeated 30 times.
- From -70 mV with bias current applied, apply hyper to depolarizing current steps of 500 ms duration (-100 to +400 pA, 25 pA steps) with 3 repetitions of a family of traces.
- Apply a sinusoidal wave of 100 pA peak-to-peak amplitude, with variable frequency from 0.1 to 20 Hz. Repeat 3 times.
- Apply a 5x 2 nA, 2 ms stimuli to drive action potentials at 20, 40, 60, 80, 100 Hz. 10 sweeps per frequency.
- From a -70 mV voltage-clamp, apply 5 minutes of spontaneous excitatory postsynaptic currents (EPSC) for recording.
- To reseal cells for histological analysis following successful recording, produce outside-out patches by slowly retracting the patch pipette. When an increase in series resistance is observed from experimental levels to >1 GΩ as measured by a -5 mV test pulse, raise the holding potential to -40 mV and retract the pipette fully.
- Perform additional recordings in the same slice to satisfy the required statistical power of the experimental design.
- Remove brain slices containing recorded neurons from the recording chamber, place in 24-well plate, replace the ACSF with 4% paraformaldehyde (in 0.1 M phosphate buffer) and leave overnight.
- The next day, replace the PFA with 0.1 M phosphate buffer and store until histological processing. Visualize cells with fluorescent-conjugated streptavidin as previously described22.
Representative Results
The protocol described above allows for the preparation of viable slices from the septal pole of the dorsal hippocampus in mature rats. A key factor in this protocol is the perfusion of chilled sucrose-ACSF, prior to slice preparation, resulting in healthy CA1 PCs proximal to the slice surface. The quality of the slice produced is assessed visually under IR-DIC optics, and healthy cells identified as having large, ovoid-shaped cell bodies are located throughout the full extent of stratum pyramidale, from the compact layer, into stratum oriens (Figure 2A, black arrow). Unhealthy slices are identified as having dead cells on the surface and rarely in the depths of the slice (Figure 2A, red arrow), which are identified on the basis of having either condensed and highly contrasted somata, or large “ballooned” somata with condensed nuclei.
Confirmation of slice health is achieved by performing whole-cell patch clamp recordings from putative healthy neurons. Whole-cell patch-clamp recordings are achieved with rapid, spontaneous gigaohm seal formation (15.5 ± 2.9 s; Figure 2B), comparable to those previously reported31. When the membrane is ruptured, healthy neurons in mature rats possess hyperpolarized resting membrane potentials (Mean: -65.6 ± 1.5 mV, Range: -55.6 to -73.9 mV; 15 PCs from 4 rats) and relatively low input resistances (Mean: 90.3 ± 5.2 MΩ, Range: 54.9 to 134.2 MΩ; 19 PCs from 4 rats). General slice quality is confirmed by high-fidelity spontaneous EPSCs (Figure 2C,2D), given low electrical noise (<10 pA peak-to-peak) when filtered at 10 kHz. Furthermore, stable cell recordings of hyperpolarized neurons require typically a <200 pA holding current, which is stable over long periods, due to the absence of network activity in the submerged recording conditions of this slice preparation.
Whole-cell patch clamp recordings from dorsal CA1 PCs allow for direct measurement of action potential discharge properties. Provided that the slice quality is sufficiently high, many cells can be recorded from a single slice within a short time frame (~1 hour). A key determinant of cell viability is the presence of an intact dendritic tree, and the axon surviving beyond the initial segment. The slicing angle of 10° from vertical allows for the preservation of this cellular anatomy, with cells preserved within the plane of slicing (Figure 3A). Healthy CA1 PCs from adult rats typically have a hyperpolarized membrane potential of -60 mV to -70 mV, input resistances of 100-200 MΩ and membrane time-constants of 20-40 ms when measured at the soma (Figure 3B). A key requirement for neuron inclusion in datasets is the presence of action potentials in response to depolarizing stimuli. CA1 PCs in adult rats present increasing numbers of action potentials to depolarizing stimuli, from the rheobase current to the maximum tested currents (400 pA), at which trains of action potentials display both adaptation of inter-spike times and accommodation of action potential amplitude (Figure 3C). The use of a variable frequency sinusoidal wave (0.1 -20 Hz over 20 s) allows for characterization of the membrane resonance of the recorded neurons (Figure 3D). Finally, temporally controlled trains of action potential discharge over a range of frequencies allow for comparison of accommodation and recruitment of K+ channels associated with the resulting hyperpolarization (Figure 3E). Following post-hoc confirmation of intact dendrites using streptavidin visualization of biocytin labelling performed during recordings, the spontaneous EPSC frequency measured from the continuous recording (Figure 2B, upper) allows for characterization of CA1 PC integration into the local network.
In summary, optimization of slice quality of the dorsal extent of the hippocampus allows for whole-cell recordings from multiple neurons per slice. This slice preparation facilitates the collection of large datasets of intrinsic excitability, the establishment of intra-animal variability measures, and the production of slices of sufficient quality to perform paired recordings from synaptically-coupled neurons.
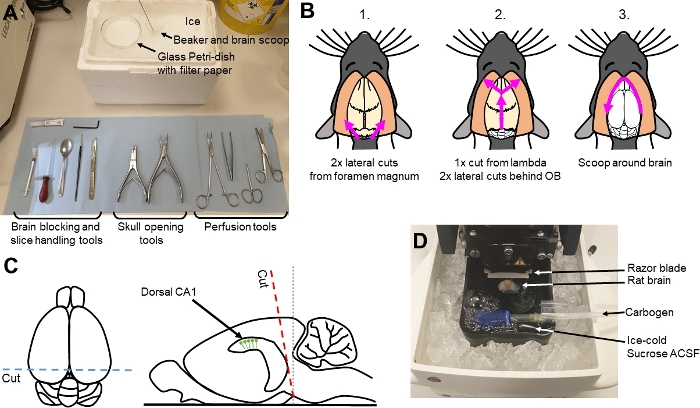
Figure 1: Overview of experimental setup and dissection schematic. (A) Experimental tools for all aspects of slice preparation, labelled according to use. (B) Cartoon depicting the directions of cuts with bone snippers and the movement of the spatula (pink arrows) to remove the brain from the skull. (C) Overview of the cutting angle (dashed red line) to allow preservation of dorsal CA1. (D) Overview of the slicing chamber with the brain mounted, anterior aspect facing up. Please click here to view a larger version of this figure.
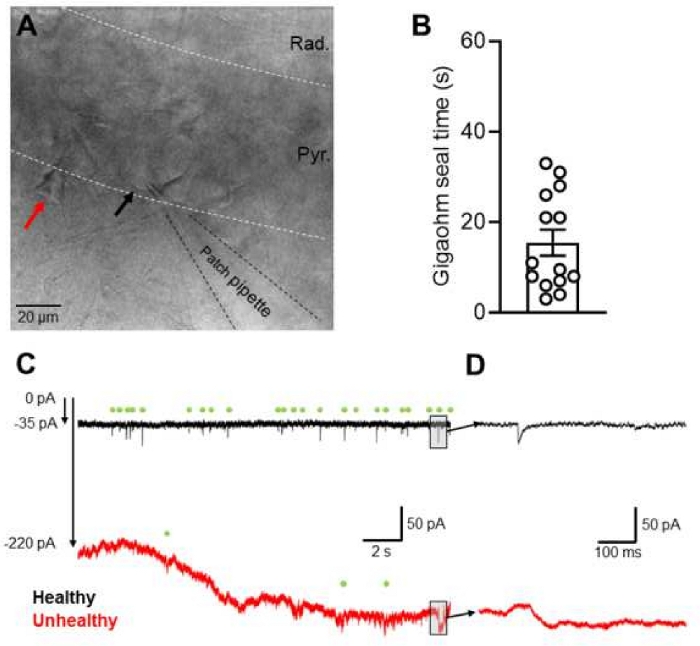
Figure 2: Identification of healthy neurons from the CA1 region of dorsal hippocampus. (A) Micrograph of area CA1 from an acute dorsal hippocampal slice, produced from a near coronal brain slice. The patch pipette is shown in a whole-cell configuration from a healthy neuron in the slice (indicated with black arrow). A nearby highly contrasted neuron to be avoided for recording is indicated (red arrow). (B) Representative continuous recordings of spontaneous EPSCs performed at -70 mV voltage-clamp from a stable recording of a healthy CA1 PC (black), with spontaneous EPSCs identified (green circles) and an unstable/unhealthy cell recorded under the same conditions (red). The holding current required to maintain -70 mV voltage-clamp is indicated. (C) Expanded view from the region of the trace in (B) indicated with a shaded box. Note the EPSC present in the top, stable trace (black) and the unstable, noisy trace (red). Please click here to view a larger version of this figure.
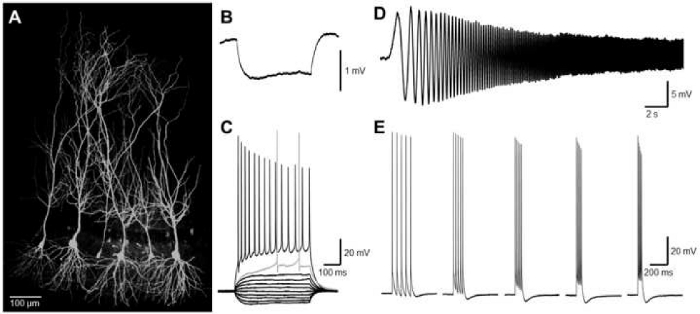
Figure 3: Cell identification and intrinsic electrophysiology, as measured by whole-cell patch-clamp recording from dorsal hippocampal CA1 PCs. (A) Visualization of biocytin with fluorescent-conjugated streptavidin labelling, followed by confocal imaging, from a slice containing multiple (6) CA1 PCs recorded sequentially, confirming the cellular identity of neurons recorded. (B) Average response to a -10 pA, 500 ms small hyperpolarizing step to ascertain passive membrane properties. (C) Voltage response of an identified CA1 PC to hyper- to depolarizing current steps (-100 to +400 pA, 500 ms duration). Action potential discharge is shown at both the rheobase current (grey sweep) and maximal discharge at 400 pA. (D) Membrane response to a 100 pA sinusoidal wave, frequency modulated from 0.1 – 20 Hz. Note the larger voltage response at the lowest cycle rates. (E) Trains of action potentials generated in response to trains of 5 stimuli (2 nA, 2 ms duration) over a range of frequencies (indicated). Please click here to view a larger version of this figure.
| Solution | Composition (in mM) | Notes | |
| Sucrose-ACSF | 87 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 75 sucrose, 7 MgCl2, 0.5 CaCl2 | Chill before use | |
| Recording-ACSF | 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 1 MgCl2, 2 CaCl2 | Pre-warm before use | |
| Intracellular Solution | 142 K-gluconate, 4 KCl, 0.5 EGTA, 10 HEPES, 2 MgCl2, 2 Na2-ATP, 0.3 Na2-GTP, 1 Na2-Phosphocreatine, 2.7 Biocytin (Osm ≈ 300 mOsm) | pH to 7.35 with 10 M KOH | |
| (K-gluconate) | |||
Table 1: List of solutions used in the preparation and recording of brain slices. Solutions are listed with their components reported as mM concentration. Specific notes prior to use are listed.
Discussion
Here, a protocol is described to produce high-quality brain slices from the dorsal extent of the CA1 of the hippocampus, allowing for recordings from multiple viable neurons within this region. The combinatorial approach of whole-cell recording from near-coronal slices followed by neuron visualization is critical to the confirmation of cell viability and identity.
This protocol reliably produces viable slices for 2 major reasons. Firstly, the modification to the cutting angle, as a deviation from true coronal, allows for greater preservation of somatodendritic axis and thus biologically relevant function of neurons. Given that the orientation of the somatodendritic axis of CA1 PCs in the most dorsal extent of the hippocampus is not in plane with a true coronal section1, this modification allows for greater tissue preservation. Alternatively, it is possible to remove the hippocampus fully and use a tissue chopper to prepare brain slices, as previously described for acute slices4,33 and for slice culture34,35. A drawback to this approach is the potential for damage to the hippocampus during its extraction and chopping (in inexperienced hands), which is avoided by keeping the brain intact. This approach more closely resembles that of earlier studies that maintain the hippocampal neurons in the transverse plane, with respect to the septal/temporal axis21,22. The second major factor that contributes to viable neurons in mature rats is the use of ice-cold sucrose-ACSF perfusion immediately prior to decapitation, dissection, and slice preparation. Given that the rat skull at ages beyond 3 months is typically thick and much harder to cut with traditional brain slice preparation tools (i.e., fine scissors, scalpels, and forceps), the duration of dissection using Rongeur’s and bone cutters is by its very nature longer, thus pre-cooling of the brain affords the researcher more time for dissection and slicing. The speed at which the brain can be cooled and sliced has long been understood to be advantageous to slice quality36, especially when the ice-cold ACSF is perfused before the cardiovascular system has been isolated from the brain30,31,37,38. Nevertheless, it has been suggested recently, that more physiological temperatures may also be useful for studying some brain regions39.
The slice quality produced by the combination of ice-cold sucrose-ACSF perfusion and the modified near-coronal cutting angle provides slice quality near comparable to that of horizontal slices prepared at the same developmental stage. Indeed, the optimization of this technique, using a sucrose-ACSF composition with similar ionic composition to that used for recording, allows for great consistency between conditions used within the experiment and slice preparation approaches used for neonatal rats. One major drawback of the use of submerged slice storage and recording conditions, as described here, is that the activity of neuronal networks in brain slices is significantly reduced compared to the in vivo setting. This is overcome by alternatively transferring the cut slices into an interface chamber flowing with recording ACSF at 35 °C for storage. This approach significantly improves the activity of local circuit, allowing measurement of neuronal oscillations and functionally relevant neuronal firing40,41. Other methods of slice production from older rodents, such as the use of NMDG recovery can similarly produce very high-quality slices30,31, which are suitable for the same recordings described. The specific advantage of this approach here is that it allows for direct comparison between recordings performed in younger rodents, based on slice preparations described previously21,22 due to the identical ionic basis of the solutions and slice recovery conditions. A combination of this approach with NMDG-based recovery could also yield high quality slices.
Overall, the brain slice preparation and recording protocol described here allows for a direct comparison of neuronal physiology and anatomy in a high-throughput manner, with other experimental modalities performed in the dorsal hippocampus, as performed in other brain areas, such as the neocortex. Indeed, there is an increasing number of studies that address the neurophysiological differences between the dorsal and the ventral hippocampus27,28,38,42,43. However, few studies perform recordings at an age comparable to that used for behavioral, anatomical, or in vivo electrophysiological studies. As such, the combination of improved slicing procedures and an appropriate choice of rodent age will allow for more realistic correlation of neuronal physiology to brain function. The above protocols have been performed on rats up to 1 year of age, but there is no reason to believe that this could not be performed on older rats given the appropriate permissions.
In summary, the protocol presented here provides a reliable method to produce brain slices from adult rats, thus allowing comparison of electrophysiological properties of neurons to in vivo and anatomical experiments.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The author wishes to thank Prof. David JA Wyllie, Dr. Emma Perkins, Laura Simoes de Oliveira, and Prof. Peter C Kind for helpful comments on the manuscript and protocol optimisation, and The Simons Initiative for the Developing Brain for providing publication costs.
Materials
| Acquisition software | Molecular Devices | pClamp 10 | |
| Adult rats | Various | n/a | Any strain of adult rat (60 days and older) |
| Amplifier | Molecular Devices | Axopatch 700B | |
| Analysis software | Freeware | Stimfit | https://github.com/neurodroid/stimfit |
| Bone Scissors | FST | 16152-12 | Littauer style |
| Capillary Glass | Harvard Apparatus | 30-0060 | Borosilicate glass pipettes with filament 1.5 mm outer diameter, 0.86 mm inner diameter. |
| Carbogen | BOC | Various | 95% O2/5% CO2 |
| CCD camera | Scientifica | SciCamPro | https://www.scientifica.uk.com/products/ |
| Chemicals/Reagents | Sigma Aldrich | Various | All laboratory reagents procured from Sigma Aldrich. |
| Cyanoacrylate (i.e. RS Pro 3) | RS Components | 918-6872 | Avoid gel based cyanoacrylate formulations |
| Digitizer | Molecular Devices | Digidata 1550B | |
| Dissection pins/needles | Various | Various | Use 16 gauge needles (above) |
| Electrophysiology system | Scientifica | SliceScope | https://www.scientifica.uk.com/products/ scientifica-slicescope |
| Fine iris scissors | FST | 14568-12 | With Tungsten-Carbide tips |
| Glass Petri dish | Fisher Scientific | 12911408 | |
| Hypodermic needles | BD Healthcare | Various | 16, 18, and 22 gauge |
| Isofluorane 100% W/W (i.e.IsoFlo) | Zoetis | 50019100 | |
| Mayo-type scissors | FST | 14110-17 | Blunt tips |
| Micromanipulators | Scientifica | Microstar | https://www.scientifica.uk.com/products/scientifica-microstar-micromanipulator |
| Paintbrush | Art store | n/a | A fine bristled paintbrush, procured from a local art shop. |
| Pasteur pipette | Fisher Scientific | 11546963 | A glass Pasteur pipette, but cut so that the blunt end is used to transfer pipette. |
| Peristaltic pump | Watson Marlow | 12466260 | Single channel peristaltic pump |
| Pipette puller | Sutter Instruments | P-97 | Other models and methods of pipette production would work equally well. |
| Plastic syringes (1, 2, 5 mL) | BD Healthcare | Various | |
| Rongeur bone tool | FST | 16021-14 | |
| Slice holding chamber | Homemade | ||
| Slice weight/harp | Harvard Apparatus | SHD-22L/15 | Alternatives would be suitable. |
| Sodium Pentobarbital (i.e. Pentoject) | Animalcare Ltd | 10347/4014 | 200 mg/mL; other formulations of pentobarbital would be suitable |
| Spatula | Bochem | 3213 | Available from Fisher Scientific |
| Syringe filters | Fisher Scientific | 10482012 | Corning brand syringe filters, 0.22 µm pore diameter. |
| Vibtratome | Leica | 1491200S001 | VT1200S model with Vibrocheck |
| Water Bath | Fisher Scientific | 15167015 | 5 Litre water bath, for example: Grant Instruments™JBA5 scientifica-scicam-pro |
Referenzen
- Amaral, D. G., Witter, M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neurowissenschaften. 31 (3), 571-591 (1989).
- Moser, M. B., Moser, E. I. Functional differentiation in the hippocampus. Hippocampus. 8 (6), 608-619 (1998).
- Babiec, W. E., Jami, S. A., Guglietta, R., Chen, P. B., O’Dell, T. J. Differential regulation of NMDA receptor-mediated transmission by SK channels underlies dorsal-ventral differences in dynamics of schaffer collateral synaptic function. Journal of Neuroscience. 37 (7), 1950-1964 (2017).
- Papatheodoropoulos, C. Striking differences in synaptic facilitation along the dorsoventral axis of the hippocampus. Neurowissenschaften. 301, 454-470 (2015).
- O’Reilly, K. C., et al. Identification of dorsal-ventral hippocampal differentiation in neonatal rats. Brain Structure and Function. 220 (5), 2873-2893 (2015).
- Cembrowski, M. S., et al. Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 89 (2), 351-368 (2016).
- Cembrowski, M. S., Wang, L., Sugino, K., Shields, B. C., Spruston, N. Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. elife. 5, 14997 (2016).
- Leonardo, E., Richardson-Jones, J., Sibille, E., Kottman, A., Hen, R. Molecular heterogeneity along the dorsal–ventral axis of the murine hippocampal CA1 field: a microarray analysis of gene expression. Neurowissenschaften. 137 (1), 177-186 (2006).
- Kheirbek, M. A., et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 77 (5), 955-968 (2013).
- Kjelstrup, K. B., et al. Finite scale of spatial representation in the hippocampus. Science. 321 (5885), 140-143 (2008).
- Klausberger, T., Somogyi, P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 321 (5885), 53-57 (2008).
- Booker, S. A., Vida, I. Morphological diversity and connectivity of hippocampal interneurons. Cell and Tissue Research. 373 (3), 619-641 (2018).
- Freund, T. F., Buzsaki, G. Interneurons of the hippocampus. Hippocampus. 6 (4), 347 (1996).
- Pelkey, K. A., et al. Hippocampal GABAergic inhibitory interneurons. Physiological Reviews. 97 (4), 1619 (2017).
- Barnes, C. A., Rao, G., Foster, T. C., McNaughton, B. L. Region-specific age effects on AMPA sensitivity: Electrophysiological evidence for loss of synaptic contacts in hippocampal field CA1. Hippocampus. 2 (4), 457-468 (1992).
- Potier, B., Jouvenceau, A., Epelbaum, J., Dutar, P. Age-related alterations of GABAergic input to CA1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neurowissenschaften. 142 (1), 187-201 (2006).
- Ekonomou, A., Pagonopoulou, O., Angelatou, F. Age-dependent changes in adenosine A1 receptor and uptake site binding in the mouse brain: An autoradiographic study. Journal of Neuroscience Research. 60 (2), 257-265 (2000).
- Pyapali, G. K., Turner, D. A. Increased dendritic extent in hippocampal CA1 neurons from aged F344 rats. Neurobiology of Aging. 17 (4), 601-611 (1996).
- Blalock, E. M., et al. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. Journal of Neuroscience. 23 (9), 3807-3819 (2003).
- Sakmann, B., Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annual Review of Physiology. 46 (1), 455-472 (1984).
- Bischofberger, J., Engel, D., Li, L., Geiger, J. R., Jonas, P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nature Protocols. 1 (4), 2075 (2006).
- Booker, S. A., Song, J., Vida, I. Whole-cell patch-clamp recordings from morphologically-and neurochemically-identified hippocampal interneurons. Journal of Visualized Experiments. (91), e51706 (2014).
- Geiger, J., et al. Patch-clamp recording in brain slices with improved slicer technology. Pflügers Archive. 443 (3), 491-501 (2002).
- Aitken, P., et al. Preparative methods for brain slices: a discussion. Journal of Neuroscience Methods. 59 (1), 139-149 (1995).
- Siwani, S., et al. OLMα2 cells bidirectionally modulate learning. Neuron. 99 (2), 404-412 (2018).
- Dong, W. Q., Schurr, A., Reid, K. H., Shields, C. B., West, C. A. The rat hippocampal slice preparation as an in vitro model of ischemia. Stroke. 19 (4), 498-502 (1988).
- Dougherty, K. A. Differential developmental refinement of the intrinsic electrophysiological properties of CA1 pyramidal neurons from the rat dorsal and ventral hippocampus. Hippocampus. 30 (3), 233-249 (2020).
- Foggetti, A., Baccini, G., Arnold, P., Schiffelholz, T., Wulff, P. Spiny and Non-spiny Parvalbumin-Positive Hippocampal Interneurons Show Different Plastic Properties. Cell Reports. 27 (13), 3725-3732 (2019).
- Newman, G. C., Qi, H., Hospod, F. E., Grundmann, K. Preservation of hippocampal brain slices with in vivo or in vitro hypothermia. Brain Research. 575 (1), 159-163 (1992).
- Ting, J. T., Daigle, T. L., Chen, Q., Feng, G. . Patch-Clamp Methods and Protocols. , 221-242 (2014).
- Ting, J. T., et al. Preparation of acute brain slices using an optimized N-methyl-D-glucamine protective recovery method. Journal of Visualized Experiments. (132), e53825 (2018).
- Hajos, N., et al. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. European Journal of Neuroscience. 29 (2), 319-327 (2009).
- Papaleonidopoulos, V., Trompoukis, G., Koutsoumpa, A., Papatheodoropoulos, C. A gradient of frequency-dependent synaptic properties along the longitudinal hippocampal axis. BMC Neuroscience. 18 (1), 79 (2017).
- Fuller, L., Dailey, M. E. . Preparation of rodent hippocampal slice cultures. (10), 4848 (2007).
- Gee, C. E., Ohmert, I., Wiegert, J. S., Oertner, T. G. Preparation of slice cultures from rodent hippocampus. Cold Spring Harbor Protocols. 2017 (2), (2017).
- Andersen, P. Brain slices – a neurobiological tool of increasing usefulness. Trends in Neurosciences. 4, 53-56 (1981).
- Moyer, J. R., Brown, T. H. . Patch-Clamp Analysis. , 135-193 (2002).
- Dougherty, K. A., et al. Differential expression of HCN subunits alters voltage-dependent gating of h-channels in CA1 pyramidal neurons from dorsal and ventral hippocampus. Journal of Neurophysiology. 109 (7), 1940-1953 (2013).
- Huang, S., Uusisaari, M. Y. Physiological temperature during brain slicing enhances the quality of acute slice preparations. Frontiers in Cellular Neuroscience. 7, 48 (2013).
- Booker, S. A., et al. Presynaptic GABAB receptors functionally uncouple somatostatin interneurons from the active hippocampal network. Elife. 9, 51156 (2020).
- Gloveli, T., et al. Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proceedings of the National Academy of Sciences. 102 (37), 13295-13300 (2005).
- Ordemann, G. J., Apgar, C. J., Brager, D. H. D-type potassium channels normalize action potential firing between dorsal and ventral CA1 neurons of the mouse hippocampus. Journal of Neurophysiology. 121 (3), 983-995 (2019).
- Arnold, E. C., McMurray, C., Gray, R., Johnston, D. Epilepsy-induced reduction in HCN channel expression contributes to an increased excitability in dorsal, but not ventral, hippocampal CA1 neurons. eNeuro. 6 (2), (2019).

.
