In vitro Monitoring of Extracellular pH in Real-Time
Summary
This article represents a useful in vitro assay to measure changes in extracellular pH during neutrophil (PMN) transepithelial migration (TEM)
Abstract
Early accumulation of neutrophils (PMN) is a hallmark of acute intestinal inflammation. This acute inflammation is either resolved or progresses to chronic inflammation. Without efficient PMN clearance at sites of infiltration, PMN can accumulate and contribute to chronic inflammatory conditions, including the intestinal diseases ulcerative colitis (UC) and Crohn’s Disease (CD). The pH in the distal colon in individuals with active UC can range between a pH of 5 and 6, whereas healthy individuals maintain colonic pH in the range of 6.8-7.4. Extracellular pH has been shown to influence both intestinal epithelial cells and the infiltrating immune cells. More specifically, extracellular acidosis significantly impacts PMN. At pH below 6.5, there are increases in the production of H2O2, inhibition of apoptosis, and increases in the functional lifespan of PMN. Given the significant presence of PMN and extracellular acidification at sites of inflammation, we developed a novel model that allows for the monitoring of extracellular pH during PMN transepithelial migration in real time. Here, we describe this model and how it can be utilized to measure both the apical and basal pH during PMN trafficking. This model can be utilized to monitor extracellular pH under a wide range of conditions; including, hypoxia, PMN transepithelial migration, and for extended periods of time.
Introduction
The extracellular microenvironment has been shown to play a significant role in modulating the inflammatory response. One aspect of the microenvironment which is often underappreciated is extracellular acidification. Extracellular acidification is often observed at sites of active inflammation, including mucosal disorders such as inflammatory bowel diseases. The luminal pH in the distal colon from patients with UC can range between a pH of 5 and 6, whereas healthy individuals have colonic pH’s in the range of 6.8-7.41,2. This decrease in colonic pH is of particular interest because extracellular pH has been shown to broadly influence IEC and infiltrating immune cell function. Acidic microenvironments, for example, have been shown to extend the functional life spawns of infiltrating PMN, stimulate H2O2 production, and inhibit PMN apoptosis3,4. The exact mechanism by which the extracellular environmental becomes acidic remains unclear, highlighting the need to develop techniques to study acidification over time.
In a recent study, it was demonstrated that PMN TEM results in a significant acidification of the microenvironment and that IEC rapidly respond to this acidification through the adaptive upregulation of SLC26A3, the major chloride-HCO3 transporter in mucosal tissue5,6, thereby promoting pH homeostasis7. The exact mechanism(s) by which PMN TEM induces extracellular acidification remains unclear. It is currently believed that tissue acidification is caused by increased accumulation of lactic acid resulting from enhanced glycolysis and it was recently observed that PMN TEM stimulates the release of lactate from IEC7,8. However, the levels of secreted lactate do not fully account for the acidification observed during PMN TEM. A better understanding of the mechanisms involved in extracellular acidification will allow for the potential identification of novel therapeutic targets. To better understand the mechanism involved there needs to be a system which allows for monitoring of pH over an extended period of time, while allowing PMN to transmigration in the physiologically relevant apical to basal direction. Using a pH probe is time intensive and requires repeated manipulation of the culture system. Currently, there are several non-invasive techniques to monitor pH over time. One commonly used assay utilizes 2′,7′-bis(2-carboxyethyl)-5,6-carboxyfluorescein (BCECF), a dye which is internalized by the cell, however this assay is limited to the study of internal pH 9. There are several pH plate assays, but most are only available in a 96-well format or require the addition of pH sensitive dyes. The protocol described below is based off a commercially available pH sensing plate which was designed for non-invasive monitoring of pH over an extended period of time10. Cells are grown as a monolayer in a 24-well plate that contains a pre-calibrated pH sensor. This sensor contains a luminescent dye which is excited by specialized plate reader. The luminescence lifetime, which is dependent on pH, is also measured by the plate reader. However, this model lacks an apical and basal compartment, preventing the study of transmigration or basal/apical differences in acidification.
Described is a protocol that allows for in vitro evaluation of extracellular acidification during PMN TEM, using human T84 intestinal epithelial cells grown on transwell inserts, human PMN, and a non-invasive fluorescent based pH sensor. Provided is a successful example where the extracellular pH is examined over the course of 10 hr and demonstrate active PMN TMN results in extracellular acidification. Although a specific example is presented, this protocol can be utilized to evaluate the effect of any number of factors; including, metabolites, cell types, and potential therapeutics, on extracellular pH.
Protocol
1. Cell Preparation
Day 1
- Warm cell culture media (DMEM/F12 with 5% FBS, 2 mM L-alanyl-L-glutamine dipeptide, and Pen Strep) in a 37 °C water bath for 20 min.
- Prepare the tissue culture hood by spraying it with 70% ethanol and wiping down the surfaces with paper towels.
- Spray and wipe tissue culture flasks and media, PBS, and trypsin bottles with 70% ethanol and bring them into the tissue culture hood.
- Aspirate the media from the cell culture flask and wash the cells with 12 mL of phosphate buffered saline (PBS).
- Aspirate the PBS and add 1 mL of trypsin to the cells. Incubate at RT for 10-20 min, until the cells detach.
- Add 11 mL of cell culture media to the trypsin and mix by pipetting up and down 3-5 times.
- Invert a 24-well cell culture dish containing 5 μm pore inserts and gently pipette 100 μL of the cell suspension to the underside of the insert (Figure 1).
- Place the inverted plate in a 37 °C incubator with 5% CO2.
Day 2 - Warm cell culture media in a 37 °C water bath for 20 min.
- Prepare the tissue culture hood by spraying it with 70% ethanol and wiping down the surfaces with paper towels.
- Spray and wipe tissue culture flasks and media, PBS, and trypsin bottles with 70% ethanol and bring them into the tissue culture hood.
- Right the tissue culture plate and gently add 1 mL of media to the basal compartment and 180 μL to the apical compartment.
- Place the plate in a 37 °C incubator with 5% CO2.
Day 3-7: - Monitor the transepithelial resistance (TER) daily, using a epithelial volt/Ohm meter, until the TER have stabilized and the cells have formed a confluent monolayer.
2. PMN Isolation
- Room temperature gradient solutions, 50 mM K2EDTA, and Hanks' Balanced Salt Solution (HBSS) without calcium/magnesium (HBSS-) to room temperature.
- Prepare double density gradients in 50 mL conical tubes.
- Coat a 60 mL syringe with 6 mL of 50 mM K2EDTA and collect 54 mL of blood from donor.
- Overlay 15 mL of blood to the double density gradients prepared above.
- Centrifuge at 700 x g for 30 min at room temperature, with the brake off.
- Aspirate the serum, peripheral blood monocyte, interphases and transfer the layer containing PMN to a new 50 mL conical tube and resuspend to 50 mL in HBSS-.
- Centrifuge at 700 x g for 10 min at room temperature.
- Aspirate the supernatant and resuspend the pellet in 40 mL of cold red blood cell lysis buffer.
- Centrifuge at 700 x g for 10 minutes at 4 °C.
- Aspirate the supernatant and combine the pellets from all tubes into 3 mL of HBSS-.
- Determine the concentration of PMN and dilute to 5×104 using the appropriate about of HBSS-.
3. In Vitro pH Monitoring Assay
- Warm HBSS with calcium/magnesium (HBSS+) in a 37 °C water bath for 20 min.
- Prepare the tissue culture hood by spraying it with 70% ethanol and wiping down the surfaces with paper towels.
- Spray and wipe HBSS+ with 70% ethanol and bring them into the tissue culture hood.
- h before the assay is performed, equilibrate the pH sensing plates by adding 1 mL of HBSS+ to each well and placing in a 37 °C incubator.
- min before the assay remove the pH sensing plates from the incubator and add 1 μM fMLP to each well.
- Place the pH sensing plate on the SDS reader at 37 °C and record the pH to establish a baseline for the experiment.
- Once the pH reading has stabilized remove media from inserts and add 180 μL HBSS+ to the top well.
- Add 20 μL HBSS+ to control wells or 20 μL PMN.
- Remove the HBSS+ and transfer the inserts to the pH sensing plate.
- To the bottom well, add 1 mL of HBSS+ with and without 1 μM fMLP.
- To the top well, add 180 μL ofHBSS+.
- To the top well, add 20 μl of HBSS- containing 0 or 1 x 106 PMN.
- Place the pH sensing plate on the SDS reader at 37 °C (Figure 2).
- On the attached computer, open the pH monitoring software and set up the software to record the pH at designated time intervals (every 1-10 min).
Representative Results
The results are usually via line graph to show change in pH over time (example shown in Figure 3A) or as a scatter plot showing extracellular pH at a single point in time (example shown in Figure 3B). Depending on experimental need, additional controls and treatments can be included. Additionally, this assay can be modified to monitor extracellular under a wide range of conditions. For example, the SDR reader can be placed in a hypoxic chamber and the extracellular pH can be monitored.

Figure 1: Inverted plating of T84 IEC. 100 μl of T84 IEC cell suspension was plated on the underside of 5 μm pore permeable inserts. Please click here to view a larger version of this figure.

Figure 2: Image of plate reader and 24-well pH sensing plate Please click here to view a larger version of this figure.
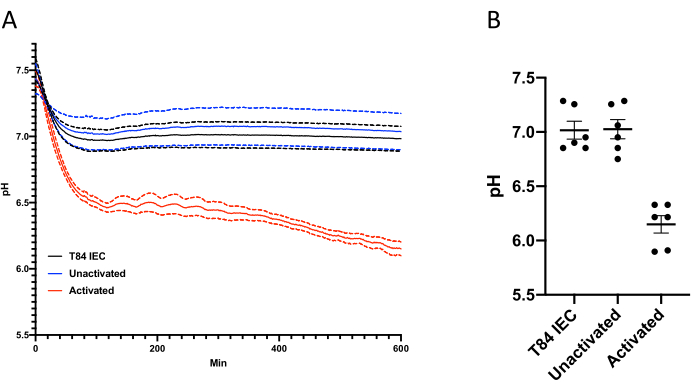
Figure 3: Change in extracellular pH over time. (A) Changes in extracellular pH over time. Extracellular pH in T84 cultures was recorded every minute for 600 min in the presence or absence of 1 x 106 PMN ± fMLP. (B) At 600 min the difference in pH between 0 min and 600 min were calculated. Data expressed as the mean ±SEM. Please click here to view a larger version of this figure.
Discussion
In this protocol, there are several key steps. Monolayers should be confluent, but not overconfluent. For T84 IEC, they should be used 7-10 days after plating. Human and murine enteroids grow at different rates than T84 IEC and the researchers should determine how long it takes each line to reach confluency. It is important that the researchers need to use minimally buffered media to ensure shifts in extracellular pH are observed. HBSS+ contains glucose and is suitable for experiments shorter than 12 h. T84 IEC incubated in HBSS+ for periods longer than ~16 h begin to loss barrier function. If researchers are interested in extended experiments complete media, containing FBS, should be used and the plates incubated in a 37 °C incubator with 5% CO2.
Researchers can modify this assay for different applications. For example, both IEC and PMN can be pre-treated with various compounds targeting metabolic or signaling pathways. Additionally, this assay can be expanded to include the use of other immune cells or the impact of various stimuli on IEC alone. Likewise, a similar model using an oxygen-sensitive fluorescent probe has been used to monitor oxygen consumption in real-time during PMN TEM11,12. Finally, researchers can use cell lines other than T84 human IEC. Human/murine enteroids can be grown as 2D monolayers and assayed as described here13.
All assays have their limitations. For this assay, the experiment is being carried out in a closed system. Extended incubations might be influenced by the depletion of a crucial nutrient or the accumulation of metabolites. Additionally, this assay lacks potential signaling from other parenchymal cells within the tissue environment. Therefore, further confirmation of the in vitro findings using other in vitro and in vivo methods would be beneficial.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
NA
Materials
| 10 mL serological pipettes | Corning | 4101 | |
| 24-well plate | Corning | CLS3527-100EA | |
| 5 mm pore inserts | Corning | 3421 | |
| 50 ml sterile conical tube | Corning | 0553855A | |
| 75 cm2 flask | Corning | 430641U | |
| DMEM/F12 | Gibco | 10565-018 | |
| FBS | Gibco | 26140 | |
| GlutaMax | ThermoFisher | 35050061 | |
| HBSS- | Sigma-Aldrich | H4891-10X1L | |
| HBSS+ | Sigma-Aldrich | H1387-10L | |
| Histopaque T1077 | Sigma-Aldrich | 10771-6X100ML | |
| Histopaque T1119 | Sigma-Aldrich | 11191-6X100ML | |
| HydroDish HD24 | PreSens | NA | https://www.presens.de/products/detail/hydrodish-hd24 |
| PBS | Gibco | 14190-144 | |
| Pen Strep | Gibco | 15140-122 | |
| RBC lysis buffer | ThermoFisher | 00-4333-57 | |
| SDR Reader | PreSens | NA | https://www.presens.de/products/detail/sdr-sensordish-reader-basic-set |
| Trypsin | Fisher Scientific | 25200114 |
Referenzen
- Roediger, W. E., Lawson, M. J., Kwok, V., Grant, A. K., Pannall, P. R. Colonic bicarbonate output as a test of disease activity in ulcerative colitis. Journal of Clinical Pathology. 37 (6), 704-707 (1984).
- Nugent, S. G., Kumar, D., Rampton, D. S., Evans, D. F. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 48 (4), 571-577 (2001).
- Trevani, A. S., et al. Extracellular acidification induces human neutrophil activation. Journal of Immunology. 162 (8), 4849-4857 (1999).
- Cao, S., et al. Extracellular Acidification Acts as a Key Modulator of Neutrophil Apoptosis and Functions. PLoS One. 10 (9), 0137221 (2015).
- Schweinfest, C. W., Henderson, K. W., Suster, S., Kondoh, N., Papas, T. S. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proceedings of the National Academy of Sciences of the United States of America. 90 (9), 4166-4170 (1993).
- Schweinfest, C. W., et al. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. Journal of Biological Chemistry. 281 (49), 37962-37971 (2006).
- Cartwright, I. M., et al. Adaptation to inflammatory acidity through neutrophil-derived adenosine regulation of SLC26A3. Mucosal Immunology. 13 (2), 230-244 (2020).
- Pucino, V., Bombardieri, M., Pitzalis, C., Mauro, C. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. European Journal of Immunology. 47 (1), 14-21 (2017).
- Ozkan, P., Mutharasan, R. A rapid method for measuring intracellular pH using BCECF-AM. Biochimica et Biophysica Acta. 1572 (1), 143-148 (2002).
- Naciri, M., Kuystermans, D., Al-Rubeai, M. Monitoring pH and dissolved oxygen in mammalian cell culture using optical sensors. Cytotechnology. 57 (3), 245-250 (2008).
- Campbell, E. L., et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 40 (1), 66-77 (2014).
- Abaci, H. E., Truitt, R., Luong, E., Drazer, G., Gerecht, S. Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. American Journal of Physiology-Cell Physiology. 298 (6), 1527-1537 (2010).
- Braverman, J., Yilmaz, O. H. From 3D Organoids back to 2D Enteroids. Developmental Cell. 44 (5), 533-534 (2018).

.
