Using Phage Display to Develop Ubiquitin Variant Modulators for E3 Ligases
Summary
Ubiquitination is a critical protein post-translational modification, dysregulation of which has been implicated in numerous human diseases. This protocol details how phage display can be utilized to isolate novel ubiquitin variants that can bind and modulate the activity of E3 ligases that control the specificity, efficiency, and patterns of ubiquitination.
Abstract
Ubiquitin is a small 8.6 kDa protein that is a core component of the ubiquitin-proteasome system. Consequently, it can bind to a diverse array of proteins with high specificity but low affinity. Through phage display, ubiquitin variants (UbVs) can be engineered such that they exhibit improved affinity over wildtype ubiquitin and maintain binding specificity to target proteins. Phage display utilizes a phagemid library, whereby the pIII coat protein of a filamentous M13 bacteriophage (chosen because it is displayed externally on the phage surface) is fused with UbVs. Specific residues of human wildtype ubiquitin are soft and randomized (i.e., there is a bias towards to native wildtype sequence) to generate UbVs so that deleterious changes in protein conformation are avoided while introducing the diversity necessary for promoting novel interactions with the target protein. During the phage display process, these UbVs are expressed and displayed on phage coat proteins and panned against a protein of interest. UbVs that exhibit favorable binding interactions with the target protein are retained, whereas poor binders are washed away and removed from the library pool. The retained UbVs, which are attached to the phage particle containing the UbV’s corresponding phagemid, are eluted, amplified, and concentrated so that they can be panned against the same target protein in another round of phage display. Typically, up to five rounds of phage display are performed, during which a strong selection pressure is imposed against UbVs that bind weakly and/or promiscuously so that those with higher affinities are concentrated and enriched. Ultimately, UbVs that demonstrate higher specificity and/or affinity for the target protein than their wildtype counterparts are isolated and can be characterized through further experiments.
Introduction
Understanding the molecular details of protein-protein interactions is critical for delineating the signal transduction mechanisms of biological processes, particularly those that contribute to clinically important diseases. In recent years, phage display has been utilized as a practical and accessible method to isolate proteins/peptides with much improved binding to a desired target protein1,2,3,4, which in turn can be used as intracellular probes of protein-protein interactions.
Ubiquitination is a cascade of enzymatic activities (E1 activating enzyme → E2 conjugating enzyme → E3 ligases) that covalently conjugate ubiquitin (Ub) to protein substrates to target them for degradation or to mediate cell signaling changes. In addition, deubiquitinases catalyze the removal of ubiquitin from proteins. Therefore, in cells, there are thousands of Ub-dependent protein-protein interactions, the vast majority of which recognize a common surface with low affinity but high specificity to allow weak interactions through large and diverse surfaces.
Ernst et al. introduced mutations into known binding regions of Ub in order to see if they could enhance binding affinity for a protein of interest while still maintaining high selectivity5. A combinatorial library of over 10 billion (7.5 x 1010) Ub variants (UbVs) with mutations at positions across the Ub surface that mediate the known Ub-protein interactions was developed. This library consisted of phagemids that express the M13 bacteriophage pIII coat protein fused to diversified UbVs. Therefore, individual UbVs can be displayed on the phage surface via the coat protein upon expression. During the selection process, phage that display UbVs with considerable binding interactions with the target protein will be retained and enriched in subsequent rounds of phage display, whereas phage displaying UbVs that bind poorly to the target protein are washed away and removed from the phage pool. The retained phage particles contain the phagemid corresponding to their displayed UbV, allowing them to be sequenced and further characterized once isolated.
Using this protein engineering strategy, UbV inhibitors were developed for human deubiquitinases5 and viral proteases6. Importantly, we have generated inhibitory UbVs for human HECT-family E3 ligases through hijacking the E2-binding site and activating UbVs that occupy a Ub-binding exosite on the HECT domain7. We can also inhibit monomeric RING-family E3s by targeting the E2 binding site and induce UbV dimerization to activate homodimeric RING E3s8. For multi-subunit RING E3s, UbVs can achieve inhibition by targeting the RING subunit (e.g., for APC/C complex9) or disrupting complex formation (e.g., for SCF E3s10). Collectively, UbVs can be leveraged to systematically interrogate protein-protein interactions in the Ub-proteasome system (UPS) so that we can better decipher biochemical mechanisms of UPS enzymes and to identify and validate functional sites for therapeutic intervention.
The following protocol describes how to employ a previously generated phage displayed UbV library to target a protein of interest and how to enrich the UbV binders that interact with the target protein through successive rounds of phage display.
Protocol
1. Reagent preparation
- PBS (phosphate buffered saline): Mix 50 mL of 10x PBS solution with 450 mL of ultrapure H2O. Sterilize by filtration and store at 4 °C or room temperature (~20-25 °C).
- 10% BSA (bovine serum albumin): Slowly add 1 g of BSA to 7 mL of ultrapure H2O and mix until fully dissolved (no clumps). Top up with ultrapure H2O until the final volume is 10 mL. Sterilize by filtration and store at 4 °C.
- PB buffer (PBS supplemented with 1% BSA): Slowly add 5 g of BSA to 400 mL of ultrapure H2O and 50 mL of PBS and mix until fully dissolved (no clumps). Top up with ultrapure H2O until the final volume is 500 mL. Sterilize by filtration and store at 4 °C.
- PBT buffer (PBS supplemented with 1% BSA and 0.05% Tween 20): Slowly add 5 g of BSA to 400 mL of ultrapure H2O and 50 mL of PBS and mix until fully dissolved (no clumps). Add 250 µL of Tween 20. Top up with ultrapure H2O until the final volume is 500 mL. Sterilize by filtration and store at 4 °C.
- PT buffer (PBS supplemented with 0.05% Tween 20): Mix 1 mL of Tween 20 with 400 mL of PBS. Top up with ultrapure H2O until the final volume is 2 L. Sterilize by filtration and store at 4 °C or room temperature (~20-25 °C).
- 2YT broth: Add 16 g of tryptone, 10 g of yeast extract, and 5 g of NaCl to 800 mL of ultrapure H2O and mix until fully dissolved (no clumps). Top up with ultrapure H2O until the final volume is 1 L. Sterilize by autoclaving and store at room temperature (~20-25 °C).
- LB/carb plates: Add 12.5 g of pre-made LB mixture and 7.5 g of agar to 400 mL of H2O. Top up with ultrapure H2O until the final volume is 500 mL and sterilize by autoclaving. Make sure agar is fully dissolved and wait for it to cool to below 60 °C. Add 500 µL of 100 mg mL carbenicillin, mix well, and pour into plate. Store at 4 °C.
- LB/tet plates: Add 12.5 g of pre-made LB mixture and 7.5 g of agar to 400 mL of ultrapure H2O. Top up with ultrapure H2O until the final volume is 500 mL and sterilize by autoclaving. Ensure agar is fully dissolved and wait for it to cool to below 60 °C. Add 500 µL of 10 mg mL tetracycline, mix well, and pour into plate. Store at 4 °C.
- 20% PEG (polyethylene glycol)/2.5 M NaCl: Add 50 g of PEG-8000 and 36.5 g of NaCl to 200 mL of ultrapure H2O. Mix until fully dissolved.
NOTE: This may take a while; heating can help. Top up with ultrapure H2O until the final volume is 250 mL. Sterilize by filtration or autoclaving. - 0.1 M HCl: Mix 20 mL of 1 M HCl with 180 mL of ultrapure H2O. Sterilize by filtration and store at room temperature (~20-25 °C).
- 1 M Tris (pH 11.0): Add 6.1 g of Tris base to 40 mL of ultrapure H2O. Adjust pH to 11.0 with HCl and mix until Tris is fully dissolved. Top up with ultrapure H2O until the final volume is 50 mL. Sterilize by filtration and store at room temperature (~20-25 °C).
- 100 mg/mL (1000x) carbenicillin: Add 2 g of the carbenicillin disodium salt to 20 mL of ultrapure H2O. Mix until fully dissolved. Sterilize by filtration and store at -20 °C.
- 50 mg/mL (1000x) kanamycin: Add 1 g of kanamycin sulfate to 20 mL of ultrapure H2O. Mix until fully dissolved. Sterilize by filtration and store at -20 °C.
- 10 mg/mL (1000x) tetracycline: Add 0.2 g of tetracycline hydrochloride to 20 mL of 70% ethanol. Mix until fully dissolved. Sterilize by filtration and store at -20 °C.
2. Protein preparation
- Determine the concentration of the target protein stock in µM. The most convenient way to assess protein concentration is to measure the absorbance of the protein stock at 280 nm. If the concentration is known in mg/mL, convert it to µM using the molecular weight of the target protein. A range of methods can be used depending on the protein of interest; see the Discussion section for details.
NOTE: If the protein is truncated (specific protein domain/motif) or tagged, remember to account for these changes in molecular weight when converting from mg/mL to µM. - Prepare three microcentrifuge tubes labeled "round 1", "round 2/3", and "round 4/5".
- Calculate the volume of protein necessary to dilute it to 1 µM in 800 µL PBS and aliquot this volume into the tubes without adding PBS.
NOTE: If the amount of target protein available is low, the concentration can be lowered to as little as 0.25 µM.
- Calculate the volume of protein necessary to dilute it to 1 µM in 800 µL PBS and aliquot this volume into the tubes without adding PBS.
3. Preparation for round one of selection
- Prepare a seed culture for culturing the phage input for round two of selection.
- Inoculate 5 mL of 2YT/tet with a well-isolated Escherichia coli colony and incubate overnight at 37 °C with 200 rpm orbital shaking.
- Coat the plate for the first round of selection.
- Dilute the target protein in the "round 1" tube with the appropriate amount of PBS and aliquot 100 µL into eight wells of a 96-well binding plate (i.e., the target plate).
NOTE: Phage display can be done for four different proteins simultaneously on a single plate by coating the wells in the corners of the plate. - Optional control: If the target protein is tagged, such as with GST or MBP (maltose binding protein), coat eight wells in another 96-well binding plate with 100 µL of a 1 µM solution containing the appropriate epitope tag. This will be used to remove undesired phage that bind to the tag non-specifically.
- Shake plate(s) overnight at 4 °C with 200 rpm orbital shaking.
- Dilute the target protein in the "round 1" tube with the appropriate amount of PBS and aliquot 100 µL into eight wells of a 96-well binding plate (i.e., the target plate).
4. Round one of selection
- Prepare the round two input.
- Inoculate 30 mL of 2YT/tet with 200 µL of the seed culture from step 3.1.
- Incubate at 37 °C with 200 rpm orbital shaking until the bacteria are in mid-log phase (OD600
 0.6 – 0.8). This takes approximately three h.
0.6 – 0.8). This takes approximately three h.
- Block target plate.
- Remove the coating solution from the plate, or both plates if a control plate has been made, by inverting and shaking over a sink. Pat dry on paper towels.
- Add 300 µL of PB buffer to each coated well.
- Remove the PB buffer as in step 4.2.1 and add another 200 µL of PB buffer.
- Incubate at room temperature (~20-25 °C) for 1 h with 300 rpm orbital shaking.
- Prepare phage library.
- Thaw the phage library on ice and dilute it to 100x the library diversity in PBS. For example, if the library diversity is 1 x 1010 and the library concentration is 1 x 1013, dilute the library so that the concentration is 1 x 1012. For the libraries used here, dilute them 10-fold in PBS by combining 1 mL of the library with 9 mL of PBS.
NOTE: Library diversity should have been determined during library creation and cannot be easily assessed otherwise. Library concentration can be determined by inoculating E. coli cells in mid-log phase (OD600 0.6 – 0.8) and plating on LB/carb.
0.6 – 0.8) and plating on LB/carb. - Add 1/5 volume of PEG/NaCl. For example, for 10 mL of the previously diluted library, add 2 mL of PEG/NaCl.
- Incubate on ice for 30 min.
- Centrifuge at 11,000 x g for 30 min at 4 °C, discard supernatant, and recentrifuge for 2 min to pull down the remaining supernatant.
NOTE: Put the tube in the rotor in the same orientation the second time as it was the first to keep the pellet in the same place, therefore, making it easier to see. - Gently resuspend the phage pellet in 1 mL of PBT per protein. For four proteins, resuspend the pellet in 4 mL of PBT.
NOTE: Try not to touch the pellet and do not introduce air bubbles.
- Thaw the phage library on ice and dilute it to 100x the library diversity in PBS. For example, if the library diversity is 1 x 1010 and the library concentration is 1 x 1013, dilute the library so that the concentration is 1 x 1012. For the libraries used here, dilute them 10-fold in PBS by combining 1 mL of the library with 9 mL of PBS.
- Display phage to the target protein(s).
- Optional control: Add 100 µL of phage library to each coated well in the control plate. Incubate at room temperature (~20-25 °C) for 1 h with 300 rpm orbital shaking and transfer the library from the control plate to the target plate. Skip step 4.4.2.
- Remove the PB buffer from the target plate as in step 4.2.1 and add 100 µL of the phage library to each coated well.
- Incubate at room temperature (~20-25 °C) for 1 h with 300 rpm orbital shaking.
- Elute round one phage.
- Remove phage library and wash the coated wells four times with PT buffer. Invert the plate and tap on a paper towel to remove the last drops.
NOTE: If multiple target proteins are coated on one plate, try to minimize the flow of all subsequent solutions between the wells. - Add 100 µL of 0.1 M HCl to each coated well and incubate at room temperature for 5 min with 300 rpm orbital shaking.
- Neutralize the pH by adding 12.5 µL of 1 M Tris-HCl (pH 11) to each coated well.
- Pool the eluted phage from all 8 wells into a single 1.5 mL microcentrifuge tube. Pipette up and down during the transfer to make solutions homogenous and aspirate all liquid from the wells.
- Add 10% BSA to the pooled eluted phage to a final concentration of 1%. For a typical round one elution volume of 950 µL, add 95 µL of 10% BSA. Store at 4 °C. This is the round one output.
- Remove phage library and wash the coated wells four times with PT buffer. Invert the plate and tap on a paper towel to remove the last drops.
5. Preparation for subsequent rounds of selection
- Prepare a seed culture for culturing the phage input for the next round of selection as in step 3.1.
- Coat the plate for the third round of selection as in step 3.2 with the changes noted below.
- Only coat four wells per protein in a 96-well binding plate. Use half of the contents of the "round 2/3" or "round 4/5" tubes for the appropriate round. Dilute the contents of these tubes as needed, in order to avoid proteins settling out of the solution.
- Prepare the phage input for the next round of selection.
- Use half of the round one output from step 4.5.5 to inoculate 3 mL of mid-log phase cells from step 4.1. For a typical round one output, inoculate with 500 µL of the output.
- Incubate at 37 °C for 30 min with 200 rpm orbital shaking.
- Add M13K07 helper phage to a final concentration of 1 x 1010 PFU/mL.
- Incubate at 37 °C for 1 h with 200 rpm orbital shaking.
- Transfer the entire 3 mL of culture to 30 mL of 2YT/carb/kan. Grow overnight at 37 °C with 200 rpm orbital shaking.
- The next day, transfer the culture into a 50 mL centrifuge tube and centrifuge at 11,000 x g for 10 min at 4 °C to precipitate cells.
- Decant the supernatant into a new 50 mL centrifuge tube and mix with 8 mL of PEG/NaCl and incubate on ice for 10 min.
- Centrifuge at 11,000 x g for 10 min at 4 °C, discard the supernatant, and centrifuge again for 2 min.
NOTE: Put the tube in the rotor in the same orientation the second time as it was the first to keep the pellet in the same place, therefore, making it easier to see. - Resuspend the phage pellet in 800 µL of PBT so that it is fully homogenous and no clumps are visible.
- Transfer the phage solution to a 1.5 mL microcentrifuge tube and centrifuge at 16,200 x g for 4 min at 4 °C to pellet debris.
- Transfer the supernatant to a new microcentrifuge tube. This is the input for the next round of selection.
- Optional: Titer the input and output solutions.
- Use 500 µL of an E. coli seed culture to inoculate 5 mL of 2YT/tet and incubate at 37 °C with 200 rpm orbital shaking until bacteria is in mid-log phase (OD600
 0.6 – 0.8). This takes approximately 1 h.
0.6 – 0.8). This takes approximately 1 h. - Dilute phage input/output solutions to 10-3 - 10-5 in PBS and add 10 µL of each dilution to 90 µL of cultured cells.
NOTE: Use diluted phage solutions immediately because phage will adsorb to the tube over time, and transformations may produce false negatives. - Incubate at 37 °C for 20 min with 200 rpm orbital shaking.
- Plate 5 µL on separate LB/carb plates.
NOTE: Amount plated is variable depends on previous results/personal preference.
- Use 500 µL of an E. coli seed culture to inoculate 5 mL of 2YT/tet and incubate at 37 °C with 200 rpm orbital shaking until bacteria is in mid-log phase (OD600
6. Subsequent rounds of selection
- Perform all subsequent rounds along with the preparation as done in steps 3 and 4, respectively, with differences noted below.
- Use the input produced in the previous round as the phage source instead of a library. Coat 4 wells per target protein with 100 µL of the phage input acquired from the previous round. Store remaining phage inputs at 4 °C.
- Make the washing step in 4.5.1 more stringent with each round of selection. Round one requires washing 4 times, round two requires 6 times, round three requires 8 times, and rounds four and five both require washing 10 times.
- Reduce some volumes compared to those used in round one because subsequent rounds only coat four wells instead of eight. For example, in step 4.5.5 only 45 µL of 10% BSA is added to the phage output, and in step 5.3.1 only 250 µL of the phage output is used to inoculate cells.
7. Post selection processing and phage Isolation
- Titer the input and output solutions for rounds four and five.
- If not already done in step 5.4, follow the same steps to titer rounds four and five phage outputs, ideally producing a range of 30-300 colonies across a few plates.
- Culture and isolate phage.
- Aliquot 450 µL of 2YT/carb/M13K07 into every tube of a 96 mini tube culture box.
- Pick well-isolated colonies from any of the plates from step 7.1 to inoculate each tube.
NOTE: Use the top half of the box for round four outputs and the bottom half for round five outputs. - Incubate overnight at 37 °C with 200 rpm orbital shaking.
- Centrifuge at 1,200 x g for 10 min at 4 °C.
- Transfer as much supernatant as possible to a new 96 mini tube culture box without disturbing the cell pellet and discard the old one. Store at 4 °C.
- From the new box, transfer 100 µL from each tube to a 96 well non-binding plate containing 100 µL of 50% glycerol in all wells. Mix well and store at -80 °C as a backup stock.
Representative Results
Binders produced from phage display can be verified and analyzed in many ways. It is recommended to first proceed with sequencing the phage with primers that flank the diversified insert in the phagemid library. An ideal phage display experiment will show a clear bias towards several sequences (Figure 1). Other sequences will also be present but with a lower count, appearing more as background noise. In the example provided, where phage display was performed between ubiquitin variants (UbVs) and wildtype UBE4B, there is a particular bias towards sequences #1-4. An example Python script for organizing and analyzing sequencing files has been provided ("phageDisplaySeqAnalysis.py"). To confirm the significance of the binders, enzyme-linked immunosorbent assays (ELISA) can be used as a quick measure of relative binding affinity to the wildtype target protein, mutated target protein, as well as off-target proteins (Figure 1). Differences in binding affinities between the new binders and the wildtype binding protein, upon which the new binders were based, can be determined by normalizing the ELISA absorbance of the binders to that of the wildtype protein (not shown) or other proteins that demonstrate no appreciable binding with the target protein (Figure 1). This example demonstrates the tendency of the enriched sequences to have higher binding affinity for the target protein. An example Python script for organizing and analyzing sequencing files has been provided ("phageDisplayElisaAnalysis.py"). Additionally, an example Python script specifically for analyzing UbV results has been provided ("phageDisplayUbvAnalysis.py"). Ideally, binder sequences that are more numerous will possess higher relative binding affinity than less numerous sequences, which are presumably background noise. It is possible that unstable binders will be low in number but demonstrate appreciable binding through ELISAs. These binders should be further investigated to determine if the ELISA scores are artifacts or if they are indeed binders worthy of further characterization.
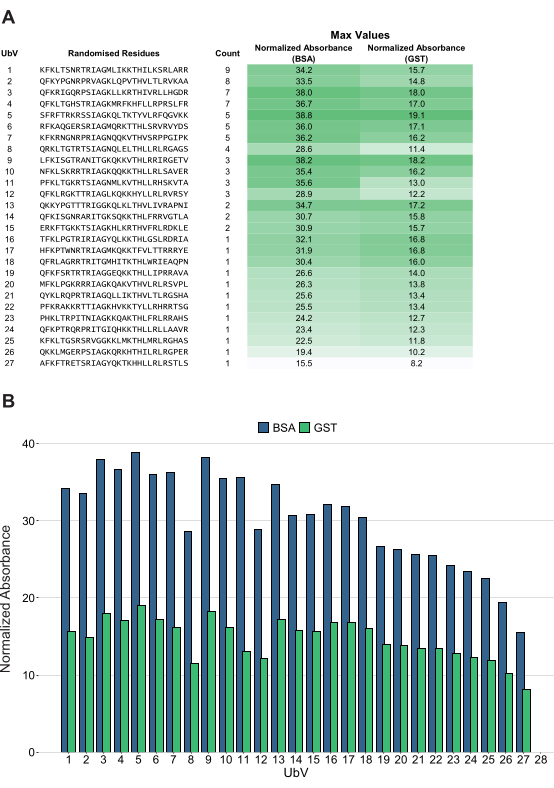
Figure 1: Representative results for a ubiquitin variant (UbV) selection against UBE4B. (A) UbVs were ordered from highest to lowest frequency (counts). Sequences represent the diversified ubiquitin region in the UbV with all the randomized residues (specifically, residues 2, 4, 6, 8-12, 14, 42, 44, 46-49, 62-64, 66, 68, and 70-78). All ELISA absorbances were normalized against 96 averaged BSA ELISA scores and 96 averaged GST ELISA scores. Darker green represents stronger relative binding. GST was included as a control for non-specific binding due to the fact that the target protein is GST-tagged. (B) Graphical summary of the ELISA results from panel A. Please click here to view a larger version of this figure.
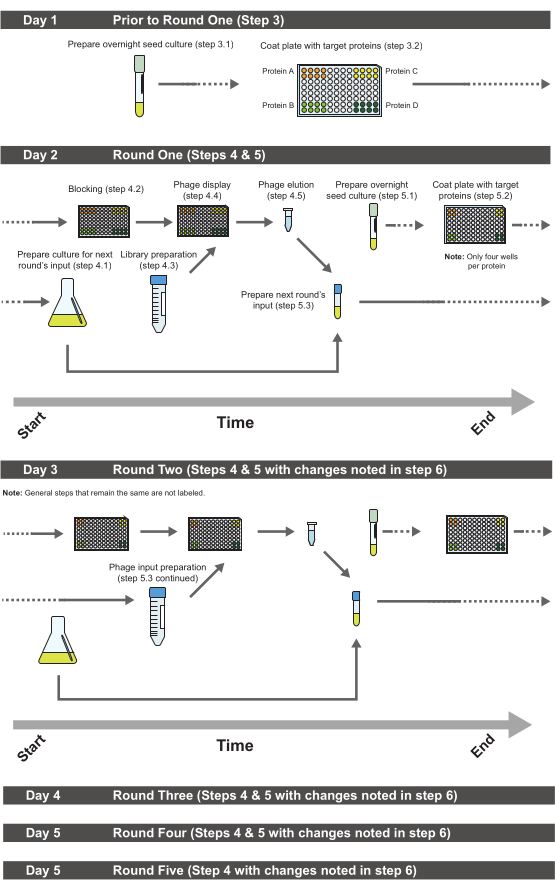
Figure 2. Suggested order of steps for performing phage display. Dashed lines indicate processes that are carried over to the next day or from the previous day. All subsequent rounds after round three proceed similarly to round three, excluding round five where no phage input preparation is necessary unless more rounds are being performed. Please click here to view a larger version of this figure.
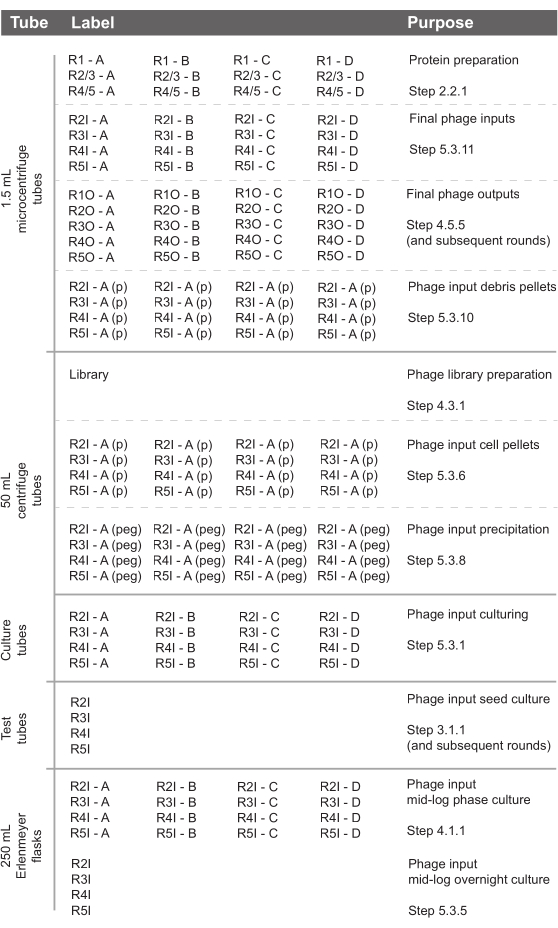
Figure 3. Suggested labware and labels for setting up a phage display experiment. Purpose and relevant steps in the protocol are indicated. R: round; I: input; O: output. Please click here to view a larger version of this figure.
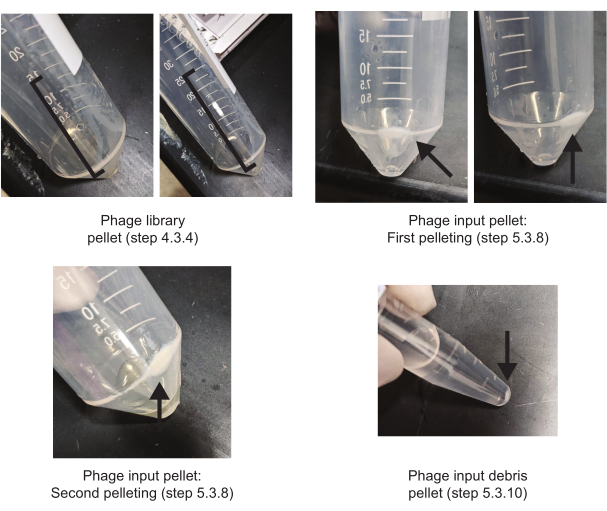
Figure 4. Appearances of typical pellets encountered during the phage display procedure. The phage library pellet presents as a streak along the side of the tube and can be recentrifuged to concentrate the pellet in the bottom of the tube. Phage input pellets and debris pellets appear more typical. Please click here to view a larger version of this figure.
Python Scripts. Please click here to download this File.
Discussion
As mentioned in step 2.1 (protein preparation), a variety of methods can be used to assess the protein concentration, and each will have unique benefits and drawbacks based on the specific target protein used for phage display. A source of detailed descriptions and protocols for popular methods has been provided previously11.
Using the phage retained by a previous round of phage display as the input for a subsequent round enriches the good binders by gradually removing binders that bound weakly, transiently, or by chance. By rounds four and five there will ideally be a clear bias towards a small and specific set of peptide sequences, demonstrating binding preferences of the target protein.
This protocol has been optimized for use with a UbV library. While these steps may generally apply for other kinds of libraries (e.g., peptide, antibody, etc.), they will likely need to be adapted to accommodate such non-UbV libraries. As mentioned in step 4.3.1, library diversity and concentration should be known prior to proceeding with phage display. For more information on how these library attributes are determined, please see the protocol used to create the UbV libraries used in this procedure12.
The phage display results can be very clear cut and present obvious candidate binders to pursue further characterization. For example, binders that are both highly frequent and have significantly higher binding, as measured by ELISA, are clear candidates for further study. However, when the frequency of a particular binder does not positively correlate with the ELISA data, this may present some confusion as to how to single out interesting binders. In the example provided (Figure 1), it would be recommended to pick a combination of the most common binders, even if they have a low binding affinity, and those with higher binding affinity, even if they appear infrequent. Infrequent binders with high ELISA scores may not be as common due to instability during phage display, which would negatively influence their prevalence in this final data. As such, these are worth investigating as much as the highly frequent binders are.
Isolated phage solutions can be contaminated easily. It is recommended to proceed with DNA sequencing as soon as possible using primers that flank the region of the diversified peptide. Another typical post-display analysis is to perform ELISAs of the binders with their target protein. Additionally, ELISAs can be performed with the binders and mutated/truncated versions of their target proteins, which can give a rough idea of their probable binding mode. It is very important to note that phage solutions ought to be mixed well prior to use in any experiment if they have been sitting for a while. The phage can adsorb to the walls of the tube or settle out of the solution and may produce false negatives.
The most time-efficient way of carrying out this procedure is illustrated in Figure 2. Begin every round with preparing the bacterial culture necessary for growing the phage input for the subsequent round the next day. While this culture is growing, coated plates can be blocked. While the plates are being blocked, the library can be prepared (for round one) or the phage input can be prepared (for subsequent rounds). On the day of round four there is no need to prepare a seed culture and thus on the day of round five there is no need to do any preparations for the next round's phage input unless one intends to do more than five rounds of selection. To further economize time, a suggested labware setup has also been provided (Figure 3). Additionally, phage pellets are not always distinct, so pictures of typical pellet appearances encountered during this procedure have been provided (Figure 4).
If there are any issues with pelleting the phage during the preparation of the input for a subsequent round, the experiment may need to be halted for a day while new phage are grown. These can be recovered by going back to the phage saved in the tubes for the input for that round. For example, if the phage necessary for the third round of display cannot be pelleted for some reason, you can return to the "R3I" tube that contains 400 µL of phage input for round three. This can only be repeated once if coating four wells with 100 µL.
The titer of output phage for rounds four to five is typically in the range of 106 to 108 PFU/mL. If titering is not of interest, simply having enough colonies present to fill up a 96 tube mini culture box should be sufficient to provide an accurate representation of the phage diversity and titer does not necessarily matter. However, if the titer of the output phage is low and more colonies are desired, phage can be reamplified by repeating step 5.3. Essentially, the phage can be reamplified by taking the output for the desired selection round, inoculating mid-log phase cells, adding helper phage and carbenicillin, growing the culture overnight, and harvesting the phage via PEG precipitation as previously described.
Other display methods do exist, and each possesses their own advantages and drawbacks relative to phage display. In vivo display methods, such as cell-surface display, may increase the likelihood of proper protein folding and also permit post-translational modifications to occur; however, these methods are constrained by having to use considerably smaller library sizes13 and expressing proteins polyvalently. Polyvalent expression introduces avidity effects that interfere with and mask the intrinsic affinity of the peptide, which is of greater interest when generating novel binders. Phage display bypasses this issue because it has been adapted for monovalent display, thereby facilitating the selection of binders with genuinely improved affinities14,15,16. Other in vitro display methods are similarly not impeded by the limitations of in vivo display methods but present their own unique challenges. For example, ribosome display may be used to probe larger libraries (1013-14)17, however, the output of the selections is in the form of mRNA molecules which are inherently less stable than the phage-encapsulated DNA output of phage display18. Other in vitro display methods, such as mRNA/cDNA display, cis activity-based display (CIS) and covalent antibody display (CAD) have demonstrated problems with efficiency, stability, and inconsistency19,20,21,22,23.
Phage display itself is limited by library sizes being restricted by the efficiency of bacterial transformation and by not permitting libraries with sequences that interfere with phage/bacterial growth16, but generally, these limitations are negligible, and phage display has been successful at producing highly specific and potent binders of target proteins2,5,10,24,25. This can not only be utilized in medical research to develop new therapeutics but also to elucidate protein and enzyme characteristics and learn more about protein interactions involved in important biological pathways.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The ubiquitin variant technology was devised in the laboratory of Dr. Sachdev Sidhu (University of Toronto). WZ is currently a CIFAR Azrieli Global Scholar in the Humans & The Microbiome Program. This research was funded by NSERC Discovery Grants awarded to WZ (RGPIN-2019-05721).
Materials
| Axygen Mini Tube System (0.65 mL, sterile, 96/Rack, 10 Racks/pack) | Fisher Scientific | 14-222-198 | Culturing phage outputs after phage display. |
| BD Difco Dehydrated Culture Media: LB Broth, Miller (Luria-Bertani) | Fisher Scientific | DF0446-17-3 | Preparing plates for titering. |
| Bovine Serum Albumin (BSA), Fraction V | BioShop Canada | ALB001 | Buffer component. |
| Carbenicillin disodium salt 89.0-100.5% anhydrous | Millipore-Sigma | C1389-5G | Culturing phagemid-infected cells. |
| Compact Digital Microplate Shaker | Fisher Scientific | 11-676-337 | Shaking plates during incubation with the phage library. |
| Corning Microplate Aluminum Sealing Tape | Fisher Scientific | 07-200-684 | Sealing phage glycerol stocks. |
| Dehydrated Agar | Fisher Scientific | DF0140-01-0 | Preparing plates for titering. |
| DS-11 Spectrophotometer/Fluorometer | DeNovix | DS-11 FX+ | Protein concentration measurement. |
| Greiner Bio-One CellStar 96-Well, Non-Treated, U-Shaped-Bottom Microplate | Fisher Scientific | 7000133 | Storing phage glycerol stocks. |
| Hydrochloric Acid | Fisher Scientific | A144-500 | Phage elution. |
| Invitrogen One Shot OmniMAX 2 T1R Chemically Competent E. coli | Fisher Scientific | C854003 | Bacterial strain for phage infection. |
| Kanamycin Sulfate | Fisher Scientific | AAJ1792406 | Culturing M13K07 helper phage-infected cells. |
| M13KO7 Helper Phage | New England Biolabs | N0315S | Permit phagemid packing and secretion. |
| MaxQ 4000 Benchtop Orbital Shaker | Fisher Scientific | 11-676-076 | Bacterial cell culture. |
| Nunc MaxiSorp 96 well microplate, flat bottom | Life Technologies | 44-2404-21 | Immobilizing proteins. |
| Phosphate Buffered Saline (PBS) 10X Solution | Fisher Scientific | BP3994 | Buffer component/phage resuspension medium. |
| Polyester Films for ELISA and Incubation | VWR | 60941-120 | Covering the microplates during incubation. |
| Polyethylene Glycol 8000 (PEG) | Fisher Scientific | BP233-1 | Phage precipitation. |
| Sodium chloride | Millipore-Sigma | S3014 | Phage precipitation. |
| Sterile Plastic Culture Tubes: Translucent Polypropylene | Fisher Scientific | 14-956-1D | Culturing phage inputs. |
| Tetracycline Hydrochloride | Fisher Scientific | BP912-100 | Culturing E. coli OmniMax cells. |
| Tris Base | Fisher Scientific | BP1525 | Neutralizing eluted phage solution. |
| Tryptone Powder | Fisher Scientific | BP1421-2 | Cell growth media component. |
| Tween 20 | Fisher Scientific | BP337500 | Buffer component. |
| Yeast Extract | Fisher Scientific | BP1422-2 | Cell growth media component. |
Referenzen
- Karlsson, O. A., et al. Design of a PDZbody, a bivalent binder of the E6 protein from human papillomavirus. Scientific Reports. 5 (1), 9382 (2015).
- Veggiani, G., et al. Engineered SH2 domains with tailored specificities and enhanced affinities for phosphoproteome analysis. Protein Science. 28 (2), 403-413 (2019).
- Kaneko, T., et al. Superbinder SH2 domains act as antagonists of cell signaling. Science Signaling. 5 (243), (2012).
- Wiechmann, S., et al. Site-specific inhibition of the small ubiquitin-like modifier (SUMO)-conjugating enzyme Ubc9 selectively impairs SUMO chain formation. Journal of Biological Chemistry. 292 (37), 15340-15351 (2017).
- Ernst, A., et al. A strategy for modulation of enzymes in the ubiquitin system. Science. 339 (6119), 590-595 (2013).
- Zhang, W., et al. Potent and selective inhibition of pathogenic viruses by engineered ubiquitin variants. PLOS Pathogens. 13 (5), 1006372 (2017).
- Zhang, W., et al. System-wide modulation of HECT E3 ligases with selective ubiquitin variant probes. Molecular Cell. 62 (1), 121-136 (2016).
- Gabrielsen, M., et al. A general strategy for discovery of inhibitors and activators of RING and U-box E3 ligases with ubiquitin variants. Molecular Cell. 68 (2), 456-470 (2017).
- Brown, N. G., et al. Dual RING E3 architectures regulate multiubiquitination and ubiquitin chain elongation by APC/C. Cell. 165 (6), 1440-1453 (2016).
- Gorelik, M., et al. Inhibition of SCF ubiquitin ligases by engineered ubiquitin variants that target the Cul1 binding site on the Skp1-F-box interface. Proceedings of the National Academy of Sciences. 113 (13), 3527-3532 (2016).
- Goldring, J. P. D. Protein quantification methods to determine protein concentration prior to electrophoresis. Protein Electrophoresis. 869, 29-35 (2012).
- Zhang, W., Sidhu, S. S. Generating intracellular modulators of E3 ligases and deubiquitinases from phage-displayed ubiquitin variant libraries. The Ubiquitin Proteasome System: Methods and Protocols. 1844, 101-119 (2018).
- Sheehan, J., Marasco, W. A. Phage and yeast display. Microbiology Spectrum. 3 (1), 1-17 (2015).
- Lowman, H. B., Wells, J. A. Monovalent phage display: A method for selecting variant proteins from random libraries. Methods. 3 (3), 205-216 (1991).
- Lowman, H. B., Bass, S. H., Simpson, N., Wells, J. A. Selecting high-affinity binding proteins by monovalent phage display. Biochemie. 30 (45), 1-7 (1991).
- Beaber, J. W., Tam, E. M., Lao, L. S., Rondon, I. J. A new helper phage for improved monovalent display of Fab molecules. Journal of Immunological Methods. 376 (1-2), 46-54 (2012).
- Galán, A., et al. Library-based display technologies: where do we stand. Molecular BioSystems. 12 (8), 2342-2358 (2016).
- Huang, S., et al. Ribosome display and selection of single-chain variable fragments effectively inhibit growth and progression of microspheres in vitro and in vivo. Cancer Science. 109 (5), 1503-1512 (2018).
- Yamaguchi, J., et al. cDNA display: a novel screening method for functional disulfide-rich peptides by solid-phase synthesis and stabilization of mRNA-protein fusions. Nucleic Acids Research. 37 (16), 1-13 (2009).
- Mochizuki, Y., Kumachi, S., Nishigaki, K., Nemoto, N. Increasing the library size in cDNA display by optimizing purification procedures. Biological Procedures Online. 15 (1), 1-5 (2013).
- Odegrip, R., et al. CIS display: In vitro selection of peptides from libraries of protein-DNA complexes. Proceedings of the National Academy of Sciences. 101 (9), 2806-2810 (2004).
- Reiersen, H., et al. Covalent antibody display–an in vitro antibody-DNA library selection system. Nucleic Acids Research. 33 (1), 1-9 (2005).
- Houlihan, G., Gatti-Lafranconi, P., Lowe, D., Hollfelder, F. Directed evolution of anti-HER2 DARPins by SNAP display reveals stability/function trade-offs in the selection process. Protein Engineering Design and Selection. 28 (9), 269-279 (2015).
- Zhang, W., et al. Generation and validation of intracellular ubiquitin variant inhibitors for USP7 and USP10. Journal of Molecular Biology. 429 (22), 3546-3560 (2017).
- Teyra, J., et al. Structural and functional characterization of ubiquitin variant inhibitors of USP15. Structure. 27 (4), 590-605 (2019).

