Flow Cytometry and Confocal Imaging Analysis of Low Wnt Expression in Axin2-mTurquoise2 Reporter Thymocytes
Summary
Signaling levels are known to regulate cell fate, indicating that regulation of Wnt signaling constitutes an interesting therapeutic target. Here, we describe flow cytometry and confocal microscopy analysis methods for a robust murine canonical Wnt signaling reporter model that measures distinct Wnt signaling levels.
Abstract
Measuring Wnt expression levels is essential when trying to identify or test new Wnt therapeutic targets. Previous studies have shown that canonical Wnt signaling operates via a dosage-driven mechanism, motivating the need to study and measure Wnt signaling in various cell types. Although several reporter models have been proposed to represent physiological Wnt expression, either the genetic context or the reporter protein highly influenced the validity, accuracy, and flexibility of these tools. This paper describes methods for acquiring and analyzing data obtained with the Axin2-mTurquoise2 mouse Wnt reporter model, which contains a mutated Axin2em1Fstl allele. This model facilitates the study of endogenous canonical Wnt signaling in individual cells over a wide range of Wnt activity.
This protocol describes how to fully appreciate Axin2-mTurquoise2 reporter activity using cell population analysis of the hematopoietic system, combined with cell surface markers or β-catenin intracellular staining. These procedures serve as a base for implementation and reproduction in other tissues or cells of interest. By combining fluorescence-activated cell sorting and confocal imaging, distinct canonical Wnt expression levels can be visualized. The recommended measurement and analysis strategies provide quantitative data on the fluorescent expression levels for precise assessment of canonical Wnt signaling. These methods will be useful for researchers who want to use the Axin2-mTurquise2 model for canonical Wnt expression patterns.
Introduction
Canonical Wnt signaling is a conserved signaling pathway implicated in healthy tissue homeostasis as well as in disease. Precise regulation of Wnt signaling levels has been shown to be important in embryonic development, but is also of great importance in adult tissues. Canonical Wnt signaling has been found to play an important role in tissue regeneration of several organs such as the gut, the skin, and the hematopoietic system. Hence, when Wnt signaling is deregulated, severe pathologies arise. Colorectal, liver, and skin cancer, neurological disease, as well as certain hematological malignancies are exemplary pathologies wherein deregulated Wnt signaling is the causative factor or contributor1. Therefore, several inhibitors for different Wnt targets are currently being tested in clinical trials as Wnt-associated cancer therapeutics2.
Additionally, interesting advances are taking place in Wnt therapeutic potential for neurological recovery, age-related neurological disorders, and congenital autism spectrum disorders3,4,5. Wnt signals have been explored for ex vivo expansion of stem cells for subsequent transplantation6. However, therapeutic targeting of canonical Wnt signaling is a difficult endeavor due to its importance in many basic cell functions and cross-talk with other pathways7,8,9, resulting in the need to precisely measure the effects of these Wnt therapeutic agents in an easy-to-interpret model. Canonical Wnt signaling is driven by short-range, soluble Wnt ligands, which are secreted by neighboring cells or as autocrine excretion as reported in various Wnt-responsive stem cell types.
The Wnt Frizzled receptor and lipoprotein receptor-related protein (LRP) co-receptors are responsive to these ligands, which triggers an intracellular signaling cascade. When Wnt signaling is off, a destruction complex composed of Axis Inhibitor (Axin), tumor suppressor gene product, Adenomatous Polyposis Coli (APC), Casein Kinase1 (CK1α), and Glycogen Synthase Kinase (GSK-3β), prevents the accumulation of β-catenin (CTNNB1) by proteasomal degradation. Upon Wnt ligand-receptor binding, the destruction complex is inactivated, leading to accumulation and stabilization of β-catenin in the cytoplasm. The active β-catenin can migrate to the nucleus where it binds to the Transcription Factor/Lymphoid Enhancer-binding Factor (TCF/LEF) transcription factors to initiate the transcription of Wnt target genes. Axin2 is considered a target gene as it is a direct target of the Wnt pathway10. Additionally, Axin2 serves as a negative regulator as well as a reporter gene for active canonical Wnt signaling11,12.
Several canonical Wnt signaling reporters have been described in literature and have been of great use in understanding the role of Wnt signaling in embryonic development. Most of these reporters make use of synthetically inserted TCF/LEF binding sites, which do not use an endogenous target gene13,14,15,16,17,18,19. Additionally, Axin2 knock-in strategies have been used that respect the natural location of the gene11,20,21,22,23, of which Axin2-LacZ is generally accepted as the most robust canonical Wnt reporter11. However, the reporter protein LacZ, albeit easy-to-use in most tissues, requires a β-galactosidase substrate, which is recognized to be harsh for live cells24. Especially for stem cells and thymocytes, the harsh LacZ detection conditions increase cellular death (own unreported data) when handling cell suspensions.
Although the signal amplification caused by the LacZ staining is convenient to detect low signals, it makes the quantification less direct and thus arguably less reliable. Therefore, a murine reporter model was designed to mimic the Axin2-LacZ genetic strategy, but with an mTurquoise2 reporter protein21, to provide a readout that is more direct and closer to the physiological expression levels. The mTurquoise2 fluorescent protein is an excellent substitute for LacZ due to its high brightness (quantum yield (QY)= 0.93), flexibility in combination with other fluorescent proteins for extensive cell surface characterization, and its lack of needing an exogenous substrate. Furthermore, its close genetic relationship to green fluorescent protein (GFP) offers the possibility to use most GFP-recognizing fluorescent antibodies for stronger signal detection, if necessary, in extremely Wnt-sensitive cells25.
The Axin2-mTurquoise2 model is not only a canonical Wnt reporter, but also offers the possibility to study Axin2 heterozygote and homozygote (Axin2 knock-out) phenotypes. The targeted insertion of mTurquoise2 at the start site of Axin2 results in a disrupted Axin2 protein21. As Axin2, also known as Conductin, is part of the Wnt destruction complex, and the destruction complex tightly regulates β-catenin mediated transcription, its partial or complete absence could be of interest to study diverse pathologies. For instance, in colorectal cancer, Axin2 levels are relatively high due to Wnt hyperactivation11; however, its role in other pathologies is still largely unknown. Even though Axin2 is considered to play a limited role in the degradation of β-catenin, its role in Wnt regulation can be enhanced by the addition of a small peptide, which blocks Wnt-mediated colorectal cancer growth26.
Altogether, careful Wnt regulation via Wnt therapeutic targets can open up opportunities to change the onset or development of severe pathologies and should be further investigated in models with reporter capacity. In this report, we explain our best-practice analysis method of the Axin2-mTurquoise2 murine model for flow cytometry and confocal imaging. In the context of Wnt dosage levels, very low canonical Wnt signaling levels are difficult to detect, for which advanced detection and analysis abilities provide an advantage to fully derive the benefits of this model. Thymocytes are used as a model system due to their fragile cell viability, low canonical Wnt signaling expression, and condensed cytoplasm area to represent the detection sensitivity of the Axin2-mTurquoise2 model. Additionally, a histological total β-catenin-staining procedure for thymocyte cell suspensions is explained to measure cytoplasmic β-catenin levels and verify nuclear active canonical Wnt signaling in combination with the reporter.
Protocol
NOTE: All mouse procedures were performed with the approval of the Leiden University Medical Centre (LUMC) Ethical Committee on Animal Experiments. Male and female, 6-12-week-old, wild-type (wt), which have no insertion of the Axin2-mTurquoise2 reporter construct, heterozygous (Tg/0) with one insertion of the Axin2-murquoise2 reporter construct and thus, one disrupted Axin2 gene, and homozygous (Tg/Tg) with the insertion of the Axin2-mTurquoise2 reporter construct in both alleles and thus, two disrupted Axin2 genes; Axin2-mTurquoise2 mice (B6;CBA-Axin2em1Fstl/J mice) were used in the experiments. The animals were sacrificed by CO2 euthanasia prior to organ isolation. Throughout the procedure, minimize exposure of the samples to light, and keep on ice or 4 °C at all times, unless indicated differently. Cover the samples with aluminum foil. All the steps should be performed in a standard laboratory with a biosafety cabinet.
1. Preparation of thymocyte cell suspension
- Harvest the thymus from mice carefully without blood contamination by cutting open the abdomen of the mice and extracting the thymus with forceps. Store/transport temporarily in ice-cold Iscove's Modified Dulbecco's Medium (IMDM) containing 2.5% fetal calf serum (FCS).
NOTE: To avoid blood spillage and possible thymus damage, do not sacrifice the mice by cervical dislocation. - Prepare a 50 mL tube with a 70 μm cell strainer, and wet the filter with 1 mL of cold IMDM/2.5% FCS medium.
- Mash the organ with the back tip of a 1 mL syringe plunger while washing twice with cold IMDM/2.5% FCS medium (Figure 1A). If desired, add a final concentration of 50 U/mL of DNAse I to the IMDM/2.5% FCS medium to prevent dead cell clumping. Rinse the filter 2x with cold IMDM/2.5% FCS medium, and resuspend gently in the 50 mL tube.
NOTE: Do not exceed a total end volume of 10 mL. Keep cells on ice and in the dark for subsequent steps and when not handling. - Centrifuge at 330 × g for 5 min at 4 °C, and gently aspirate the supernatant from the cell pellet. Resuspend the cell pellet gently in cold incomplete IMDM/2.5% FCS medium, and prepare for cell counting.
NOTE: If required, freeze and store the thymocytes in liquid nitrogen in FCS-10% dimethylsulfoxide for later experimentation. Proper cell freezing and thawing will reduce excessive cell death. On average, half of the thymocytes can be apoptotic after thawing due to the natural T cell selection in the thymus, which should be considered when deciding how many thymocytes to freeze down per cryo-vial. No less than 2.5 × 106 thymocytes should be frozen per vial.
2. Thymocyte flow cytometry preparation
- Prepare thymocytes for cell surface staining procedure by preparing 2.5 × 106 thymocytes per staining sample in ice-cold phosphate-buffered saline (PBS, pH 7.4) (Figure 1B). Recount the number of live cells after thawing, if thymocytes have been previously frozen. If required, add a final concentration of 50 U/mL of DNAse I to prevent dead cell clumping.
- Use the antibody staining panels for cell surface characterization of a complete thymocyte subset.
NOTE: Other combinations of fluorochromes can be selected. Select rare population markers for bright fluorescent fluorochromes and if possible, add a live-dead marker. Neither V450 nor V500 fluorochromes should be used in combination with the mTurquoise2 fluorescent reporter due to spectral overlap. Always check the fluorescence spectra of mTurquoise2 in combination with blue and green fluorochromes (Supplemental Figure 1A).- Mix the antibodies in previously defined ratios of the lineage-negative (Lin-) panels separately in PBS/0.2% bovine serum albumin (BSA)/0.1% NaN3 (sodium azide) buffer.
NOTE: All unwanted cells (non-thymocytes present in the thymus) are stained in a 2-step process with a streptavidin secondary antibody (in this example, phycoerythrin (Pe)-Cy7 and allophycocyanin (APC)-Cy7) and can be excluded by using a "dump gate" in the flow cytometry analysis. - Mix the antibodies in previously defined ratios of the Double Negative (DN) staining panel with the streptavidin secondary antibody Pe-Cy7 in PBS/0.2% BSA/0.1% NaN3 buffer. Exclude the Lin- panel from this mix (Table 1).
- Mix the antibodies in previously defined ratios of the Immature Single Positive (ISP), Double Positive (DP), and Single Positive (SP) staining panel with the streptavidin secondary antibody APC-Cy7 in PBS/0.2% BSA/0.1% NaN3 buffer. Exclude the Lin- panel from this mix (Table 1).
- First, stain the thymocytes with the unwanted non-T cell populations by using the biotin primary antibody mixes of the Lin- panels for 30 min on ice in the dark.
NOTE: Each Lin- panel is a different set of cells and should therefore not be stained together in one sample. - Spin down at 300 × g, 4 °C for 5 min and remove the supernatant. Wash the thymocytes with 150 μL of ice-cold PBS/0.2% BSA/ 0.1% NaN3 buffer, and spin down at 300 × g, 4 °C for 5 min.
- Stain with the DN panel and the ISP/DP/SP panel of the corresponding Lin- staining for 30 min on ice in the dark. Spin down at 300 × g, 4 °C for 5 min, and remove the supernatant. Wash the thymocytes with 150 μL of ice-cold PBS/0.2% BSA/0.1% NaN3 buffer, and spin down at 300 × g, 4 °C for 5 min.
- Mix the antibodies in previously defined ratios of the lineage-negative (Lin-) panels separately in PBS/0.2% bovine serum albumin (BSA)/0.1% NaN3 (sodium azide) buffer.
- Prepare the cells for flow cytometry measurement by homogenizing with a 35 μm cell strainer tube and taking the cells up in PBS/0.2% BSA/0.1% NaN3 buffer. Protect cells from light, and keep on ice until and during flow cytometer measurement.
NOTE: NaN3 (sodium azide) is highly toxic and fatal. Special care should be taken when working with this substance. Wash hands thoroughly after handling, and immediately call a poison control center or doctor/physician if NaN3 is swallowed.
3. Flow cytometer measurement
NOTE: Inexperienced users should first take flow cytometer training as the measurement of the mTurquoise2 signal in combination with several other fluorochromes requires experience and knowledgeable planification of the experiment. See the Table of Materials for information about the flow cytometer.
- Start the flow cytometer according to the user manual or other established protocol. Check, and if required adjust, the bandpass filter sets in the flow cytometer for an optimal fluorescence detection strategy.
NOTE: A recommended filter for mTurquoise2 is 470/20 nm on the ultra-violet 405 nm, 407 nm, or the less common 440 nm laser line. - Calibrate the flow cytometer by establishing compensation settings with commercially available compensation beads and stably transfected, mTurquoise2-expressing 293T cells.
NOTE: Single-stained wt thymocytes can be used instead of compensation beads. In this case, compensation with beads was equally efficient and more convenient than using cells.- Label the beads with each individual fluorochrome used in the experiment, and include unstained beads. Measure the beads to set up a compensation setting panel, and measure mTurquoise2-expressing 293T cells as there is no matching fluorochrome for mTurquoise2, which can be used on the beads. Save the compensation settings for use in the actual experiment.
NOTE: The mTurquoise2-expressing cells used for the compensation setting must be as bright or brighter than the mTurquoise2-expressing thymocytes of the actual experiment. Make sure there are also mTurquoise2-negative 293T cells present. - For the experiment, include fluorescence minus one (FMO) Tg/Tg-stained thymocytes, including a wt sample for the mTurquoise2 FMO and an unstained sample of thymocytes of each mouse genotype as positive controls for the analysis part.
NOTE: These controls are important to properly set the gates for positive cells. The rest of the experimental samples are stained with the complete staining panel (Table 1).- Create an experiment, add and name the number of tubes in the flow cytometer software, and create scatter plots to visualize the stained cells for the complete set of fluorochromes.
- Apply the previously established compensation settings to the experiment. Adjust the forward scatter (FSC) and side scatter (SSC) with unstained wt thymocytes until the complete population of cells is visible in the scatterplot. Measure the unstained Tg/Tg mTurquoise2-expressing thymocytes first to make sure the positive population is visible.
- Measure a Tg/Tg fully stained thymocyte sample, and check for all fluorochrome combinations. If necessary, adjust the previously established compensation values for the fluorochromes that show incorrect compensation. Measure the rest of the experimental samples, and do not adjust any settings during the measurement of the samples.
NOTE: Subsequent compensation adjustment can also be performed in the analysis software package. Although the number of available cells could be a limiting factor, it is advisable to perform this step during measurement. Keep the settings equal between all samples for comparison of the mTurquoise2 intensity values.
- Label the beads with each individual fluorochrome used in the experiment, and include unstained beads. Measure the beads to set up a compensation setting panel, and measure mTurquoise2-expressing 293T cells as there is no matching fluorochrome for mTurquoise2, which can be used on the beads. Save the compensation settings for use in the actual experiment.
4. Flow cytometric analysis
NOTE: Flow cytometric analysis was performed using specific software mentioned in the Table of Materials; however, other flow cytometric analysis programs are also available.
- Gate live cells and thymocyte subsets according to FSC and SSC values.
- Check the compensation settings in the compensation matrix dialog box found to the left of the sample to ensure no fluorochrome interference and that proper compensation has taken place to avoid spill-over or bleed-through.
- If the acquisition-defined matrix must be adjusted, change the values in the compensation matrix by simply increasing or decreasing the compensation value of every fluorochrome combination so that the population does not show a nearly perfect horizontal or vertical line (Supplemental Figure 1B).
- To visually spread the mTurquoise2 intensity for proper positive gating, change the mTurquoise2 X-axis display to linear (Supplemental Figure 2).
- Use the mTurquoise2 FMO control as negative control for mTurquoise2 positive signal gating. Revise the correct threshold gating per cell population (Figure 2).
NOTE: The (wt) mTurquoise2 FMO control cells do not have the mTurquoise2 marker and hence, can be used as a background threshold for Axin2 reporter activity.
- Use the mTurquoise2 FMO control as negative control for mTurquoise2 positive signal gating. Revise the correct threshold gating per cell population (Figure 2).
- Gate mTurquoise2-positive cells with the appropriate detection channel to define how many cells are Wnt-positive.
- Calculate the geometric mean and median to define the amount of fluorescent intensity in the cells of interest.
- Click on Statistics | Add statistic within the panel showing mTurquoise2-positive cells.
- Define the statistic method, the population of interest, and the detection channel of mTurquoise2, and click on Add. Represent the geometric mean and the median fluorescent intensity in Arbitrary Units (AU) to plot graphs.
NOTE: The median represents the middle value of the fluorescent intensity and hence, provides information about fluorescent intensity population shift. If required, a background correction can help to obtain clearer visualization of the dynamic range of the mTurquoise2 reporter activity. This can be done by subtracting the wt background staining frequencies from the total frequency of mTurquoise2-positive cells of the specific cell type that is gated.
5. Preparation of thymocyte cytospins for confocal imaging
NOTE: Thymocyte cytospins are recommended when working with cell suspensions of non-adhering cells. As the expression of Axin2-mTurquoise2 in thymocytes is lower than in the thymus epithelial cells, filtered thymocyte cell suspensions were used for imaging.
- Start with a cell suspension of freshly harvested or frozen thymocytes. Suspend ~20,000 thymocytes in 100 μL of cold PBS/0.5% BSA/10% FCS per thymocyte genotype.
NOTE: If required, thaw the cells gently to preserve maximum cell viability when working with previously frozen thymocytes. This will aid in less autofluorescence when imaging the cells. Check for cell viability to ensure the quality of the sample. The cytospin procedure employs a mechanical force that has been adapted to the fragile thymocyte; nonetheless, it requires a highly viable starting population. To ensure higher viability, it is advisable to start with freshly harvested instead of frozen thymocytes. - Prewet the area around the opening of the filter cards with PBS. Assemble the cytospin sample chamber holder according to the manual (Figure 1C).
- Place the filter card on the frost slide (smooth side against the glass slide). Place both items on the sample chamber holder. Take care in placing the filter card exactly on the sample chamber holder hole, and place the complete sample chamber holder in the rotor.
- Carefully resuspend the thymocytes, and add 100 μL of the cell suspension in the sample chambers. Spin the thymocyte suspension for 4 min at ~350 × g onto the frost slides. Remove the filter card carefully from the frost slide without touching the cells. Air-dry the cytospins for a period ranging from 1 h up to overnight at room temperature.
NOTE: When working with other cell types, test different cell densities for optimal results. Cytospins can be frozen at -20 °C in a sealed box for later experimentation. Thaw the cytospins for further handling for 1 h at room temperature.
6. Cytospin immunostaining with total β-catenin
- Fix the cytospins for 15 min at room temperature in 100% methanol. Air-dry the slides for 10 min at room temperature. Draw a circle around the thymocyte population on the glass slide with a hydrophobic pen.
NOTE: This fixation step is optimized specifically for β-catenin staining. - Place the slides in PBS/0.05% Tween-20 for 10 min at room temperature, and then, transfer them to a dark humid box during the blocking and incubation steps. Add 100 μL of PBS/10% normal mouse serum (NMS) per slide, and leave in the humid box for 10 min at room temperature. Tap the slide to remove the 10% NMS, add 100 μL of PBS/10% normal goat serum (NGS) per slide, and leave in the humid box for 30 min at room temperature.
NOTE: Incubation with PBS/10% NMS blocks non-specific primary antibody binding (Figure 1D), while PBS/10% NGS blocks non-specific secondary antibody binding. - Prepare additional antibodies for cellular staining. Mix 0.5 μg of the total β-catenin antibody with the AF568-labeled fragments.
NOTE: In this setting, a commercially available labeling kit was used for pre-labeling of the primary anti-mouse total β-catenin with secondary goat-anti-mouse IgG1 Fab fragments with Alexa Fluor 568 (AF568) fluorochrome label before adding it to the thymocytes. Perform the labeling according to the manufacturer's protocol as several concentrations might need to be tested. Use the total β-catenin-AF568-labeled antibody within 30 min. - Add 50 μL (0.5 μg) of the total β-catenin-AF568-labeled antibody per cytospin slide overnight at 4 °C in a humid box. Include a negative staining control according to the manufacturer's protocol or an isotype control in case of a direct labeling protocol.
- Wash for 20 min with PBS/0.05% Tween-20 at room temperature. Then, wash for 20 min with PBS at room temperature in a jar with stirring. Perform a second fixation step to ensure the binding of the antibody to the antigen: 10 min at room temperature with 100 μL of 4% paraformaldehyde (PFA) in PBS in a humid box.
NOTE: Keep the slides in the dark. Neither methanol nor PFA fixation will significantly affect the mTurquoise2 expression27. - Dip the slides in PBS. Perform nuclear staining with 50 μL of TO-PRO-3 (1:1500) for 10 min at room temperature in the humid box. Wash the slides for 20 min with PBS at room temperature in a jar with stirring.
NOTE: The TO-PRO3 concentration can be titrated depending on the use of other fluorochromes with nearby fluorescent spectra. - Embed the specimens with an antifade reagent according to the manufacturer's protocol, and cover with a coverslip. Air-dry for 24 h at room temperature. View the slides directly under a fluorescent or confocal microscope, or store at -20 °C for later imaging.
7. Confocal microscopic measurement
NOTE: See the Table of Materials for information about the confocal microscope.
- Switch on the confocal microscope according to the manual or established protocol. Use negative and positive stably transfected mTurquoise2 293T cell line controls for primary adjustment of the confocal settings. Subsequently, use wt Axin2-mTurquoise2 and Tg/Tg (knock-out) Axin2-mTurquoise2 thymocytes as negative and positive controls, respectively, to ensure no underexposure of the mTurquoise2 signal.
- Prepare the software for sequential scanning by programming the lasers and filter widths. Start with the highest wavelength laser line first, and work toward the lowest wavelength. When all sequential scanning steps are installed, load the sample on the microscope stage, focus the sample, and press Live to optimize the laser power and Smart Gain using the respective buttons on the confocal software or optional manual panel.
NOTE: The specimen section of the slide should not be imaged for signal quantification as potential photobleaching could potentially occur. However, mTurquoise2 has high photostability25. - In case of very low mTurquoise2 expression, increase the laser power and Smart Gain until a fluorescent signal is observed, and check with the negative control sample to ensure a true positive signal. Visualize the thymocytes with a 40x 1.4 oil lens, 63x 1.4 oil lens, or 100x 1.4 oil lens.
NOTE: A Leica SP5 microscope was used for this study. - Use these confocal imaging settings on the microscope before measuring the sample.
- Adjust the intensity value range to a 12-bit image by clicking on Configuration | Settings | change to 12-bit in the Bit depth option to create a broader scale of intensity and thus, more distinction between low and high fluorescent signals.
- Adjust the imaging resolution by clicking on XY, and increase the Format auf 1024 x 1024, which will also double the scanning time. Adjust the scan speed to 400-600 Hz by clicking on Speed | More to manually change the settings. Additionally, activate the Bidirectional scanning option.
- Adjust the sensitivity slider to reduce the background signal. Optimize the correct laser power and Smart Gain with the Quick LUT (Look-Up Table) option.
NOTE: In the 12-bit image, the slider has a grey scale intensity value from 0 to 4095. This can also be done afterwards with the free offline Las X software. The green color will show the black background, and the blue color shows saturated pixels of the sample.
- When all the imaging settings are optimized, measure the sample by clicking on Start, which will initiate the sequential imaging of all three channels.
- Measure the TO-PRO-3 nuclear fluorescent signal. Detect TO-PRO-3 with the 633 nm laser and HyD 640-750 nm.
NOTE: In this setup, 6% laser power was used at 15% smart gain. This setting can change depending on the intensity of TO-PRO-3 staining. If very bright, it could influence lower-intensity fluorochromes when overexcited. In such a case, reduce the staining concentration. - Measure the β-catenin nuclear and cytoplasmic fluorescent signals. Detect β-catenin with the 561 nm laser and HyD 580-605 nm. Accumulate up to 2 scans for one image by adjusting the setting in the XY box in the software with line average 2 in the case of a low AF568 signal.
NOTE: In this setup, 85% laser power was used at 87% smart gain. - Measure the mTurquoise2 cytoplasmic fluorescent signal. Detect mTurquoise2 with the 458 nm laser and HyD 490-600 nm. Accumulate up to 4 scans for one image by adjusting the setting in the XY box in the software with line average 4 in the case of a low mTurquoise2 signal.
NOTE: Due to old lasers on the confocal microscope used for this protocol and the low mTurquoise2 signal, 405 nm was used at 90% laser power, along with the 458 nm and 476 nm lasers at 100% laser power with HyD 490-550 nm at 100% smart gain. A 440 nm laser is most optimal, albeit less commonly present on a confocal microscope. High laser power should be handled with care and only performed with sequential imaging. Make sure emergency settings are in place to avoid detector overexposure. The laser power on other confocal microscopes could be inferior to the ones proposed in this protocol due to more potent or newer lasers. A bleaching test could be performed to ensure no fluorescent signal is lost before imaging. In the proposed setup, the photobleaching of mTurquoise2 was acceptable. - Perform brightfield imaging for total cell visualization. Detect thymocytes with the 488 nm laser and PMT Scan-DIC. Export the Lif files for image analysis.
NOTE: In this setup, 59% laser power was used with a gain of 212 V and data offset of -4.3%. Lif files can be read in the offline LAS x software for image correction.
- Measure the TO-PRO-3 nuclear fluorescent signal. Detect TO-PRO-3 with the 633 nm laser and HyD 640-750 nm.
8. Confocal microscopy analysis
- Analyze the images using an image processing software28 (Supplemental Figure 3). Load the images in the software.
NOTE: Multiple formats are accepted, but TIFF files with LUT or direct import of Lif files into the image processing software are recommended. - Measure the active β-catenin signal in the thymocyte nuclei.
- Select the nuclei of the TO-PRO-3-stained thymocytes in the red grey value image for the analysis of active β-catenin. Do this manually, or use automated cell selection in the software.
NOTE: Automated cell selection might need image processing for proper thresholding and particle analysis. Manual cell selection can be laborious, but generally does not require any image processing and is recommended in this protocol. - Activate manual selection with any of the selection tools in the work bar.
- Select the contour of the nucleus, and add it to the Region of Interest (ROI) manager. Activate the ROI manager by clicking on Analyze | Tools | ROI Manager, and when a new ROI manager window opens, click on the first option Add (t), or use the keyboard shortcut t. Repeat the previous step until all the nuclei are defined and added to the ROI manager. Use the Show all option in the ROI manager to visualize the selected cells.
- Select >3 background areas where no cells are present, and add these to the ROI manager.
NOTE: Size and shape are unimportant in this case. These regions will serve as background noise measurements for the final calculation.- Define the measurement on the image by clicking on Analyze | Set Measurements. In the new window that opens with different measurement options, activate Area, Integrated Density, and Mean grey value | click on OK.
- Activate the β-catenin grey value image by clicking on it, and visualize the selected nuclei and background areas by clicking on Show all in the ROI manager. Observe all selected areas that are now visible in the β-catenin image.
- Click on Measure in the ROI Manager or click on Analyze | Measure. Observe the new Ergebnisse window that opens, showing the results of the β-catenin signal within the ROIs.
- Transfer the results to a spreadsheet calculation program by clicking on Edit | Select all; and copy/paste the list into the spreadsheet for further calculation. Save the ROI manager for future reference without having to repeat the nuclei selection by clicking on More | Save….
- For automated cell selection, make Duplicates (Keyboard Ctrl D) of the image to be processed as many image processing settings cannot be undone.
NOTE: Automated nuclear labelling requires image processing, in most cases, to define the nuclei automatically and accurately. Image processing should only be done for area selection purposes. Processed images are not useful for fluorescent intensity measurement because the pixel values are altered.- Perform a Gaussian filter by clicking on Process | Filters | Gaussian Blur to smoothen the image. Test multiple Sigma (Radius) values, and activate the Vorschau option to visualize the effect before clicking on OK.
- Invert the image by clicking on Edit | Invert. Check the Brightness and Contrast by clicking on Image | Adjust | Brightness/Contrast. Use the Auto option or preferably, manually change the values.
NOTE: Do not Apply the changes as this would alter the image properties. Simply close the B&C window when the desired image has been obtained. - Create a Threshold by clicking on Image | Adjust | Threshold, and define the best Threshold settings where all cells are mostly visible. Click on Apply to apply the Threshold settings.
NOTE: If a threshold has not been applied to the cells, which show holes, click on Process | Binary | Fill Holes to fill in the gaps within the cells. If cells are fused together with the threshold settings, click on Process | Binary | Watershed to separate these cells. A fine 1-pixel line will separate any cell the program interprets as fused. - Define the smallest and largest nucleus in the image by manually selecting a nucleus of choice with the Freehand selection tool, add it to the ROI manager, and measure the Area.
- Analyze the particles (nuclei) by clicking on Analyze | Analyze Particles, and insert the smallest Area and the largest Area in the Size (^2) box with a hyphen (–) in between. Activate the boxes Display results, Add to manager, and Exclude on edges before clicking on OK. Continue with step 8.2.3 to complete the protocol.
- Select the nuclei of the TO-PRO-3-stained thymocytes in the red grey value image for the analysis of active β-catenin. Do this manually, or use automated cell selection in the software.
- Measure the cytoplasmic mTurquoise2 and β-catenin signals in the thymocytes.
- Select the contour of whole thymocytes in the brightfield image following the same steps as above.
- Select >3 background areas where no cells are present, and add these to the ROI manager.
NOTE: Size and shape are unessential in this case. These regions will serve as background noise measurements for the final calculation.- Activate the mTurquoise2 grey value image by clicking on it, and visualize the selected total cell ROIs and background areas by clicking on Show all in the ROI manager. Observe all the selected areas that are visible in the mTurquoise2 image.
- Click on Measure in the ROI Manager, or click on Analyze | Measure. Observe the new Ergebnisse window that opens up with the measurement results of the mTurquoise2 signal.
- Transfer the results to a spreadsheet calculation program by clicking on Edit | Select all, and copy/paste the list into the spreadsheet for further calculation.
- Activate the β-catenin grey value image by clicking on it, and visualize the selected total cell ROIs and background areas by clicking on Show all in the ROI manager. Observe all the selected areas that are visible in the β-catenin image.
- Click on Measure in the ROI Manager, or click on Analyze | Measure. Note the new Ergebnisse window that opens up with the measurement results of the total cellular β-catenin signal.
- Transfer the results to a spreadsheet calculation program by clicking on Edit | Select all, and copy/paste the list into the spreadsheet for further calculation. Save the ROI manager for future reference without having to repeat the cell selection by clicking on More | Save….
- Calculate the Corrected Total Nuclear Fluorescence (CTNF) for active β-catenin using equation 1.
CTNF = Integrated density – (Area × average of Mean background areas) (1) - Calculate the Corrected total Cell Fluorescence (CTCF) for mTurquoise2 using equation 2.
CTCF = Integrated density – (Area × average of Mean background areas) (2) - Differentiate between active nuclear β-catenin and cytoplasmic β-catenin by subtracting the nuclear β-catenin values obtained in step 8.2.3.3. from the total cell β-catenin values obtained in step 8.3.2.5 to obtain the cytoplasmic inactive β-catenin.
NOTE: Make sure the measurements are done within the same cell.- Calculate the average of the Mean intensity of the background areas. Calculate CTNF and CTCF using equations 1 and 2. Consider IntDen (the sum of all the pixels within the selected area) as the Integrated density and not the RawIntDen.
- If needed, calculate the Standard deviation of the IntDen values for plotting graphs. Consider up to 200 separate cells for statistical analysis using the Mann-Whitney U-test.
- Plot the results in an individual data point graph, and label the y-axis with the CTNF or CTCF values as Relative Fluorescent Units (RFU).
Representative Results
To investigate the role of canonical Wnt signaling, an Axin2-mTurquoise2 canonical Wnt reporter model has been tested in combination with β-catenin protein expression. Thymocytes are known to be fragile, show low canonical Wnt signaling at several stages in the thymocyte maturation process, and have a low cytoplasmic to nuclear ratio; all these factors hinder the detection of cytoplasmic mTurquoise2 or β-catenin. By following the protocol, murine Axin2-mTurquoise2 thymocytes were harvested from the thymus and processed into single-cell suspensions for flow cytometric and cytospin confocal analysis (Figure 1) of both Axin2-mTurquoise2 and total β-catenin.
Flow cytometric analysis facilitates the characterization of the different thymocyte maturation stages to measure the presence of the mTurquoise2 fluorochrome per cell subset as reporter protein for active canonical Wnt signaling. In the Axin2-mTurquoise2 genotypes-wildtype (wt), heterozygote (Tg/0), and homozygote (Tg/Tg)-mTurquoise2 signal was present in increasing levels, which represents the activation level of canonical Wnt signaling within double-positive (DP) thymocytes (Figure 2). As there is no inserted mTurquoise2 protein, wt mTurquoise2 levels demonstrate background noise; however, canonical Wnt signaling could still occur in these cells, but is simply not visualized through a reporter. Notwithstanding, the lack of one (Tg/0) or two (Tg/Tg) of the Axin2 genes can affect the canonical Wnt signaling activity as Axin2 plays an important role in the destruction complex to negatively regulate active canonical Wnt signaling.
Either mean or median fluorescent intensity can be examined to investigate the expression levels of the Axin2-mTurquoise2 reporter model. The median fluorescent intensity and geometric mean (Figure 2C) are the first and second most preferred graphical representations for fluorescence histograms. The increase in Axin2 expression in the Tg/Tg compared to the Tg/0, hints at incremented activation of canonical Wnt signaling due to the lack of functional Axin2 and thus, dysfunctional destruction complex. To further verify the activation levels of the canonical Wnt signaling pathway, a cytospin immunostaining was performed with total β-catenin within Axin2-mTurquoise2 thymocytes. As the cellular location of β-catenin indicates whether the canonical Wnt signaling is activated, we have measured the presence of either nuclear or cytoplasmic β-catenin.
mTurquoise2 is expressed in the cytosol and is primarily visible surrounding the nuclei (depicted in TO-PRO-3 red in Figure 3A). As thymocytes have very little cytoplasm, area selection should be done carefully to measure all of the signal (Supplemental Figure 3). Special care should be taken with false-positive staining or autofluorescence signal, as indicated by the white arrows. These fluorescence signals are normally produced by cell debris and were both visible in the mTurquoise2 and AF568 images (Figure 3B,C and Figure 3D,E). Unstained wt control images show that mTurquoise2 is also visible in these thymocytes although they do not contain the Axin2-mTurquoise2 reporter construct. This background noise is probably due to autofluorescence and the compact cytoplasm in thymocytes29. However, with careful area selection and correct background correction using the CTCF formula, Figure 3B shows an increasing Axin2-mTurquoise2 expression in pan-thymocytes as seen in the flow cytometric analysis in DP thymocytes.
To further understand the influence of the damaged Axin2 gene due to the Axin2-mTurquoise2 reporter construct on the destruction complex and thus, the presence of β-catenin, we measured the expression of either nuclear or cytoplasmic β-catenin AF568 in thymocytes. Active canonical Wnt signaling is driven by β-catenin migration into the nucleus where it will interact with TCF/LEF transcription factors and subsequently activate Axin2 as a target gene to dampen the pathway activation. As Axin2 forms part of the destruction complex that plays an important role in targeting the cytoplasmic β-catenin to proteasomal degradation, the absence of, or disruption of Axin2 protein, could cause an accumulation of either nuclear and/or cytoplasmic β-catenin.
We show that heterozygote (Tg/0) Axin2-mTurquoise pan-thymocytes have less nuclear and cytoplasmic β-catenin expression compared to wildtype (wt), suggesting that the regulation of β-catenin itself is altered. However, in homozygote (Tg/Tg) Axin2-mTurquoise pan-thymocytes, the nuclear β-catenin is higher than in wt, although the cytoplasmic β-catenin is similar between both genotypes (Figure 3C). This suggests that measuring total β-catenin levels can give additional information on the canonical Wnt pathway as opposed to directly measuring unphosphorylated β-catenin, which specifically detects β-catenin that is not destined for proteasomal degradation. Nonetheless, it should be borne in mind that the regulation of β-catenin toward canonical Wnt driven gene expression, such as of Axin2, is regulated by several other proteins that have not been tested in this protocol.
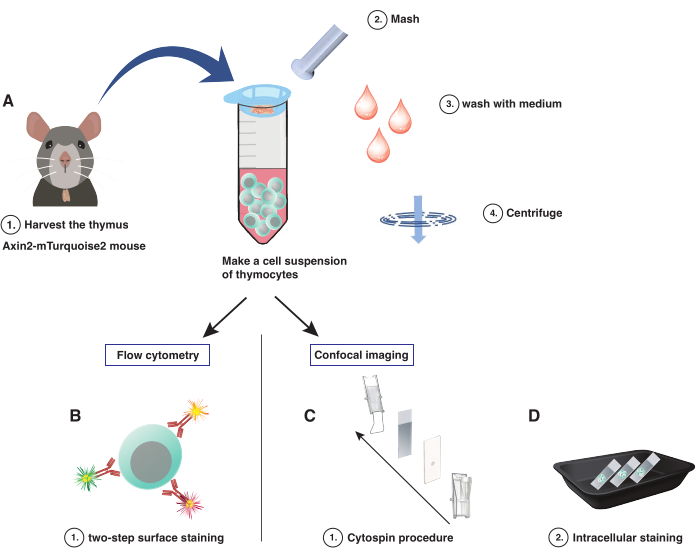
Figure 1: Schematic and simplified overview of the protocol. (A) Thymus processing into cell suspension, (B) flow cytometry protocol, (C) cytospin assembly, (D) intracellular staining protocol. Please click here to view a larger version of this figure.
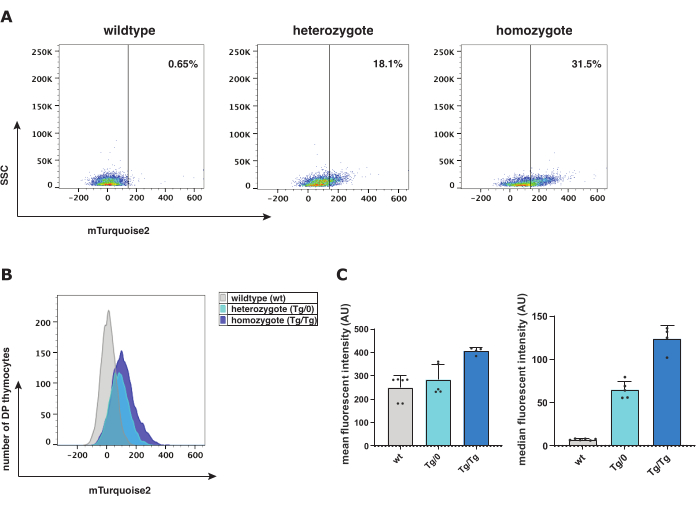
Figure 2: Flow cytometry analysis of wildtype (wt), heterozygote (Tg/0), and homozygote (Tg/Tg) Axin2-mTurquoise2 mice. (A) Representation of dot plot with mTurquoise2 DP thymocyte gating and population displacement according to Axin2-mTurquoise2 genotype. (B) Representation of a mTurquoise2 histogram showing the fluorescence intensity ranges between the mouse genotypes of DP thymocytes. (C) Bar graph representation of the mean and median fluorescent intensity with standard deviation error bars of the Axin2-mTurquoise2 genotypes in DP thymocytes (total of 5 wt; 5 Tg/0, and 4 Tg/Tg mice). Abbreviations: DP = double-positive. Please click here to view a larger version of this figure.
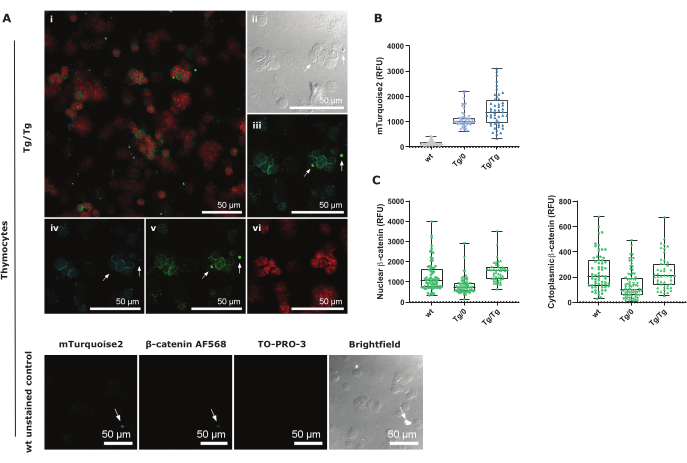
Figure 3: Confocal image representation of total thymocyte cytospin procedure and controls imaged at 40x with 1.5 zoom factor. (A) Tg/Tg (homozygote) Axin2-mTurquoise2 thymocytes stained with nuclear TO-PRO-3 and total β-catenin AF568, as well as endogenous cytoplasmic mTurquoise2 expression. i is an overlay confocal image of all three colors; ii is a brightfield image; iii is Axin2-mTurquoise2 and total β-catenin AF568 overlay; iv is a mTurquoise2 close-up; v is a total β-catenin AF568 close-up; vi is a nuclear TO-PRO-3 close-up. The lower panel contains wt unstained control images for each imaged channel. White arrows represent an identical location in all images and demonstrate the false-positive signal due to debris. Scale bars = 50 µm. (B) Boxplot representation of CTCF mTurquoise2 intensity values for all Axin2-mTurquoise2 genotypes (50-70 cells per genotype). (C) Boxplot representation of the total β-catenin AF568 CTNF and CTCF-CTNF intensity values for nuclear active β-catenin and inactive cytosolic β-catenin, respectively, for all Axin2-mTurquoise2 genotypes (50-70 cells per genotype). Abbreviations: CTCF = Corrected Total Cell Fluorescence; CTNF = Corrected Total Nuclear Fluorescence; RFU = Relative Fluorescent Units. Please click here to view a larger version of this figure.
| DN staining panel | Fluorochrome | Antibody |
| FITC | CD127 | |
| PE | CD25 | |
| PE-Cy7 | Streptavidin (SAV) | |
| APC | CD117 | |
| APC-Cy7 | CD44 | |
| PerCP | CD135 | |
| V450 | x | |
| V500 | x | |
| Lin- | ||
| Biotin | Ter119 | |
| GR1 | ||
| CD11b | ||
| B220 | ||
| NK1.1 | ||
| CD3 | ||
| CD4 | ||
| CD8 | ||
| ISP/DP/SP staining panel | ||
| FITC | TCRb | |
| PE | TCRgd | |
| PE-Cy7 | CD4 | |
| APC | CD3 | |
| APC-Cy7 | Streptavidin (SAV) | |
| PerCP | CD8 | |
| V450 | x | |
| V500 | x | |
| Lin- | ||
| Biotin | Ter119 | |
| GR1 | ||
| CD11b | ||
| B220 | ||
| NK1.1 | ||
| 1. Stain the thymocytes with the Biotin lineage-negative (Lin-) panel. | ||
| 2. Stain the thymocytes with the thymocyte cell marker panel. | ||
Table 1: Cell surface characterization antibody panels for flow cytometry. Two-step DN thymocyte staining, two-step ISP, DP, and SP thymocyte staining. Abbreviations: DN = double-negative; DP = double-positive; SP = single-positive; ISP = immature single-positive; PE = phycoerythrin; APC = allophycocyanin; FITC = fluorescein isothiocyanate.
Supplemental Figure 1: mTurquoise2 and FITC fluorochrome compatibility (A) Fluorescence excitation and emission spectra of mTurquoise2 (blue) and FITC (green), representing minimal spectral overlap. Thin lines represent the laser lines to excite mTurquoise2 (405 nm) and FITC (488 nm). Unfilled curves represent the excitation spectra as the filled curves represent the emission spectra of the specified fluorochromes. Blue corresponds to mTurquoise2, and green corresponds to FITC. The bandpass filters (grey area overlaying the emission spectra) 470/20 and 530/30 for mTurquoise2 and FITC, respectively, were used during flow cytometry. (B) Software fluorochrome emission compensation matrix within the flow cytometric analysis program of Axin2-mTurquoise2 DP thymocytes showing the fluorochrome spectral bleed-through between mTurquoise2 (y-axis) and the other fluorochromes (x-axis) used for DP thymocyte characterization. No spectral bleed-through was detected between mTurquoise2 and FITC (green box), whereas spectral bleed-through problems were detected between mTurquoise2 and AmCyan cyan-like fluorochrome (red box). The V450 flow cytometer channel was used to measure the mTurquoise2 fluorochrome, which is represented on the y-axis as comp-mTurquoise2. Abbreviations: FITC = fluorescein isothiocyanate; DP = double-positive; V450 = Violet 450. Please click here to download this File.
Supplemental Figure 2: Flow cytometry software analysis workflow scheme for mTurquoise2 positive cells gating strategy. Stepwise explanation of adjusting the transform settings for better mTurquoise2 gating strategy. A representation of an Axin2-mTurquoise2 Tg/Tg (homozygote) double-positive thymocyte population. Please click here to download this File.
Supplemental Figure 3: Image analysis software workflow scheme for fluorescent intensity measurement. Stepwise explanation of selecting and measuring the fluorescent intensity data for the CTCF calculation of mTurquoise2 or CTNF calculation of active β-catenin-AF568. (A) Brightfield image; (B) an mTurquoise2 image; (C) nuclear TO-PRO-3 staining; (D) a total β-catenin AF568 image. Square boxes are background signal areas to be used in the CTCF and CTNF calculations. Scale bars = 50 µm. Abbreviations: CTCF = Corrected Total Cell Fluorescence; CTNF = Corrected Total Nuclear Fluorescence. Please click here to download this File.
Discussion
Several canonical Wnt reporters are available with differing reporter sensitivity and actual reporter proteins. Reporter models using synthetically introduced multimerized TCF/LEF binding sites are available with fluorescent reporter proteins; however, such repeats of transgenes can be lost during breeding or long in vivo experiments and can be sensitive to non-Wnt signals from surrounding genomic sequences that influence reporter expression. Therefore, the most used reporter remains the older variant Axin2-LacZ, despite the difficulty to use this in live cells.
The Axin2-mTurquoise2 canonical Wnt reporter model, offers the same reporter reliability as Axin2-LacZ, albeit with the simplicity of a bright and relatively stable fluorescent reporter protein. This cyan variant fluorescent protein is useful for long-term imaging and can be easily combined with the most commonly used antibody fluorochromes25. However, possible limitations regarding 3D penetration and autofluorescence should be considered when using this model29. As β-catenin nuclear stabilization is a canonical Wnt reporter driver, most molecular experiments require the detection of increased total or active β-catenin to verify active Wnt signaling. However, β-catenin expression is notoriously low and difficult to detect for which Axin2 might actually be a better marker. In this protocol, we explain how to combine the Axin2-mTurquoise2 reporter model with single-cell thymocyte cytological staining of nuclear total β-catenin-AF568 despite low fluorescent signaling.
Critical steps with this model are mainly related to the proper detection of the low Axin2-mTurquoise2 and β-catenin-AF568 expression. Hence, this protocol describes the maximum possible signal detection in thymocytes, which are known to have low cell viability, leading to increased autofluorescence. This is relevant for thymocytes, which undergo natural apoptosis during thymocyte selection under physiological setting in the thymus. Therefore, we believe that demonstrating the detection of low expression of both Axin2-mTurquoise2 and β-catenin-AF568 in these cells will promote the applicability of the Axin2-mTurquoise2 model.
To obtain reliable results, special care should be taken with the proper fine-tuning of the equipment. To ensure discrimination between true signal and background signal, the inclusion of several positive and negative controls are required to calibrate the flow cytometry and confocal imaging equipment correctly. We propose the use of stable mTurquoise2-expressing cell lines, such as 293T cells, as positive control due to their ease of transfection, steady-state canonical Wnt expression over a broad intensity spectrum, and sensitivity to Wnt pathway-activating compounds such as lithium chloride (LiCl), 6-bromoindirubin-3'-oxime, or CHIR9902131,32,33. It is of utmost importance to use controls with the exact same mTurquoise2 reporter protein, as the excitation, the emission spectra and fluorescent intensity determinate the compensation values against spectral spill-over of other fluorochromes in flow cytometry or the definition of the detection filter ranges in confocal microscopy.
Additionally, a second Axin2-mTurquoise2 homozygote positive control of the cells of interest, which contains 2 times the Axin2-mTurquoise2 reporter construct, is recommended to adjust to physiologically expressed mTurquoise2 fluorescent intensity ranges, especially in the case of lowly expressing cells. Considering that canonical Wnt signaling is dosage-dependent which leads to fluctuating reporter expression, a negative control is necessary to exclude over-exposure of laser power, to define a reasonable signal/noise ratio and to define the true positive mTurquoise2 expression threshold.
As in flow cytometry, the addition of multiple characterization markers is conventional practice; matching fluorochromes should be chosen with minimal spectral spillover. The combination of FITC or Alexa Fluor 488 (AF488) with the mTurqoise2 reporter protein should give minimal spectral interference in the flow cytometer setting presented in this protocol. When comparing the fluorescent spectra of both fluorochromes, mTurquoise2 is minimally excited by the 488 laser (1% efficiency), which can be neglected especially in lowly expressing mTurquoise2 reporter cells. Therefore, any significant false positive FITC signal in thymocytes is unexpected. In the case of confocal microscopy and especially with the proposed confocal settings, the use of FITC or AF488 fluorochromes is unadvised as there is no possibility for compensation other than significant signal unmixing in an image processing software. Instead, other fluorochromes, such as AF568, should be selected to fully detect the low mTurquoise2 expression without any spectral overlap problems.
When working with high-mTurquoise2-expressing cells or having the availability of a 440 nm laser on the confocal microscope and narrowing of the emission filter range, the use of FITC or AF488 could be possible, however, Axin2 expression is known to be low in most adult tissues. In our protocol, we have measured total β-catenin expression with a pre-labelled two-step high performance AF568 labeling procedure that ensures effective immunostaining of low conjugate-stability proteins such as β-catenin. The steps in the immunostaining protocol have been optimized to measure true positive β-catenin in either cytoplasm or nucleus without the presence of high background signal. A similar staining protocol can be used on primary cultures and cryosections, however, when working with different cell types, the fixation steps should be tested. The Axin2-mTurquoise2 model only has a reporter function and therefore, would not be useful for cell tracing experiments such as other Axin2 knock-in models22. In fact, these elegant Cre-recombining Wnt models are mostly useful for tissue imaging experiments and not for cell suspensions that lose their environmental context. Even though the Axin2-mTurquoise2 model disrupts the Axin2 gene functionality due to its genetic insertion, this feature is useful for studying Axin2 knock-out models for Wnt therapeutic targets.
A homozygote mouse lacks Axin2 functionality, which impedes its protein interaction for the phosphorylation of β-catenin in the destruction complex34; however, the mTurquoise2 reporter expression helps to show whether canonical Wnt signaling remains active through an alternate pathway. Of note, Axin2 also plays an important role in the Wnt frizzled/LRP receptor complex upon Wnt ligand binding, offering another interesting Wnt regulation point in the signaling cascade35. Apart from the Axin2-mTurquoise2 murine model, a similar reporter construct is useful for transient transgenesis and can be specifically targeted to the endogenous Axin2 locus through CRISPR-Cas9 technology21. In summary, this report describes an easy and robust manner to analyze the Axin2-mTurquoise2 reporter model for low-Axin2-expressing thymocytes. This protocol can be applied to other canonical Wnt expressing cell types for drug screenings and functional Wnt therapeutic target definition.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by a grant from Leiden University for the profiling Area Regenerative Medicine to develop novel mouse models.
Materials
| BD FACScantoII flow cytometer | BD Biosciences | not aplicable | Serial number V96300710. The flow cytometer setup in this protocol contains a 405 nm laser line with 505 longpass filter and 530/30 nm bandpass filter, and 470/20 nm bandpass filter; a 488 nm laser with 735 nm longpass filter and 780/60 nm bandpass filter, 670 nm longpass filter and 655 nm longpass filter, 610 nm longpass filter, 550 nm longpass filter and 575/26 nm bandpass filter, 505 nm longpass filter and 530/30 nm bandpass filter, and 488/10 nm bandpass filter; and a 633 nm laser line with 735 nm longpass filter and 780/60 nm bandpass filter, 685 nm longpass filter, and 660/20 nm bandpass filter. |
| BSA | Sigma | A9647 | |
| Corning 70 μm cell strainer | Falcon/Corning | 352350 | |
| Cytospin 4 Type A78300101 | Thermo Scientific | not aplicable | |
| DMSO | Sigma Aldrich | D5879-1L | |
| DNAse I | Sigma | A9647 | |
| Falcon 50 mL Conical Centrifuge tubes | Greiner bio-one | 227261 | |
| Falcon round-bottom Polystyrene Test tubes with cell strainer snap cap | Fisher Scientific | 352235 | |
| Fetal Calf Serum (FCS) | Greiner Bio-One B.V. | not aplicable | Depends on origin |
| Fiji software | ImageJ | not aplicable | Version 1.53 |
| Filter card white (for cytospin) | VWR | SHAN5991022 | |
| FlowJo 10 software | Treestar | not aplicable | Version 10.5.3 |
| Frost slides | Klinipath | ||
| Gibco IMDM medium | Fisher Scientific | 12440053 | |
| HCX PL APLO 40x 1.4 OIL lens | Leica microsystems | not aplicable | |
| Hydrophobic pen: Omm Edge pen | Vector | not aplicable | |
| Leica TCS SP5 DMI6000 | Leica microsystems | not aplicable | The microscope setup in this protocol consisted of an HCX PL APO 40x/1.2 oil-immersion objective with 8-bit resolution, 1024 pixels x 1024 pixels, 400 Hz speed, pinhole 68 µm, and zoom factor of 1.5 at room temperature. This system contains a 405 diode laser, argon laser, DPSS 561 laser, HeNe 594 laser and HeNe 633 laser with 4 hybrid detectors (HyDs) and 5 photomultiplier tubes (PMTs). |
| Methanol | VWR | 1060091000 | |
| NaN3/sodium azide | Hospital farmacy | not aplicable | |
| Normal mouse serum | Own mice | not aplicable | |
| PBS | Lonza | BE17-517Q | |
| ProLong Diamond Antifade Mountant | Fisher Scientific | P36965 | |
| Purified mouse anti-β-catenin (CTNNB1) | BD Biosciences | 610154 | |
| TO-PRO-3 Iodide | Thermofisher | T3605 | |
| Transparent nailpolish | at any drugstore | not aplicable | |
| Tween-20 | Sigma Aldrich | P1379-500ml | |
| Zenon Alexa Fluor 568 Mouse IgG1 labeling kit | Thermofisher | Z25006 |
Referenzen
- Kahn, M. Can we safely target the WNT pathway. Nature Reviews. Drug Discovery. 13 (7), 513-532 (2014).
- Jung, Y. S., Park, J. I. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond beta-catenin and the destruction complex. Experimental & Molecular Medicine. 52 (2), 183-191 (2020).
- Gao, K., Zhang, T., Wang, F., Lv, C. Therapeutic Potential of Wnt-3a in neurological recovery after spinal cord injury. European Neurology. 81 (3-4), 197-204 (2019).
- Jia, L., Pina-Crespo, J., Li, Y. Restoring Wnt/beta-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Molecular Brain. 12 (1), 104 (2019).
- Bae, S. M., Hong, J. Y. The Wnt signaling pathway and related therapeutic drugs in autism spectrum disorder. Clinical Psychopharmacology and Neuroscience. 16 (2), 129-135 (2018).
- Tajer, P., Pike-Overzet, K., Arias, S., Havenga, M., Staal, F. J. T. Ex vivo expansion of hematopoietic stem cells for therapeutic purposes: Lessons from development and the niche. Cells. 8 (2), 169 (2019).
- Yanai, K., et al. Crosstalk of hedgehog and Wnt pathways in gastric cancer. Cancer Letters. 263 (1), 145-156 (2008).
- Blank, U., et al. An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Developmental Biology. 8, 86 (2008).
- Duncan, A. W., et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nature Immunology. 6 (3), 314-322 (2005).
- Jho, E. H., et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and Cellular Biology. 22 (4), 1172-1183 (2002).
- Lustig, B., et al. Negative feedback loop of Wnt signaling through upregulation of conductin/Axin2 in colorectal and liver tumors. Molecular and Cellular Biology. 22 (4), 1184-1193 (2002).
- Bernkopf, D. B., Hadjihannas, M. V., Behrens, J. Negative-feedback regulation of the Wnt pathway by conductin/axin2 involves insensitivity to upstream signalling. Journal of Cell Science. 128 (1), 33-39 (2015).
- Vassar, R., Rosenberg, M., Ross, S., Tyner, A., Fuchs, E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 86 (5), 1563-1567 (1989).
- DasGupta, R., Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 126 (20), 4557-4568 (1999).
- Maretto, S., et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proceedings of the National Academy of Sciences of the United States of America. 100 (6), 3299-3304 (2003).
- Mohamed, O. A., Clarke, H. J., Dufort, D. beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Developmental Dynamics. 231 (2), 416-424 (2004).
- Moriyama, A., et al. GFP transgenic mice reveal active canonical Wnt signal in neonatal brain and in adult liver and spleen. Genesis. 45 (2), 90-100 (2007).
- Currier, N., et al. Dynamic expression of a LEF-EGFP Wnt reporter in mouse development and cancer. Genesis. 48 (3), 183-194 (2010).
- Ferrer-Vaquer, A., et al. A sensitive and bright single-cell resolution live imaging reporter of Wnt/beta-catenin signaling in the mouse. BMC Developmental Biology. 10, 121 (2010).
- Jho, E. H., et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and Cellular Biology. 22 (4), 1172-1183 (2002).
- de Roo, J. J. D., et al. Axin2-mTurquoise2: A novel reporter mouse model for the detection of canonical Wnt signalling. Genesis. 55 (10), (2017).
- van de Moosdijk, A. A. A., van de Grift, Y. B. C., de Man, S. M. A., Zeeman, A. L., van Amerongen, R. A novel Axin2 knock-in mouse model for visualization and lineage tracing of WNT/CTNNB1 responsive cells. Genesis. 58 (9), 23387 (2020).
- Choi, Y. S., et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 13 (6), 720-733 (2013).
- Nolan, G. P., Fiering, S., Nicolas, J. F., Herzenberg, L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proceedings of the National Academy of Sciences of the United States of America. 85 (8), 2603-2607 (1988).
- Goedhart, J., et al. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93. Nature Communications. 3, 751 (2012).
- Bernkopf, D. B., Bruckner, M., Hadjihannas, M. V., Behrens, J. An aggregon in conductin/axin2 regulates Wnt/beta-catenin signaling and holds potential for cancer therapy. Nat Commun. 10 (1), 4251 (2019).
- Joosen, L., Hink, M. A., Gadella, T. W., Goedhart, J. Effect of fixation procedures on the fluorescence lifetimes of Aequorea victoria derived fluorescent proteins. Journal of Microscopy. 256 (3), 166-176 (2014).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnology Annual Reviews. 11, 227-256 (2005).
- Henriksson, J., et al. Endrov: an integrated platform for image analysis. Nature Methods. 10 (6), 454-456 (2013).
- Hedgepeth, C. M., et al. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Entwicklungsbiologie. 185 (1), 82-91 (1997).
- Sato, N., Meijer, L., Skaltsounis, L., Greengard, P., Brivanlou, A. H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine. 10 (1), 55-63 (2004).
- Ring, D. B., et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 52 (3), 588-595 (2003).
- Liu, C., et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 108 (6), 837-847 (2002).
- Zeng, X., et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 135 (2), 367-375 (2008).

.
