Growth, Purification, and Titration of Oncolytic Herpes Simplex Virus
Summary
In this manuscript, we describe a simple method of growth, purification, and titration of the oncolytic herpes simplex virus for preclinical use.
Abstract
Oncolytic viruses (OVs), such as the oncolytic herpes simplex virus (oHSV), are a rapidly growing treatment strategy in the field of cancer immunotherapy. OVs, including oHSV, selectively replicate in and kill cancer cells (sparing healthy/normal cells) while inducing anti-tumor immunity. Because of these unique properties, oHSV-based treatment strategies are being increasingly used for the treatment of cancer, preclinically and clinically, including FDA-approved talimogene laherparevec (T-Vec). Growth, purification, and titration are three essential laboratory techniques for any OVs, including oHSVs, before they can be utilized for experimental studies. This paper describes a simple step-by-step method to amplify oHSV in Vero cells. As oHSVs multiply, they produce a cytopathic effect (CPE) in Vero cells. Once 90-100% of the infected cells show a CPE, they are gently harvested, treated with benzonase and magnesium chloride (MgCl2), filtered, and subjected to purification using the sucrose-gradient method. Following purification, the number of infectious oHSV (designated as plaque-forming units or PFUs) is determined by a "plaque assay" in Vero cells. The protocol described herein can be used to prepare high-titer oHSV stock for in vitro studies in cell culture and in vivo animal experiments.
Introduction
Oncolytic viruses (OVs) are an emerging and unique form of cancer immunotherapy. OVs selectively replicate in and lyse tumor cells (sparing normal/healthy cells)1 while inducing anti-tumor immunity2. Oncolytic herpes simplex virus (oHSV) is one of the most extensively studied viruses among all OVs. It is furthest along in the clinic, with Talimogene laherparepvec (T-VEC) being the first and only OV to receive FDA approval in the USA for the treatment of advanced melanoma3. In addition to T-VEC, many other genetically engineered oHSVs are being tested preclinically and clinically in different cancer types3,4,5,6,7,8. The current advanced recombinant DNA biotechnology has further increased the feasibility of engineering new oHSVs coding for therapeutic transgene(s)3,5. An efficient system of oHSV propagation, purification, and titer determination is critical before any (newly developed) oHSV can be tested for in vitro and in vivo studies. This paper describes a simple step-by-step method of oHSV growth (in Vero cells), purification (by the sucrose-gradient method), and titration (by an oHSV plaque assay in Vero cells) (Figure 1). It can be easily adopted in any Biosafety Level 2 (BSL2) laboratory setting to achieve a high-quality viral stock for preclinical studies.
Vero, an African green monkey kidney cell line, is the most commonly used cell line for oHSV propagation9,10,11,12,13 as Vero cells have a defective antiviral interferon signaling pathway14. Other cell lines with inactivated stimulator of interferon genes (STING) signaling can also be used for oHSV growth12,13. This protocol utilizes Vero cells for oHSV growth and plaque assay. Following propagation, oHSV-infected cells are harvested, lysed, and subjected to purification, wherein lysed cells are first treated with benzonase nuclease to degrade host cell DNA, prevent nucleic acid-protein aggregation, and reduce the viscosity of the cell lysate. As proper activation of benzonase often requires Mg2+, 1-2 mM MgCl2 is used in this protocol15. The host cell debris from the benzonase-treated cell lysate is further eliminated by serial filtration before high-speed sucrose-gradient centrifugation. A viscous 25% sucrose solution cushion helps to ensure a slower rate of virus migration through the sucrose layer, leaving host cell-related components in the supernatant, thus improving purification and limiting virus loss in the pellet16. The purified oHSV is then titrated on Vero cells, and viral plaques are visualized by Giemsa staining17 or X-gal staining (for LacZ encoding oHSVs)18.
Protocol
1) oHSV growth
NOTE: Ensure institutional biosafety committee approval before working with oHSV. This study was conducted under approved IBC Protocol no. 18007. Maintain BSL2 precautions: bleach all pipets, tips, tubes, and other materials that come into contact with the virus. Spray gloves with 70% isopropyl alcohol before hands leave the BSL2 cell culture hood. Always thoroughly wash hands with soap water after working with a virus.
- On day -1, seed low-passage Vero cells in 20 T-150 cm2 flasks at a density of 7-8 × 106 cells/flask in regular Vero cell medium.
NOTE: Vero medium is prepared by supplementing Dulbecco's modified eagle medium (DMEM) with 10% heat-inactivated fetal calf serum (IFCS). - On Day 0 (cells are 80-90% confluent), add oHSV inoculum on the Vero cells.
- Preparation of virus inoculum
- Use a multiplicity of infection (MOI) of 0.01 (MOI can vary from 0.01 to 0.1 depending on the replication capacity of virus) for virus amplification. Use formula (1) to calculate the amount of virus (mL) for 20 flasks.
Amount of virus (mL) for 20 flasks = amount of virus needed (pfu)/titer of virus stock (pfu/mL)(1) - Use high-glucose Dulbecco's phosphate-buffered saline (DPBS) supplemented with 1% IFCS to prepare the virus inoculum (7 mL per T-150 cm2 flask, i.e., ~140 mL for 20 flasks). Add the required amount of oHSV to 140 mL of high-glucose DPBS/1% IFCS solution, vortex for 1 min, and keep the mixture ready for addition to Vero cells.
- Use a multiplicity of infection (MOI) of 0.01 (MOI can vary from 0.01 to 0.1 depending on the replication capacity of virus) for virus amplification. Use formula (1) to calculate the amount of virus (mL) for 20 flasks.
- Wash the T-150 cm2 flasks 2x with high-glucose DPBS supplemented with 1% IFCS (10 mL/wash). Aspirate the DPBS/1% IFCS and add 7 mL of virus inoculum/T-150 cm2 flask.
- Gently rock the flasks for 5 min using a flask rocker for proper distribution of the inoculum over the Vero monolayer, and then incubate the flasks at 37 °C for 1.5-2 h. Make sure the incubator shelf is level.
- Remove the inoculum and add DMEM supplemented with 1% IFCS (25 mL/flask). Incubate the flasks for 2-4 days.
- Preparation of virus inoculum
- Check the flasks daily for 90-100% CPE (see Figure 2).
- Harvest the oHSV-infected Vero cells.
- Collect the culture supernatant (~20 mL) from each flask (leave ~5 mL in each flask) in 50 mL conical centrifuge tubes or media container.
- Use a cell scraper to scrape the cells from the bottom of the flasks gently.
NOTE: The cells should quickly come off the flasks. - Add ~15 mL of the culture supernatant (collected in step 1.4.1) to each flask (which brings the volume of ~20 mL in each flask, i.e., 400 mL for 20 flasks) and gently wash the bottom of the flasks a few times using a 10 mL sterile serological pipet.
NOTE: Do not pipet vigorously. Aim to keep all cells intact. - Collect the cells (+ medium) into 50 mL conical centrifuge tubes on ice (use 8 tubes to hold 400 mL of harvested cells from 20 flasks).
- Spin the cells at 300 g for 10 min at 4 °C and aspirate the supernatant.
- Add 1.25 mL (50%) of Virus Buffer (VB) and 1.25 mL (50%) of culture supernatant (collected in step 1.4.1) to each centrifuge tube and re-suspend each pellet thoroughly. Transfer the re-suspended cells from eight 50 mL centrifuge tubes to one 50 mL conical centrifuge tube.
NOTE: Refer to the preparation of VB solution in Table 1. Filter-sterilize the VB solution using a media sterilization filter. Use 0.5 mL of VB + 0.5 mL of supernatant per T-150 cm2 flask for re-suspension, i.e., for 20 flasks (20 mL), use 10 mL of VB and 10 mL of culture supernatant. - Snap-freeze the re-suspended cells using dry ice/100% ethanol and store at -80 °C.
2) oHSV purification
- Snap-freeze (in dry ice and 100% ethanol)/thaw (in a 37 °C warm water bath) the cells followed by water bath-sonication for 1 min for a total of 3 cycles to ensure proper lysis of the cells to release virus in the supernatant.
NOTE: Perform sonication for 1 min using 40 kHz, 120 V power. If a tunable sonicator is not available, use an ultrasonic water bath sonicator. Take a 50 μL aliquot for titration in section 3, which will help to identify which of the following step(s) is responsible for potential virus loss during the purification procedure. - Treat the cell lysate with Benzonase Nuclease (175 units/mL) + 2 mM MgCl2 (1 M stock = 2 µL/mL), vortex, and incubate for 30 min at 37 °C. Place the tube on ice and perform the following steps at 4 °C.
- Pellet the cell debris by low-speed centrifugation.
- Spin the cell lysate at 300 g for 10 min.
- Collect the supernatant in a new 50 mL conical centrifuge tube (re-suspend the cell pellet in 0.5 mL of VB, designate it as pellet-1, and store at 4 °C for use in step 2.3.5; see Figure 1).
- Spin the supernatant (obtained in step 2.3.2) again at 500 g for 10 min.
- Collect the supernatant in a new 50 mL conical centrifuge tube (re-suspend the cell pellet in 0.5 mL of VB, designate it as pellet-2, and store at 4 °C for use in step 2.3.5; see Figure 1).
- Combine the re-suspended pellet-1 (from 2.3.2) and pellet-2 (from 2.3.4) into a new 1.7 mL centrifuge tube, vortex/sonicate (water bath) 2x, and spin at 400 g for 10 min. Collect the supernatant and combine it with the supernatant obtained in step 2.3.4; see Figure 1).
NOTE: Perform sonication for 1 min using 40 kHz, 120 V power to prevent viral aggregation before the filtration procedure in step 2.4. Take a 50 μL aliquot of the combined supernatant for titration in section 3.
- Filter the combined supernatant (~21 mL, i.e., 20 mL from 1.4.6, 0.5 mL from 2.3.2, and 0.5 mL from 2.3.4) using the following 3-step filtration method.
- Draw 21 mL of the supernatant using a 10 mL syringe (5-7 mL each time for easy passage through the filter) and pass it through a sterile 5 μm polyvinylidene difluoride (PVDF) membrane filter placed on a new 50 mL conical centrifuge tube (labeled as TUBE 1).
- Add 1 mL of VB to a 50 mL conical centrifuge tube emptied in 2.4.1 (to collect the remaining trace amount of virus supernatant), vortex, and pass through same 5 μm PVDF filter placed on TUBE 1 (bringing the total to 22 mL of filtrate). Proceed to step 2.4.2.
- Draw 22 mL of the filtrate from TUBE 1 (as in 2.4.1) and pass it through a sterile 0.8 μm mixed cellulose ester (MCE) membrane filter placed on a new 50 mL conical centrifuge tube (labeled as TUBE 2).
- Add 1 mL of VB to TUBE 1 emptied in step 2.4.2, vortex, and pass it through the same 0.8 μm MCE filter placed on TUBE 2 (bringing the total to 23 mL of filtrate). Proceed to step 2.4.3.
- Draw 23 mL of filtrate from TUBE 2 (as in 2.4.1) and pass it through a sterile 0.45 µm PVDF filter placed on a new 50 mL conical centrifuge tube (labeled as TUBE 3).
- Add 1 mL of VB to TUBE 2 emptied in step 2.4.3, vortex, and pass it through the same 0.45 μm PVDF filter placed on TUBE 3 (bringing the total to 24 mL of filtrate).
NOTE: Take a 50 μL aliquot of the filtrate for titration in section 3.
- Add 1 mL of VB to TUBE 2 emptied in step 2.4.3, vortex, and pass it through the same 0.45 μm PVDF filter placed on TUBE 3 (bringing the total to 24 mL of filtrate).
- Draw 21 mL of the supernatant using a 10 mL syringe (5-7 mL each time for easy passage through the filter) and pass it through a sterile 5 μm polyvinylidene difluoride (PVDF) membrane filter placed on a new 50 mL conical centrifuge tube (labeled as TUBE 1).
- High-speed centrifugation using the sucrose-gradient method
NOTE: A fixed angle F13-14x50cy rotor was used in this protocol. Both the rotor and the centrifuge must be at 4 °C before proceeding with the following steps.- Add 10 mL of an ice-cold, sterile-filtered 25% sucrose solution (prepared by dissolving 25 g of sucrose powder in 100 mL of Hank's Balanced Salt Solution) in a new 50 mL conical centrifuge tube.
- Slowly (3 mL/min) add 24 mL of the virus filtrate (obtained from step 2.4.3.1) on top of the sucrose layer. Take care to maintain separate layers of the virus filtrate and the sucrose solution.
NOTE: Up to 30 mL of the virus layer can be added over 10 mL of the sucrose solution. - Centrifuge the tube for 90 min at 22,620 g at 4 °C.
- Remove the supernatant and the sucrose layer from the 50 mL conical centrifuge tube.
NOTE: The virus pellet should be whitish. Take a 50 μL aliquot of the supernatant and a 50 μL aliquot of the sucrose layer for titration in section 3.
- Re-suspend the pellet in 10% glycerol/PBS solution.
- Add 1 mL of sterile 10% glycerol (diluted in PBS) to the 50 mL conical centrifuge tube to cover the pellet.
NOTE: The amount of the re-suspended mixture can vary (such as 0.8-1.2 mL) depending on the pellet size. A smaller volume would give a higher concentration of virus. - Place the 50 mL conical centrifuge tube on ice for 2-4 h. During this period, sonicate/vortex the pellet every 15 min for 30 s to help dislodge/re-suspend the pellet.
- Collect the re-suspended pellet (1 mL of 10% glycerol/PBS + the pellet size will bring the total volume to ~1.3 mL) in a 2 mL microcentrifuge tube. [Optional: Add another 0.3 mL of 10% glycerol/PBS to the same 50 mL conical centrifuge tube to collect the remaining trace amount of the pellet, pipet up and down, and combine with the re-suspended pellet in step 2.6.3 to bring the total volume to ~1.6 mL.]
- If the suspension in step 2.6.3 is turbid or cloudy (due to cell debris), centrifuge the tube at 500 g for 10 min, transfer the supernatant to a new 2 mL microcentrifuge tube, and proceed to step 2.6.4.
- Take a 50 μL aliquot for oHSV titration in section 3. Aliquot the rest of the solution (250 μL/aliquot) into sterile microcentrifuge tubes with screw caps (sealed well for long-term storage), snap-freeze, and store at -80 °C until use (ready for experimental studies once the oHSV titer is determined).
NOTE: All materials that come in contact with the virus must be bleached or treated with ultraviolet radiation before removal from the hood or disposal.
- Add 1 mL of sterile 10% glycerol (diluted in PBS) to the 50 mL conical centrifuge tube to cover the pellet.
3. oHSV titration and plaque assay
- Seed 1.7-1.8 x 105 Vero cells/well in a 6-well cell culture plate in Vero cell medium (DMEM with 10% IFCS; see step 1.1).
NOTE: Make sure that the cells are homogeneously distributed throughout the well; do not swirl the plate, which can cause accumulation of cells in the middle of the wells. To prevent swirling, slowly rock the plate by hand vertically, then horizontally, and then gently place the plate in the incubator. - The next day (when the cells reach 70-80% confluency), aspirate the culture medium, and add 1 mL/well of high-glucose PBS supplemented with 1% IFCS. Leave the plate in the cell culture hood until step 3.3 is complete.
- Serially dilute the virus in 5 mL polypropylene tubes using PBS/1% IFCS (Figure 3).
NOTE: Use the 50 μL aliquot collected in step 2.6.4 for serial dilution. In the 1st tube (10-3 dilution), add 2 μL of the virus in 1998 μL of PBS/1%IFCS, vortex. In the 2nd tube (10-5 dilution), take 10 μL from the 1st tube in 990 μL of PBS/1% IFCS, vortex. In the 3rd tube (10-6 dilution), take 100 μL from the 2nd tube in 900 μL of PBS/1% IFCS, vortex; continue this 10-fold serial dilution until 10-9 dilution. See the details in Figure 3. - Aspirate PBS/1% IFCS, and add 0.7 mL/well of the serially diluted virus (starting from 10-5 to 10-9) to Vero cells.
NOTE: Do not let the cells dry. - Gently rock the plate on a rocker for 5 min at room temperature (to ensure homogeneous distribution of the virus inoculum).
- Incubate the plate for 1.5 h at 37 °C.
NOTE: During this incubation period, prepare 1:1000 dilution of human immunoglobulin G (IgG) in DMEM supplemented with 1% IFCS. For a 6-well plate, prepare 12.5 mL so that 2 mL/well can be used in step 3.7. Adjust the dilution to account for lot variations in the human IgG. - Remove the virus inoculum from the wells, and add 2 mL/well of 0.1% human IgG (to neutralize the oHSV in the culture medium and prevent the formation of secondary plaques) in DMEM supplemented with 1% IFCS. Incubate the plate at 37 °C for 3-4 days.
NOTE: Clear plaques usually form in 3 days. - Fixing and staining plates
- Remove the supernatant, and fix the cells in pure methanol (1 mL/well) for 5 min. Remove the methanol, and allow the plates to air-dry.
- Dilute Giemsa stain (1:5) with deionized water, and add 1 mL of the diluted Giemsa stain per well. Incubate the plate at room temperature for 10-15 min.
- Remove the stain, rinse with tap water, and allow the plates to air-dry.
- Count the plaques using a dissecting microscope.
- Optional for oHSVs with lacZ expression:
- Remove the supernatant, and fix the cells with cold 0.2% glutaraldehyde/2% paraformaldehyde for 5-10 min at room temperature.
- Remove the fixative solution, and wash the cells 3x with PBS.
- Add X-gal solution to the cells (1 mL/well), and incubate the plate at 37 °C for 2 h.
NOTE: The X-gal stain should be prepared freshly on the day of staining; long-term storage might lead to fading of color. See the preparation of X-gal solution in Table 1. - Remove the X-gal stain, and wash the plate with tap water for 1 min.
- Counter-stain with Neutral Red solution (1 mL/well) for 2 min at room temperature.
NOTE: See preparation of Neutral Red solution in Table 1. - Wash the plate with tap water for 1 min; allow the plates to air-dry.
- Count blue plaques using a dissecting microscope (Figure 4).
- Calculate the titer by using formula (2)
Titer in pfu/mL (plaque-forming units) = Number of plaques/0.7 mL × dilution factor (2)
NOTE: For example, if 25 plaques are found in the 10-9 dilution well, the titer is 25/0.7 × 109 = 35.7 × 109 = 3.57 × 1010 pfu/mL. This is the final oHSV titer of aliquots prepared in step 2.6.4.
Representative Results
A brief overview of the entire protocol is depicted in Figure 1, which represents the critical steps involved in the growth, purification, and titration of oHSV. CPE in Vero cells can be detected as early as 4 h post-HSV infection19. Figure 2 demonstrates CPE in Vero cells at three different time points following oHSV infection. The level of the CPE is increased over time. In this protocol, 90-100% CPE is usually observed within 48 h of low-MOI oHSV inoculation (which is the best time to harvest cells for purification). However, it can take up to 4 days depending on the oHSV MOI inoculated in step 1.2 and/or the oHSV's replication potential. Beyond this period, cells with CPE can be lysed, leading to the release of the virus in the supernatant. Thus, to obtain a high viral titer, it is critical to harvest CPE-affected cells when they are intact. Another important factor that contributes to the final virus titer is the number or size of the tissue culture flasks used for oHSV amplification. Figure 3 depicts the process of serial dilution (10-3 to 10-9) of a given virus stock (obtained in step 2.6.4) required for titer determination by the plaque assay. For oHSVs with lacZ expression, viral plaques can be visualized by X-gal staining (Figure 4). In this protocol, 20 T-150 cm2 tissue culture flasks were used, for which 1.3-1.6 mL of oHSV stock with a titer of 1 × 1010 pfu/mL can be expected.
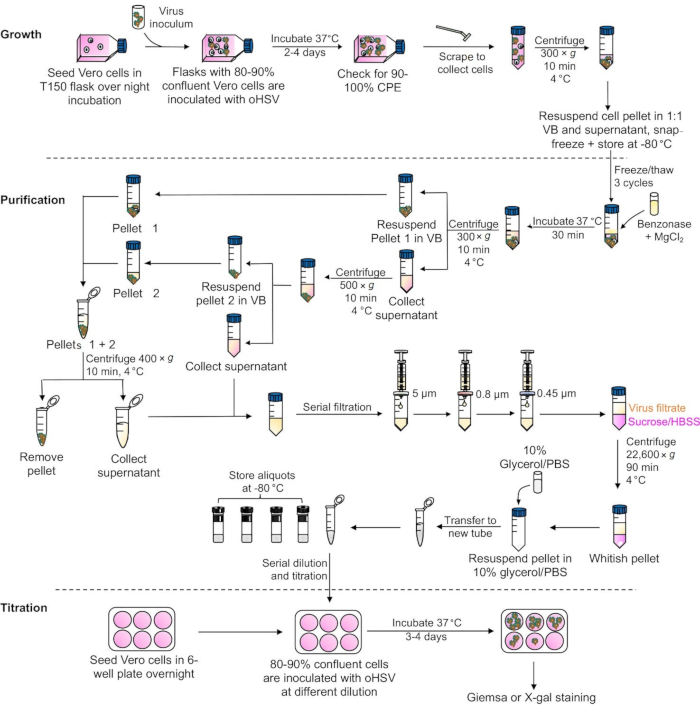
Figure 1: A schematic presentation of major steps involved in oHSV growth, purification, and the plaque assay. Abbreviations: oHSV = oncolytic herpes simplex virus; CPE = cytopathic effect; VB = Virus Buffer; HBSS = Hank's Balanced Salt Solution; PBS = phosphate-buffered saline. Please click here to view a larger version of this figure.
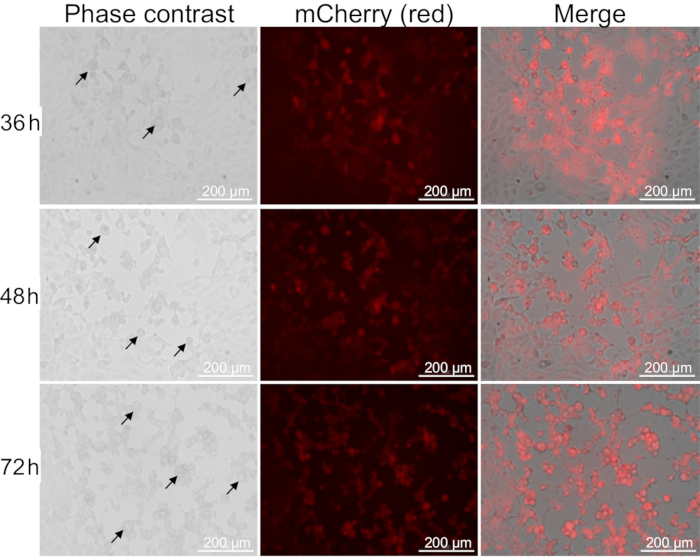
Figure 2: Cytopathic effect in Vero cells after oHSV infection. Vero cells were inoculated with oHSV coding for mCherry (shown in red fluorescence) at an MOI of 0.01 and imaged (10x magnification) at 36, 48, and 72 h post-virus infection. CPE is identified by rounding of the oHSV-infected cells (indicated by black arrows). Scale bars = 200 μm. Abbreviation: oHSV = oncolytic herpes simplex virus. Please click here to view a larger version of this figure.
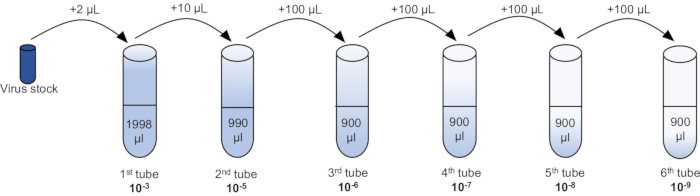
Figure 3: A serial dilution of an oHSV stock for plaque assays. See also step 3.3 of the protocol. Abbreviation: oHSV = oncolytic herpes simplex virus. Please click here to view a larger version of this figure.
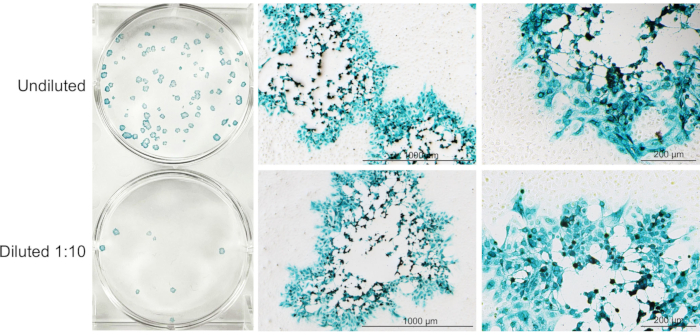
Figure 4: A representative image of X-gal-stained plaques at 72 h post-oHSV infection. Undiluted (upper well) and diluted (1:10; lower well) oHSV-infected cell culture supernatants added to Vero cells (70-80% confluent), followed by X-gal staining protocol described in section 3.8.5 (excluding counter-staining with Neutral Red). Representative images of an X-gal-stained virus plaque (from left panel) is presented in the middle (4x; scale bars = 1000 μm) and right (10x; scale bars = 200 μm) panels. Please click here to view a larger version of this figure.
| Solution Composition | |
| Preparation of virus buffer | Quantity |
| 1 M Sodium chloride | 15 mL |
| 1 M Tris-hydrochloride | 3 mL |
| Purified water | 132 mL |
| adjust pH to 6.8 | |
| Preparation of X-gal solution (~6.5 mL for one 6-well plate) | |
| 250 mM potassium ferricyanide | 130 µL |
| 250 mM potassium ferrocyanide | 130 µL |
| 1 M Magnesium chloride | 13 µL |
| X-gal pre-dissolved (20 mg/mL) in dimethyl sulfoxide (DMSO) | 162.5 µL |
| PBS | 6064.5 µL |
| Preparation of Neutral Red solution for one 6-well plate | |
| Neutral Red solution | 100 µL |
| Methanol | 1 mL |
| Purified water | 7 mL |
Table 1: Solution composition.
Discussion
The protocol starts with the growth of oHSV in low-passage Vero cells. The confluency of the Vero cell monolayer should be ~80% at the time of virus inoculation as overgrown cells can develop tight fibrous structures that can reduce oHSV entry into Vero cells20. Once 90-100% CPE is observed, the culture supernatant is removed, cells are harvested, resuspended in VB/supernatant (see step 1.4.6), snap-frozen, and stored at -80 °C for later purification. Blaho and colleagues employed a slightly different method of harvesting and storage of infected Vero cells. For instance, the flasks containing the cells and culture media (supplemented with 1% bovine serum albumin and PBS with potassium) are initially stored at -80 °C for at least 15 min, followed by a slow warming up of the flasks at room temperature. The cells are then harvested, mixed with sterile milk, and stored at -80 °C until purification20. In this case, sterile milk acts as a stabilizer, and it was demonstrated that the titer of a virus stock is dramatically higher when it is stored in sterile milk/medium than in medium alone20. However, in another study, a direct comparison between different stabilizers (including sterile milk) used for the storage of several herpesviruses at -80 °C did not show any significant impact on the final virus titers21. Here, the cell pellet was re-suspended in VB constituted with Tris-buffer saline (pH 6.8) and 10% glycerol as a storage stabilizer, which usually gives a high virus titer (as outlined in step 3.10) for experimental studies.
It is critical to remove all cellular- and media-related components from the re-suspended pellet to obtain a high-quality virus stock. The removal of non-viral particles is crucial to avoid potential immune reactions during in vivo experiments. Several methods of virus purification have been described including centrifugation22, different gradient methods23, filtration24, and affinity chromatography25. Although this protocol is based on high-speed centrifugation using a sucrose-gradient method, a variety of other gradient methods have been used by others, such as iodixanol11,26, Percoll27, and Ficoll-Nycodenz23. These gradient methods separate the virus by density and require isolation of the band from the gradient, instead of the sucrose cushion where the virus is pelleted. The sucrose-gradient method offers a gentle approach to separate viral particles because it minimizes the risk of disrupting viral envelope proteins while retaining viral infectivity. Despite these advantages, the high osmolarity of the concentrated sucrose solution might dehydrate the viral particles; therefore, the iodixanol gradient method was developed to overcome this drawback. However, the iodixanol gradient method requires ultracentrifuge and collection of the virion band. Other factors that need to be considered during oHSV purification are speed and time of centrifugation and choice of the virus buffer used for long-term storage. This protocol has the limitation that the purity of oHSV is not confirmed; however, a high number of functional virus particles were found in a given purified oHSV stock by titration on Vero cells (see section 3).
oHSV forms plaques on Vero cells (Figure 4). The viral plaques can be identified by Giemsa staining, which is an easy and convenient method. Giemsa stains Vero cells, leaving the viral plaques transparent or empty that can be easily visualized (naked eye) and counted using a dissecting microscope. While overlaying the media with agarose or methylcellulose is commonly used during plaque formation (in step 3.7) to prevent the spread of the virus and secondary infections and plaque tails28, the use of human IgG to neutralize oHSV in the culture supernatant is easier and more convenient. For oHSVs expressing lacZ, plaques can be visualized by X-gal staining (Figure 4), while fluorescent microscopy is used for fluorescent protein (i.e., green fluorescent protein)-expressing oHSVs18. Additional assays to detect oHSV-infected cells include immuno-histochemical or -fluorescence with oHSV-specific antibodies29 or laser-based scanning of near-infrared fluorophore-conjugated oHSV-specific antibodies30.
There are critical measures that must be followed to achieve a good virus stock such as maintaining sterility to prevent microbial (bacteria, yeast, or mold) contamination and healthy Vero cells. As the envelope of oHSV is extremely thermosensitive20, the oHSV stock should be handled in a cryoprotectant such as 10% glycerol. Overall, this protocol can be easily employed and practiced in a laboratory setting, but may not be useful for large-scale virus production.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Research in the Saha lab was supported in part by funds from the DOD (W81XWH-20-1-0702) and Dodge Jones Foundation-Abilene. Samuel D. Rabkin and Melissa R.M. Humphrey were partially supported by NIH (R01 CA160762).
Materials
| 1.7 mL centrifuge tubes | Sigma | CLS3620 | |
| 15 mL polypropylene centrifuge tubes | Falcon | 352097 | |
| 5 mL polypropylene tubes | Falcon | 352063 | |
| 50 mL polypropylene centrifuge tubes | Falcon | 352098 | |
| 6-well cell culture plates | Falcon | 353046 | |
| Benzonase Nuclease | Sigma | E8263-25KU | |
| Cell scraper | Fisher Scientific | 179693 | |
| Dimethyl sulfoxide | Sigma | D2650-100ML | |
| Dulbecco’s Modified Eagle Medium | Corning | MT-10-013-CV | |
| Dulbecco’s Phosphate Buffered Saline | Corning | MT-21-031-CV | |
| Fetal Bovine Serum | Hyclone | SH3007003 | |
| Giemsa Stain | Sigma | G3032 | |
| Glutaraldehyde | Fisher Scientific | 50-262-23 | |
| Glycerol | Sigma | G5516 | |
| Hank's Balanced Salt Solution (HBSS) | Corning | MT-21-021-CV | |
| High-Glucose Dulbecco’s Phosphate-buffered Saline | Sigma | D4031 | |
| Human immune globulin | Gamastan | NDC 13533-335-12 | |
| Magnesium chloride | Fisher Chemical | M33-500 | |
| Media Sterilization filter, 250 mL | Nalgene, Fisher Scientific | 09-740-25E | |
| Media Sterilization filter, 500 mL | Nalgene, Fisher Scientific | 09-740-25C | |
| Neutral Red solution | Sigma | N4638 | |
| Paraformaldehyde | Fisher scientific | 15710S | |
| Plate rocker | Fisher | 88861043 | |
| Potassium Ferricyanide | Sigma | P8131 | |
| Potassium Ferrocyanide | Sigma | P9387 | |
| Sodium chloride | Fisher Chemical | S271-3 | |
| Sorvall ST 16R Centrifuge | ThermoFisher Scientific | 75004381 | |
| Sorvall ST 21R Centrifuge | ThermoFisher Scientific | 75002446 | |
| Sterile Microcentrifuge Tubes with Screw Caps | Fisher Scientific | 02-681-371 | |
| Sucrose | Fisher Scientific | BP220-1 | |
| Syringe Filter, 0.45 PVDF | MilliporeSigma | SLHV033RS | |
| Syringe Filter, 0.8 MCE | MilliporeSigma | SLAA033SS | |
| Syringe filter, 5 µm PVDF | MilliporeSigma | SLSV025LS | |
| T150 culture flask | Falcon | 355001 | |
| Tris-HCl | MP Biomedicals LLC | 816116 | |
| Ultrasonic water bath | Branson | CPX-952-116R | |
| X-gal | Corning | 46-101-RF |
Referenzen
- Harrington, K., Freeman, D. J., Kelly, B., Harper, J., Soria, J. -. C. Optimizing oncolytic virotherapy in cancer treatment. Nature Reviews Drug Discovery. 18 (9), 689-706 (2019).
- Zhang, S., Rabkin, S. D. The discovery and development of oncolytic viruses: are they the future of cancer immunotherapy. Expert Opinion on Drug Discovery. 16 (4), 391-410 (2021).
- Bommareddy, P. K., Peters, C., Saha, D., Rabkin, S. D., Kaufman, H. L. Oncolytic herpes simplex viruses as a paradigm for the treatment of cancer. Annual Review of Cancer Biology. 2 (1), 155-173 (2018).
- Peters, C., Rabkin, S. D. Designing herpes viruses as oncolytics. Molecular Therapy-Oncolytics. 2, 15010 (2015).
- Nguyen, H. -. M., Saha, D. The current state of oncolytic herpes simplex virus for glioblastoma treatment. Oncolytic Virotherapy. 10, 1-27 (2021).
- Koch, M. S., Lawler, S. E., Chiocca, E. A. HSV-1 oncolytic viruses from bench to bedside: an overview of current clinical trials. Cancers. 12 (12), 3514 (2020).
- Menotti, L., Avitabile, E. Herpes simplex virus oncolytic immunovirotherapy: the blossoming branch of multimodal therapy. International Journal of Molecular Sciences. 21 (21), 8310 (2020).
- Nguyen, H. M., Guz-Montgomery, K., Saha, D. Oncolytic virus encoding a master pro-inflammatory cytokine interleukin 12 in cancer immunotherapy. Cells. 9 (2), 400 (2020).
- Agarwalla, P. K., Aghi, M. K. Oncolytic herpes simplex virus engineering and preparation. Methods in Molecular Biology. 797, 1-19 (2012).
- Grosche, L., et al. Herpes simplex virus type 1 propagation, titration and single-step growth curves. Bio-protocol. 9 (23), 3441 (2019).
- Sutter, S. O., Marconi, P., Meier, A. F. Herpes simplex virus growth, preparation, and assay. Methods in Molecular Biology. 2060, 57-72 (2020).
- Froechlich, G., et al. Integrity of the antiviral STING-mediated DNA sensing in tumor cells is required to sustain the immunotherapeutic efficacy of herpes simplex oncolytic virus. Cancers. 12 (11), 3407 (2020).
- Froechlich, G., et al. Generation of a novel mesothelin-targeted oncolytic herpes virus and implemented strategies for manufacturing. International Journal of Molecular Sciences. 22 (2), 477 (2021).
- Mosca, J. D., Pitha, P. M. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Molecular and Cellular Biology. 6 (6), 2279-2283 (1986).
- Gousseinoz, E., Kools, W., Pattnaik, P. Nucleic acid impurity reduction in viral vaccine manufacturing. BioProcess International. 12 (2), 59-68 (2014).
- Diefenbach, R. J., Fraefel, C. Herpes simplex virus: methods and protocols. Methods in Molecular Biology. , (2014).
- Hadi, A. M., et al. An experimental trial to prepared γ1 34.5 herpes simplex virus 1 immunogene by cloning technique. Systematic Review Pharmacy. 11 (5), 140-149 (2020).
- Kuroda, T., Martuza, R. L., Todo, T., Rabkin, S. D. Flip-Flop HSV-BAC: bacterial artificial chromosome based system for rapid generation of recombinant herpes simplex virus vectors using two independent site-specific recombinases. BMC Biotechnology. 6, 40 (2006).
- Motamedifar, M., Noorafshan, A. Cytopathic effect of the herpes simplex virus type 1 appears stereologically as early as 4 h after infection of Vero cells. Micron. 39 (8), 1331-1334 (2008).
- Blaho, J. A., Morton, E. R., Yedowitz, J. C. Herpes simplex virus: propagation, quantification, and storage. Current Protocols in Microbiology. , 1 (2005).
- Malenovska, H. The influence of stabilizers and rates of freezing on preserving of structurally different animal viruses during lyophilization and subsequent storage. Journal of Applied Microbiology. 117 (6), 1810-1819 (2014).
- Vahlne, A. G., Blomberg, J. Purification of herpes simplex virus. Journal of General Virology. 22 (2), 297-302 (1974).
- Sathananthan, B., Rodahl, E., Flatmark, T., Langeland, N., Haarr, L. Purification of herpes simplex virus type 1 by density gradient centrifugation and estimation of the sedimentation coefficient of the virion. APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 105 (3), 238-246 (1997).
- Mundle, S. T., et al. High-purity preparation of HSV-2 vaccine candidate ACAM529 is immunogenic and efficacious in vivo. PLoS One. 8 (2), 57224 (2013).
- Jiang, C., et al. Immobilized cobalt affinity chromatography provides a novel, efficient method for herpes simplex virus type 1 gene vector purification. Journal of Virology. 78 (17), 8994-9006 (2004).
- Grosche, L., et al. Herpes simplex virus type 1 propagation, titration and single-step growth curves. Bio-protocol. 9 (23), 3441 (2019).
- Svennerholm, B., et al. Separation of herpes simplex virus virions and nucleocapsids on Percoll gradients. Journal of Virological Methods. 1 (6), 303-309 (1980).
- Baer, A., Kehn-Hall, K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. Journal of Visualized Experiments: JoVE. (93), e52065 (2014).
- Miyatake, S., Iyer, A., Martuza, R. L., Rabkin, S. D. Transcriptional targeting of herpes simplex virus for cell-specific replication. Journal of Virology. 71 (7), 5124-5132 (1997).
- Fabiani, M., Limongi, D., Palamara, A. T., De Chiara, G., Marcocci, M. E. A novel method to titrate herpes simplex virus-1 (HSV-1) using laser-based scanning of near-infrared fluorophores conjugated antibodies. Frontiers in Microbiology. 8, 1085 (2017).

