Modeling Stroke in Mice: Focal Cortical Lesions by Photothrombosis
Summary
Described here is the photothrombotic stroke model, where a stroke is produced through the intact skull by inducing permanent microvascular occlusion using laser illumination after administration of a photosensitive dye.
Abstract
Stroke is a leading cause of death and acquired adult disability in developed countries. Despite extensive investigation for novel therapeutic strategies, there remain limited therapeutic options for stroke patients. Therefore, more research is needed for pathophysiological pathways such as post-stroke inflammation, angiogenesis, neuronal plasticity, and regeneration. Given the inability of in vitro models to reproduce the complexity of the brain, experimental stroke models are essential for the analysis and subsequent evaluation of novel drug targets for these mechanisms. In addition, detailed standardized models for all procedures are urgently needed to overcome the so-called replication crisis. As an effort within the ImmunoStroke research consortium, a standardized photothrombotic mouse model using an intraperitoneal injection of Rose Bengal and the illumination of the intact skull with a 561 nm laser is described. This model allows the performance of stroke in mice with allocation to any cortical region of the brain without invasive surgery; thus, enabling the study of stroke in various areas of the brain. In this video, the surgical methods of stroke induction in the photothrombotic model along with histological analysis are demonstrated.
Introduction
Ischemic stroke remains a principal cause of death and acquired adult disability in developed countries in the 21st century accounting for approximately 2.7 million deaths in 2017 worldwide1. Even with the immense efforts of the scientific community, few treatments are available. Furthermore, with such high exclusion criteria, these already limited options are not accessible to many patients, resulting in an urgent need for novel treatments to improve functional recovery after stroke.
Considering the incapability of in vitro models to replicate the complex interactions of the brain, animal models are essential for preclinical stroke research. Mice are the most frequently used animal model in the stroke research field. The majority of these mouse models aim to induce infarctions by blocking the blood flow within the middle cerebral artery (MCA) since the majority of human stroke lesions are located in the MCA territory2. Although these models better recapitulate human stroke lesions, they involve convulated surgeries with high infarct volume variability.
Since Rosenblum and El-Sabban's proposal of the photothrombotic model in 19773, and later the application of this model to rats Watson et al.4, it has become widely used in ischemic stroke research5,6. The photothrombotic stroke model induces a local and defined cortical infarct as a result of the photoactivation of a light-sensitive dye previously injected into the blood flow. This causes local thrombosis of the vessels in the areas exposed to light. Briefly, upon exposure to light from the injected photosensitive dye, localized oxidative injury of the endothelial cell membrane is induced, leading to platelet aggregation and thrombus formation, followed by local disruption of cerebral blood flow7.
The principal advantage of this technique resides in its simplicity of execution and the possibility to direct the lesion to the desired region. Unlike other experimental stroke models, minor surgical expertise is needed to perform the photothrombotic stroke model as the lesion is induced through illumination of the intact skull. Moreover, the well-delimited borders (Figure 2A and Figure 5B) and the flexibility to induce the lesion to a specific brain region can facilitate the study of cellular responses within the ischemic or intact cortical area8. For these reasons, this approach is suitable for the study of cellular and molecular mechanisms of cortical plasticity.
Over the past few decades, the growing concern regarding the lack of reproducibility between research groups has been coined the so-called replication crisis9. After the coordination of the first preclinical randomized controlled multicenter trial study in 201510, a proposed tool to improve preclinical research11,12,13, it was confirmed that one cause for failing reproducibility between preclinical studies from independent laboratories was the lack of sufficient standardization of experimental stroke models and outcome parameters14. Accordingly, when the ImmunoStroke consortium was established (https://immunostroke.de/), a collaboration which aims to understand brain-immune interactions underlying the mechanistic principles of stroke recovery, the standardization of all the experimental stroke models among each research group was essential.
Described here is the standardized procedure for the induction of the photothrombotic model as used in the above-mentioned research consortium. Briefly, an animal underwent anesthetics, received a Rose Bengal injection (10 µL/g) intraperitonally, and the intact skull, 3 mm left from bregma, was immediately illuminated by a 561 nm laser for 20 min (Figure 1). Additionally, a related histological and behavioral method to analyze the stroke outcome in this model is reported. All methods are based on standard operating procedures developed and used in the laboratory.
Protocol
The experiments reported in this video were conducted according to the national guidelines for the use of experimental animals, and the protocols were approved by the German governmental committees (Regierung von Oberbayern, Munich, Germany). The mice used in this study were male C57Bl/6J mice, 10-12 weeks old, and dispatched by Charles River Germany. The animals were housed under controlled temperatures (22 °C ± 2 °C), with a 12 h light-dark cycle period and access to pelleted food and water ad libitum.
1. Preparation of the material and instruments
- Dissolve Rose Bengal in 0.9% saline solution to reach a final concentration of 10 mg/mL. Connect the heat blanket to keep the operation area warm and maintain the mouse body temperature during anesthesia at 37 °C.
- Prepare scissors, forceps, pieces of cotton, dexpanthenol eye ointment, and suture material. Prepare a syringe with saline solution (without needle) to maintain the operation area hydrated. Prepare the anesthesia gas (100% O2 + isoflurane).
2. Preparation of the animal
- Inject analgesia 30 min before surgery (4 mg/kg Carprofen and 0.1 mg/kg Buprenorphine).
- Record the mouse body weight to adjust the dose of Rose Bengal to be injected (10 µL/g i.e., 100 µg/g).
- Place the mouse in the induction chamber with an isoflurane flow rate of 4% to anesthetize it until the spontaneous movement of the body and vibrissae stops.
- Transfer the mouse into the stereotactic frame and place it in a prone position with its nose into the anesthesia mask. Fix the animal and maintain the isoflurane concentration at 4% for 1 min. Then reduce and maintain the isoflurane concentration at 2%.
- Gently insert the rectal probe to monitor the temperature throughout the surgical procedures. Set the associated feedback-controlled heating pad to maintain the mouse body temperature at 37 °C.
- Apply dexpanthenol eye ointment to both eyes and clean the skin and surrounding fur with a disinfectant agent.
3. Photothrombosis model
- Make a 2.0-2.5 cm longitudinal incision and retract to expose the skull. Perform the skull exposure with a single cut to avoid wound complications.
- Remove the periosteum gently with cotton and identify the coronal sutures.
- Put on the protective glasses, switch on the 561 nm laser and mark the bregma +3 mm left.
- Switch off the laser, attach a sticker with a 4 mm diameter hole placed at the marked coordinates mentioned above.
- Inject the mouse with Bengal Rose (10 µL/g), intraperitoneally. Place the laser beam at 4-5 cm from the skull, switch on the 561 nm laser and illuminate the skull for 20 min.
- Apply two drops of 0.9% saline on the skull to rehydrate, suture the wound, and place the animal in a recovery chamber at 37 °C to recover from anesthesia. After 1 h, return the mice to their cages in a temperature-controlled room.
- Inject analgesia every 12 h for 3 days after surgery (4 mg/kg Carprofen and 0.1 mg/kg Buprenorphine).
4. Sham operation
- Carry out two different procedures of Sham operations as described in steps 4.1.1 and 4.1.2.
- Perform all the procedures identically to the operation described above. Inject Rose Bengal without switching on the laser. After 20 min under anesthesia, allow the animals to stay in the recovery chamber for 1 h to recover, before being returned to their cages.
- Perform all the procedures identically to the operation described above, switching on the laser. Do not inject Rose Bengal. After 20 min of laser illumination, allow the animals to stay in the recovery chamber for 1 h to recover from anesthesia, before being returned to their cages.
5. Laser speckle
- Connect the heated blanket to keep the operation area warm and maintain the mouse body temperature during anesthesia at 37 °C.
- Place the mouse into the induction chamber with an isoflurane flow rate of 4% to anesthetize it until the spontaneous movement of the body and vibrissae stops and then transfer the mouse into the stereotactic frame.
- Place the mouse in a prone position with its nose into the anesthesia mask. Fix the animal and maintain the isoflurane concentration at 4% for 1 min. T hen reduce and maintain it at 2%.
- Gently insert the rectal probe to monitor the temperature throughout the surgical procedures. Set the associated feedback-controlled heating pad to maintain the mouse body temperature at 37 °C and apply dexpanthenol eye ointment to both eyes. Clean the skin and the surrounding fur with a disinfectant agent.
- Make a 2.0-2.5 cm longitudinal incision and retract to expose the skull. Perform the skull exposure with a single cut to avoid wound complications.
- Place the sterotactic frame under the laser speckle and adjust the height to obtain a sharp image. Focus the laser speckle perfusion imaging (LSI) camera on the cranial window. Configure the high resolution laser speckle imaging (LSI) camera system as previously described15.
- Acquire data from a 1 cm x 1 cm field of view using a 785 nm wavelength and 80 mW lasers with a frame rate of 21 images/s at a working distance of 1 cm for 1 min.
- After imaging, apply two drops of 0.9% saline to the skull to rehydrate, suture the wound and place the animal in a recovery chamber at 37 °C to recover from anesthesia for 1 h. After 1 h, return the mice to their cages in a temperature-controlled room.
6. Neuroscore
NOTE: For the neurological deficit analysis, a modified neurological scale published by Eckenstein et al. in 1997 is used15.
- Score the animals for general (Table 1) and focal deficits (Table 2). This composite scale ranges from 0 (no deficits) to 46 (severe impairments).
- Perform the neuroscore at the same time each day and use surgical clothes to keep a neutral smell.
- Habituate the mice for 30 min in the room with an open cage prior to the testing and allow them to observe each item for 30 s.
7. Perfusion
- Prepare a 20 mL syringe containing PBS-heparin (2 U/mL) and place it 1 m above the bench to facilitate/ensure gravity-driven perfusion.
- Inject intraperitoneally 100 µL of ketamine and xylazine (120/16 mg/kg body weight, respectively). Wait for 5 min and corroborate the cessation of spontaneous body movement and vibrissae.
- Fix the animal in a supine position and disinfect the abdominal body surface with 100% ethanol. Make a 3 cm long incision in the abdomen; cut the diaphragm and the ribs to completely visualize the heart.
- Make a small incision in the right atrium and insert the perfusion cannula into the left ventricle and perfuse with 20 mL PBS-heparin.
- After perfusion, decapitate the animal and remove the brain, freeze it using dry ice and store them at -80 °C until further use.
8. Infarct volumetry
- Cryosectioning: Cut the brain serially on a cryostat to 20 µm thick sections every 120 µm and mount on slides. Store the slides at -80 °C until further processing.
- Cresyl violet (CV) staining
- To prepare the staining solution, mix 0.5 g of CV acetate in 500 mL of H2O. Stir and heat (60 °C) until the crystals are dissolved. Allow the solution to cool and store it in a dark bottle. Reheat to 60 °C and filter (paper filter) before every use.
- Dry the slides at room temperature for 30 min. Place them in 95% ethanol for 15 min, followed by 70% ethanol for 1 min, and afterwards in 50% ethanol for 1 min.
- Place the slides in distilled water for 2 min, refresh the distilled water, and place the slides in water again for 1 min. Then, place the slides in the pre-heated staining solution for 10 min at 60 °C. Wash the slides twice in distilled water for 1 min.
- Place the slides in 95% ethanol for 2 min. Then place them into 100% ethanol for 5 min, refresh the 100% ethanol and place the slides in 100% ethanol again for 2 min. Afterwards, cover the slides with a mounting medium.
- Analysis: Scan the slides and analyze the indirect infarct volume by the Swanson method16 to correct for edema: Ischemic area = (ischemic region)-((ipsilateral hemisphere) – (contralateral hemisphere)).
9. Tunel staining (in situ apoptosis detection kit)
- Dry the slides, post-fix in 4% paraformaldehyde in PBS (ph 7.4) for 10-20 min at RT. Wash in PBS, post-fix in precooled ethanol: acetic acid 2:1 for 5 min at -20 °C.
- Wash in PBS and apply equilibration buffer (10 s to a maximum of 60 min at RT) and apply working strength TdT enzyme (1 h at 37 °C in humidified chamber)
- Apply working strength stop/wash enzyme (10 min at RT), wash in PBS and apply warmed (RT) working strength anti-digoxigenin conjugate (30 min at RT in dark)
- Wash in PBS, incubate with DAPI for 5 min at RT and mount the slides with fluoromount media.
Representative Results
The model described here is a photothrombotic stroke model by Rose Bengal injection and intact skull illumination for 20 min, at a constant 561 nm wavelength and 25 mW output power at the fiber. Although the complete photothrombotic surgery lasts 30 min, the animal is kept under low anesthesia and the brain damage is moderate. Approximately 10 min after transfer to their cages, all the animals were awake, freely moving in the cage, and interacting with littermates.
Infarct volumetry was performed using cresyl violet stained serial coronal brain sections 24 h after stroke induction (Figure 2A). The mean infarct volume was 29.3 mm3, representing 23% of one brain hemisphere. Moreover, the variability of this stroke model is exceptionally low with a standard deviation of approximately 3.5% (Figure 2B). The lesion area encompasses the motor cortex without the affection of subcortical structures.
Photothrombosis caused a moderate, long-term sensorimotor impairment, indicated by the composite Neuroscore17 (Figure 3); general and focal deficits were measured 24 h, 3 days and 7 days after surgery. The general Neuroscore has five items, including the evaluation of the fur, ears, eyes, posture, and spontaneous activity, with a maximum score of 18 (Table 1). The focal Neuroscore comprises seven items, including the evaluation of body symmetry, gait, climbing, circling behavior, forelimb symmetry, compulsory cycling, and whiskers response, with a maximum score of 28 (Table 2). Stroke animals had a significant change in the composite neuroscore 24 h after surgery compared to Sham-operated animals. These differences persisted, although stroke mice improved over time (Figure 3).
Mortality during the observation time rarely occurs in 1-2% of the animals. In this report, none of the 10 animals studied had to be excluded and all of them survived the 7-day observation period. The body weight and temperature changes in the mice were monitored at 24 h, 3 days, and 7 days after surgery (Figure 4A,B). Data showed that body weight and temperature were decreased 24 h after surgery only in the Rose Bengal + illumination group, but recovered to the level of the Sham-operated animals in 3 days after surgery.
To confirm an induction of ischemic changes, 24 h after surgery, the animals underwent a laser imaging test. A laser speckle contrast imaging measured blood perfusion of the cortex for a duration of 1 min and an averaged color-coded picture was obtained for each animal. This demonstrates that Rose Bengal or laser illumination alone does not produce a lesion, while simultaneous application of Rose Bengal and laser illumination generates a round hypoperfused area of 4 mm diameter surrounded by a narrow oligemic zone (Figure 5A). In addition, a cresyl violet and Tunel staining for assessment of the infarct volume 24 h after surgery revealed no tissue damage either in Rose Bengal or laser illumination surgeries. On the other hand, Rose Bengal + laser illumination generated a well-demarcated lesion (Figure 5B).
Table 1: General Neuroscore. For each of the five general deficits measured, animals can receive between 0 and 4 points depending on the severity. The scores on the five areas are then summed to provide a total general score ranging from 0-18. Please click here to download this Table.
Table 2: Focal Neuroscore. For each of the seven general deficits measured, animals can receive between 0 and 4 points depending on the severity. The scores on the five areas are then summed to provide a total general score ranging from 0-28. Please click here to download this Table.
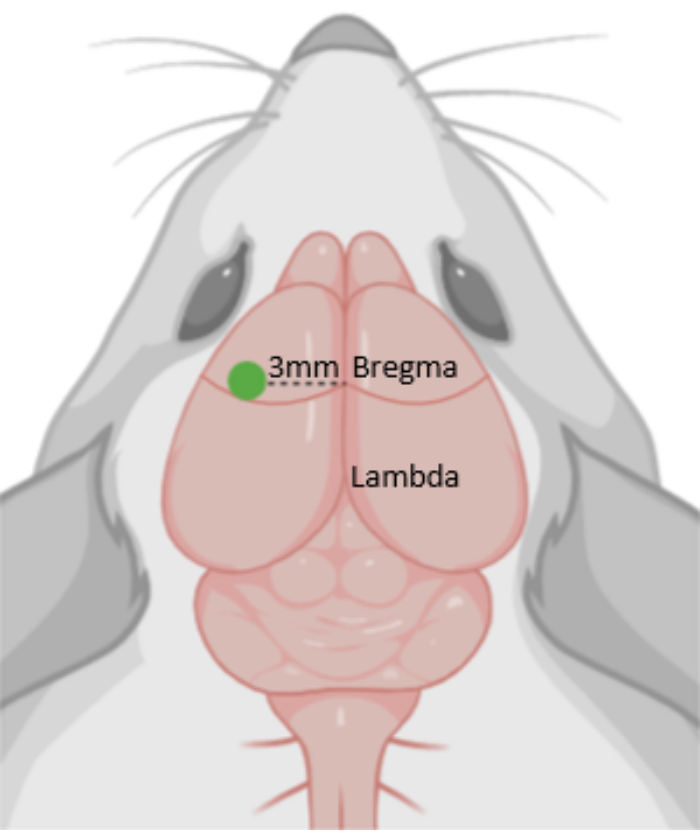
Figure 1: Photothrombosis (PT). Diagram depicting the photothrombotic area, 3 mm from Bregma. The green dot indicates the position of the laser. Please click here to view a larger version of this figure.
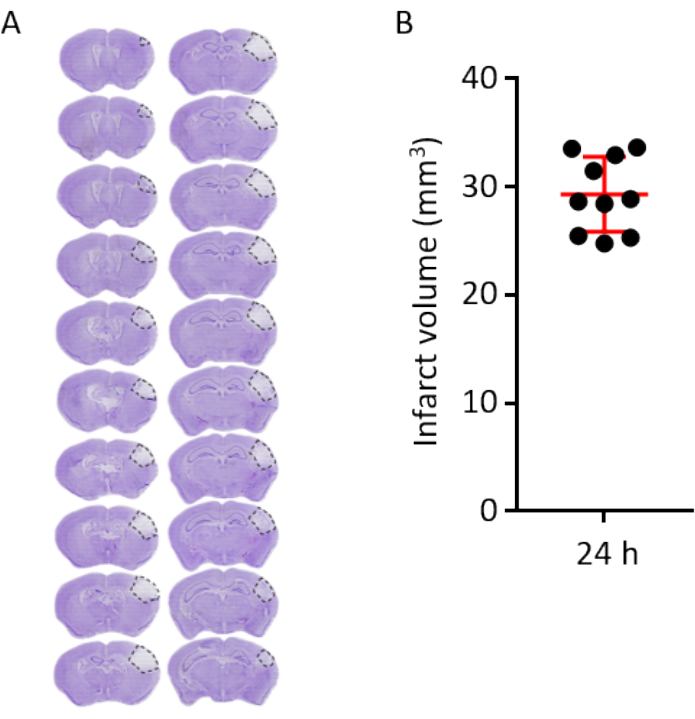
Figure 2: Volumetric infarct analysis and infarct outcome 24 h after PT. (A) Representative cresyl violet stained coronal brain, sections every 120 µm at 24 h after PT. Dashed lines demarcate the lesion area. (B) Infarct volume analysis of 10 brains (each dot representing one individual brain) 24 h after PT. The horizontal red line represents the mean (29.32 mm3), error bars indicate standard deviation (3.45 mm3). Please click here to view a larger version of this figure.
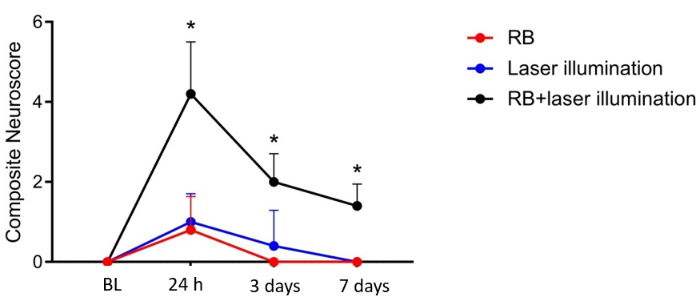
Figure 3: Neuroscore for functional deficits after PT. Composite Neuroscore before, 24 h, 3 days, and 7 days after PT. BL = before PT, RB = Rose Bengal. n = 5 per group. *p < 0.05. Please click here to view a larger version of this figure.
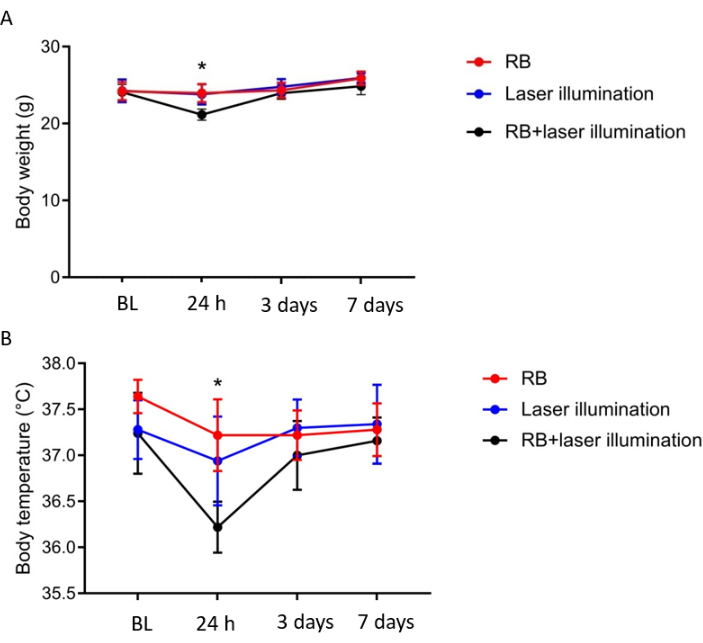
Figure 4: Body weight and temperature analysis after PT. (A) Body weight and (B) temperature was slightly reduced in PT animals compared to Sham-operated groups at 24 h and recovered 3 days after PT. BL = before PT, RB = Rose Bengal. n = 5 per group.*p < 0.05. Please click here to view a larger version of this figure.
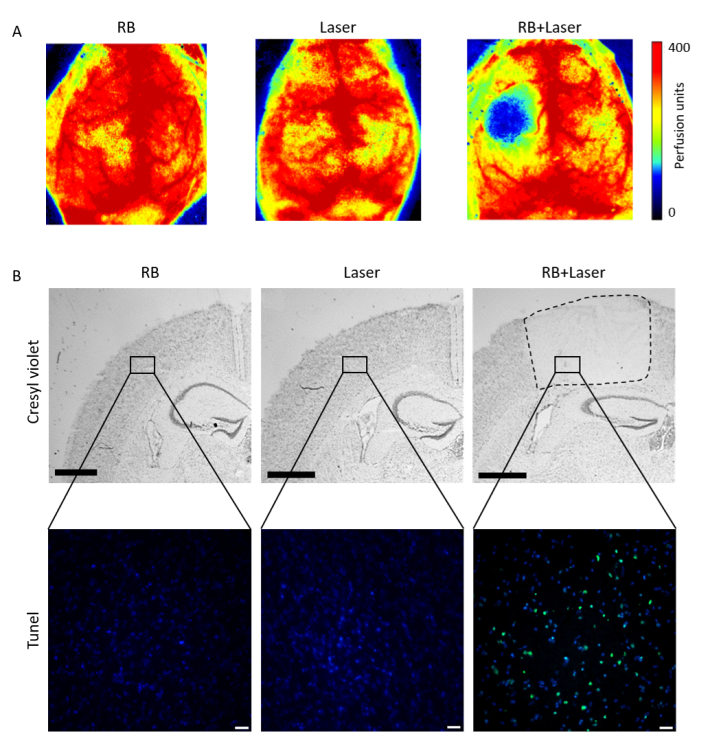
Figure 5: Lesion confirmation after PT. (A) Laser Speckle imaging (B) Cresyl violet (upper panels) and Tunel staining (lower panels) confirmed the lesion only after administration of Rose Bengal and subsequent laser illumination. RB = Rose Bengal. Scale bar = 1,000 µm in upper panel B, scale bar = 20 µm in lower panel B. Please click here to view a larger version of this figure.
Discussion
The presented protocol describes the experimental stroke model of photothrombosis by illuminating the intact skull with a 561 nm laser, with a previous intraperitoneal injection of Rose Bengal. Until recently, the use of this model has been low but is steadily increasing.
Mortality during stroke induction in this model is absent. The overall mortality of less than 5% arises during operation due to anesthesiological complications or sacrifice after meeting the exclusion criteria. To warrant the low variability of this model and its reproducibility, the following exclusion criteria are suggested: 1) operation time longer than 30 min; 2) infection of the suture; 3) bite wound; and 4) no infarct or no fore asymmetry at 24 h after PT.
A widely used experimental stroke models is the transient occlusion of the MCA, by using a suture filament, which is introduced in the internal carotid artery until the silicon-coated tip occludes the origin of the MCA. This model allows the reperfusion by removing the filament and mimics the human clinical scenario, in which there is a restoration of the cerebral blood flow after spontaneous or therapeutic (rtPA) lysis of an embolic clot18,19. However, it involves a complex surgery with high variability of the final infarct and high mortality rate10. In contrast, the permanent occlusion of the MCA distal of the lenticulostriatal arteries can be achieved by coagulation of the artery20,21, which induces locally defined lesions in the neocortex22. Although this model has a lower mortality rate, it requires invasive surgery to the animal by trepanation of the skull over the MCA to later coagulate it23. Consequently, high surgical skills are required for a successful and unbiased in vivo stroke study.
Compared to other brain ischemia models, the photothrombotic model as carried out in this video has the advantage of no craniotomy or major surgery on the animal, unlike other models that involve complex surgeries or brain craniotomy. Moreover, the simple execution of the model makes the surgery accessible to many with low time-consuming training. Low mortality, moderate infarct volume, and flexibility to induce the lesion to a specific brain region, emphasize the advantage of this experimental paradigm for brain regeneration and stroke studies24,25,26,27.
Despite the obvious advantages, a few limitations of this stroke model should be taken into consideration. The long exposure of anesthetics to the animal might be a critical factor to take into account, as the impact of anesthetics on neuroprotection and stroke outcome is already well-known28. Although the duration of this surgical procedure takes approximately 30 min, the animal can be under low anesthetic concentrations due to the minimal manipulation of the animal during the 20 min of laser illumination. Because this model induces moderate brain injuries, only minor behavioral deficits are detectable. Thus, more advanced test systems with higher sensitivity and qualitative test parameters, such as the skilled reaching test29 and Neuroscore17, as described in here, maybe more suitable for detecting long-term functional outcomes in this model. Finally, due to the permanent aggregation of the platelets into the illuminated blood vessels, no reperfusion can be obtained, which is a feature observed in a substantial percentage of stroke patients due to spontaneous clot lysis or therapy30.
A similar phototrombotic stroke model was published in 2013 by Labat-gest and Tomasi, describing a PT protocol using a cold light lamp instead of a 561 nm green laser8. Both laser and cold light sources can be used to induce Rose Bengal excitation. An advantage of laser-based light sources over cold light lamps is that lasers can be used to target individual surface arterioles for in vivo vessel-specific clotting31. Although we were not targeting specific arterioles, we used a 561 nm green laser for brain illuminationn and phototrombosis induction, because of the Rose Bengal absortion peak at 562 nm. To ensure a proper laser intensity during the illumination, the Cobolt Monitor Software-6.1.0.0 was used to calibrate the laser. Moreover, in the present study a Rose Bengal dosage of 10 µL/g (100 µg/g) was sufficient to induce phototrombosis, while the previous protocol reported a higher dose (150 µg/g)8. In addition, the protocol provides a behavioral method to analyze the stroke outcome (Neuroscore) and an additional sham-control group (laser illumination) in order to prove that the laser itself does not produce any tissue damage, so only the combination of Rose Bengal + laser illumination induce a brain lesion.
Overall, this non-invasive straightforward surgical procedure enables high reproducibility and directionality of the stroke lesion to the brain alongside the possibility of long-term observation due to minimal mortality. This photothrombotic stroke model is distinguished as a valuable experimental paradigm for basic and translational stroke research.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank all our collaboration partners of the Immunostroke Consortia (FOR 2879, From immune cells to stroke recovery) for suggestions and discussions. This work was funded by the Deutsche Forschungsgemeinschaft (DFG,German Research Foundation) under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198) and under the grants LI-2534/6-1, LI-2534/7-1 and LL-112/1-1.
Materials
| 561 nm wavelenght laser | Solna | Cobolt HS-03 | |
| Acetic Acid | Sigma Life Science | 695092 | |
| Anesthesia system for isoflurane | Drager | ||
| ApopTag Peroxidase In Situ Apoptosis Detection Kit | Millipore | S7100 | |
| Bepanthen pomade | Bayer | 1578681 | |
| C57Bl/6J mice | Charles River | 000664 | |
| Collimeter | Thorlabs | F240APC-A | |
| Cotons | NOBA Verbondmitel Danz | 974116 | |
| Cresyl violet | Sigma Life Science | C5042-10G | |
| Cryostat | Thermo Scientific CryoStarNX70 | ||
| Ethanol 70% | CLN Chemikalien Laborbedorf | 521005 | |
| Ethanol 96% | CLN Chemikalien Laborbedorf | 522078 | |
| Ethanol 99% | CLN Chemikalien Laborbedorf | ETO-5000-99-1 | |
| Filter paper | Macherey-Nagel | 432018 | |
| Fine Scissors | FST | 15000-00 | |
| Forceps | FST | 11616-15 | |
| Heating blanket | FHC DC Temperature Controller | 40-90-8D | |
| Isoflurane | Abbot | B506 | |
| Isopentane | Fluka | 59070 | |
| Ketamine | Inresa Arzneimittel GmbH | ||
| Laser Speckle | Perimed | PeriCam PSI HR | |
| Mayor Scissors | FST | 1410-15 | |
| Phosphate Buffered Saline PH: 7.4 | Apotheke Innestadt Uni Munchen | P32799 | |
| Protective glasses | Laser 2000 | NIR-ZS2-38 | |
| Rose Bengal | Sigma Aldrich | 198250-5G | |
| Roti-Histokit mounting medium | Roth | 6638.1 | |
| Saline solution | Braun | 131321 | |
| Stereomikroskop | Zeiss | Stemi DV4 | |
| Stereotactic frame | Stoelting | 51500U | |
| Superfrost Plus Slides | Thermo Scientific | J1800AMNZ | |
| Xylacine | Albrecht |
Referenzen
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 390 (10100), 1151-1210 (2017).
- Carmichael, S. T. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx: The Journal of the American Society for Experimental Neuro Therapeutics. 2 (3), 396-409 (2005).
- Rosenblum, W. I., El-Sabban, F. Platelet aggregation in the cerebral microcirculation: effect of aspirin and other agents. Circulation Research. 40 (3), 320-328 (1977).
- Watson, B. D., Dietrich, W. D., Busto, R., Wachtel, M. S., Ginsberg, M. D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Annals of Neurology. 17 (5), 497-504 (1985).
- Bergeron, M. Inducing photochemical cortical lesions in rat brain. Current Protocols in Neuroscience. , (2003).
- Lee, J. K., et al. Photochemically induced cerebral ischemia in a mouse model. Surgical Neurology. 67 (6), 620-625 (2007).
- Dietrich, W. D., Watson, B. D., Busto, R., Ginsberg, M. D., Bethea, J. R. Photochemically induced cerebral infarction. I. Early microvascular alterations. Acta Neuropathologica. 72 (4), 315-325 (1987).
- Labat-gest, V., Tomasi, S. Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. Journal of Visualized Experiments: JoVE. (76), e50370 (2013).
- McNutt, M. Journals unite for reproducibility. Science. 346 (6210), 679 (2014).
- Llovera, G., et al. Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia. Science Translational Medicine. 7 (299), (2015).
- Dirnagl, U., et al. A concerted appeal for international cooperation in preclinical stroke research. Stroke. 44 (6), 1754-1760 (2013).
- Bath, P. M., Macleod, M. R., Green, A. R. Emulating multicentre clinical stroke trials: a new paradigm for studying novel interventions in experimental models of stroke. International Journal of Stroke: Official Journal of the INternational Stroke Society. 4 (6), 471-479 (2009).
- Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., Altman, D. G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Journal of Pharmacology & Pharmacotherapeutics. 1 (2), 94-99 (2010).
- Llovera, G., Liesz, A. The next step in translational research: lessons learned from the first preclinical randomized controlled trial. Journal of Neurochemistry. 139, 271-279 (2016).
- Gnyawali, S. C., et al. Retooling laser speckle contrast analysis algorithm to enhance non-invasive high resolution laser speckle functional imaging of cutaneous microcirculation. Scientific Reports. 7, 41048 (2017).
- Swanson, G. M., Satariano, E. R., Satariano, W. A., Threatt, B. A. Racial differences in the early detection of breast cancer in metropolitan Detroit, 1978 to 1987. Cancer. 66 (6), 1297-1301 (1990).
- Clark, W. M., Lessov, N. S., Dixon, M. P., Eckenstein, F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurological Research. 19 (6), 641-648 (1997).
- Longa, E. Z., Weinstein, P. R., Carlson, S., Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20 (1), 84-91 (1989).
- Engel, O., Kolodziej, S., Dirnagl, U., Prinz, V. Modeling stroke in mice – middle cerebral artery occlusion with the filament model. Journal of Visualized Experiments: JoVE. (47), e2423 (2011).
- Tamura, A., Graham, D. I., McCulloch, J., Teasdale, G. M. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 1 (1), 53-60 (1981).
- Chen, S. T., Hsu, C. Y., Hogan, E. L., Maricq, H., Balentine, J. D. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 17 (4), 738-743 (1986).
- Tureyen, K., Vemuganti, R., Sailor, K. A., Dempsey, R. J. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. Journal of Neuroscience Methods. 139 (2), 203-207 (2004).
- Llovera, G., Roth, S., Plesnila, N., Veltkamp, R., Liesz, A. Modeling stroke in mice: permanent coagulation of the distal middle cerebral artery. Journal of Visualized Experiments: JoVE. (89), e511729 (2014).
- Cramer, J. V., et al. In vivo widefield calcium imaging of the mouse cortex for analysis of network connectivity in health and brain disease. Neuroimage. 199, 570-584 (2019).
- Heindl, S., et al. Automated morphological analysis of microglia after stroke. Frontiers in Cellular Neuroscience. 12, 106 (2018).
- Nih, L. R., Gojgini, S., Carmichael, S. T., Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nature Materials. 17 (7), 642-651 (2018).
- Rust, R., et al. Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proceedings of the National Academy of Sciences of the United States of America. 116 (28), 14270-14279 (2019).
- Kitano, H., Kirsch, J. R., Hurn, P. D., Murphy, S. J. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 27 (6), 1108-1128 (2007).
- Farr, T. D., Whishaw, I. Q. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 33 (7), 1869-1875 (2002).
- Kassem-Moussa, H., Graffagnino, C. Nonocclusion and spontaneous recanalization rates in acute ischemic stroke: a review of cerebral angiography studies. Archives of Neurology. 59 (12), 1870-1873 (2002).
- Sigler, A., Goroshkov, A., Murphy, T. H. Hardware and methodology for targeting single brain arterioles for photothrombotic stroke on an upright microscope. Journal of Neuroscience Methods. 170 (1), 35-44 (2008).

