Stereotaxic Viral Injection and Gradient-Index Lens Implantation for Deep Brain In Vivo Calcium Imaging
Summary
Miniscope in vivo calcium imaging is a powerful technique to study neuronal dynamics and microcircuits in freely behaving mice. This protocol describes performing brain surgeries to achieve good in vivo calcium imaging using a miniscope.
Abstract
A miniature fluorescence microscope (miniscope) is a potent tool for in vivo calcium imaging from freely behaving animals. It offers several advantages over conventional multi-photon calcium imaging systems: (1) compact; (2) light-weighted; (3) affordable; and (4) allows recording from freely behaving animals. This protocol describes brain surgeries for deep brain in vivo calcium imaging using a custom-developed miniscope recording system. The preparation procedure consists of three steps, including (1) stereotaxically injecting the virus at the desired brain region of a mouse brain to label a specific subgroup of neurons with genetically encoded calcium sensor; (2) implantation of gradient-index (GRIN) lens that can relay calcium image from deep brain region to the miniscope system; and (3) affixing the miniscope holder over the mouse skull where miniscope can be attached later. To perform in vivo calcium imaging, the miniscope is fastened onto the holder, and neuronal calcium images are collected along with simultaneous behavior recordings. The present surgery protocol is compatible with any commercial or custom-built single-photon and two-photon imaging systems for deep brain in vivo calcium imaging.
Introduction
Intracellular Ca2+ signaling is an essential regulator of cell growth, proliferation, differentiation, migration, gene transcription, secretion, and apoptosis1. In neurons, Ca2+ signaling is precisely controlled since its spatio-temporal pattern is related to crucial functions such as membrane excitability, neurotransmitter release, and synaptic plasticity2.
In vivo calcium imaging is a powerful technique that can be utilized to decode neural circuit representation elemental to normal animal behaviors, identify aberrant neuronal activities in animal models of brain disorders, and unravel potential therapeutic targets that may normalize these altered circuitries. The two common in vivo calcium imaging systems are two-photon laser scanning fluorescence microscopy3,4,5,6 and head-mounted miniaturized microendoscopy (miniscope)7,8,9,10,11,12,13. The conventional two-photon microscopy offers commanding advantages such as better resolution, lower noise, and lower photobleaching; however, the experimental animals are required to be head-fixed, limiting the behavior studies that can be performed3,4,5,6. By contrast, the head-mounted miniscope system is small and portable, making it possible to study a wide variety of behavior tests using freely behaving animals7,8,9,10,11,12,13.
There are two leading Ca2+ indicators, chemical indicators5,14 and genetically encoded calcium indicators (GECIs)15,16. Ca2+ imaging has been facilitated by using highly sensitive GECIs delivered with viral vectors that allow specific labeling of neurons in the targeted circuit. The continuous effort to enhance the sensitivity, longevity, and ability to label even the subcellular compartments, makes GECIs ideal for various in vivo calcium imaging studies17,18,19.
The scattering of light in brain tissue during imaging limits the optical penetration in-depth, even with the two-photon microscopy. However, the Gradient Index (GRIN) lens overcomes this issue as the GRIN lens can be directly embedded into the biological tissues and relay images from the deep brain region to the microscope objective. Unlike conventional lens made of optically homogenous material and requires a complicated shaped surface to focus and create images, GRIN lens performance is based on a gradual refractive index change within the lens material that achieves focus with a plane surface20. GRIN lens can be fabricated down to 0.2 mm in diameter. Therefore, a miniaturized GRIN lens can be implanted into the deep brain without causing too much damage.
In this article, a complete surgery protocol is presented for the deep brain in vivo calcium imaging. For the demonstration purpose, we describe brain surgeries specifically targeting the medial prefrontal cortex (mPFC) of the mouse brain and in vivo calcium imaging recording via a custom-built miniscope system developed by Dr. Lin's group at the National Institute on Drug Abuse (NIDA/IRP)7,12. The experimental procedure involves two major brain surgeries. The first surgery is stereotaxically injecting a viral vector expressing GCaMP6f (a GECI) in the mPFC. The second surgery is to implant the GRIN lens into the same brain region. After recovery from these brain surgeries, the subsequent procedure is to affix the miniscope holder (base) on the mouse skull using dental cement. In vivo Ca2+ imaging can be performed any time after mounting the miniscope onto its base. The surgery protocol for viral injection and GRIN lens implantation is compatible with any commercial or custom-built single-photon and two-photon imaging systems for the deep brain in vivo calcium imaging.
Protocol
The experimental protocol follows the animal care guidelines of the University of Wyoming. The mouse used in this study is 6 months old male C57BL/6J. The procedure can be used to target any deep brain regions for in vivo calcium imaging. Here, for demonstration, the targeted brain area is the mouse mPFC (anterior and posterior (A/P): 1.94 mm, medial and lateral (M/L): 0.5 mm, dorsal and ventral (D/V): 1.8 mm). This protocol is modified based on the previously published protocol21.
1. Stereotaxic injection of virus in the mPFC (Figure 1)
- Preparation for the surgery
- Sterilize all the surgical instruments using an autoclave and place them over a sterile surface.
- Prepare a 10 µL syringe by priming it with saline and prefilling it with 5 µL of saline. Attach the syringe to a micropump (see Table of Materials).
- Turn on the heating pad and maintain the temperature at 35 °C.
- Place the mouse in the induction chamber (5" x 10" x 4", length x width x height) with 5% isoflurane and 1 L/min of oxygen flow rate. Carefully watch and count the mouse's respiratory rate. Take out the mouse once its respiratory rate decreases to 1 breath/s.
NOTE: The breathing rate of the mouse can be readily monitored by watching the downward and upward of the back muscle movement during each inhalation. - Allow the mouse to settle on a benchtop area separated from the surgical area. With a shaving clipper, shave the hair off from the mouse head till the first cervical vertebrae.
- Settle the mouse on the stereotaxic stage (see Table of Materials) and secure its position with ear bars and nose clip. Maintain the isoflurane flow to the stereotaxic stage at 1.5% isoflurane and 0.5 L/min of oxygen flow rate.
- Apply the lubricating ophthalmic eye ointment on both eyes with a clean cotton swab to prevent dryness of the eyes during surgery. Assess pedal reflexes on the mouse to confirm that the mouse is fully anesthetized before starting the surgery.
- Disinfect the hairless area with 7.5% povidone-iodine solution (see Table of Materials) and 70% ethanol three times each using sterile cotton swabs.
- Inject a small volume (50 µL) of 2% lidocaine under the skin of the hairless area.
- Make a 2 cm incision through the skin along the midline using a scalpel to expose the lambda and bregma of the skull.
- Remove the fascia from the skull with the help of dry cotton swabs and pointed forceps.
- After the bregma and lambda are visible, use the tip of a dental drill burr (0.5 mm in diameter) to measure the Z coordinates of bregma and lambda (see Table of Materials). Adjust the height of the nose holder until the bregma and lambda lie at the same Z position.
- Locate the 0.5 mm dental drill burr to a position of A/P: 1.94 mm, M/L: 0.5 mm from the bregma. Drill through the skull.
NOTE: Here, for demonstration, the targeted brain region is the mouse mPFC. This protocol can be used to target any other deep brain region. For example, if the targeted brain area is the Nucleus Accumbens (NAc), the corresponding position should be A/P: 0.9 mm, M/L: 1.2 mm. - Remove the dura using a 30 G needle tip and clean all pieces of bone debris using 45° angled sharp forceps.
NOTE: Bleeding is common as small blood vessels may get ruptured during the cleaning step. Use sterile cotton swabs to stop bleeding and apply saline to wash the area. Apply saline to the exposed skull area to keep it moist. - Load the virus into the microliter syringe using the control panel of the micropump. Withdraw 500 nL of air bubble followed by 800 nL of the virus at a flow rate of 50 nL/s.
NOTE: For the demonstration purpose, here, an adeno-associated virus serotype 1 (AAV1) expressing GCaMP6f (a GECI), AAV1-CamKII-GCamp6f, is injected into the mPFC (see Table of Materials). The titer of the virus is 2.8 x 1013 GC/mL. It is 1:2 diluted in saline before injection. - Place the tip of the needle on the top of the bregma to just touching the bregma and note down the Z-coordinate of bregma. Move the needle above the drilled hole and inject 100 nL of the virus to ensure that the needle is not clogged.
- Slowly move down the needle into the brain tissue to the targeted Z-coordinate of D/V: 1.75 mm and then slightly move it up to the Z-coordinate of D/V: 1.65 mm.
NOTE: This is to create a small pocket for the viral solution to be infused. If the targeted brain region is the NAc, the corresponding targeted Z-coordinate of D/V should be 4.2 mm, and then slightly move up to 4.1 mm. - Use the control panel to set the micropump to injecting 500 nL of the virus at the flow rate of 50 nL/min. Hit the RUN button on the control panel to inject the virus.
NOTE: Injection will take about 10 min. After the injection is over, wait for an additional 5-10 min before taking the needle out from the brain. Frequently apply saline to keep the exposed skull area moist during the injection period. - Move the needle up and out of the brain. Inject 500 nL volume twice at the flow rate of 50 nL/s.
NOTE: This step confirms that a proper volume of the virus has been administered into the brain. During the first 500 nL injections, the virus is released, followed by air bubbles. During the second 500 nL injection, air bubbles first appear, followed by saline. The syringe is now ready to load the virus for the next mouse. Once the surgery is done, the microliter syringe and needle are thoroughly cleaned with acetone followed by saline. - Line up the skin edges and carefully close the incision with a suture (size 4.0). Apply antibiotic ointment on the stitched area to prevent infection.
- Remove the mouse from the stereotaxic stage and return it to its home cage. Place the home cage in a 33 °C incubator until the mouse is ambulatory.
NOTE: It usually takes 10-15 min for a mouse to wake up from isoflurane anesthesia before it starts moving around. - After the mouse starts moving around, administer nonsteroidal anti-inflammatory drugs for 3 postsurgical days (see Table of Materials). Let the mouse recover from the surgery for 14 days before pursuing GRIN lens implantation.
2. GRIN lens implantation in the mPFC (Figure 1)
- Preparation for the surgery
- Prepare the artificial cerebrospinal fluid (ACSF) containing 124 mM of NaCl, 2.5 mM of KCl, 1.25 mM of NaH2PO4, 1.2 mM of MgCl2, 25 mM of glucose, 26 mM of NaHCO3 and 2.4 mM of CaCl2.
- Sterilize all the surgical instruments using an autoclave and place them over a sterile surface.
- Turn on the heating pad and maintain the temperature at 35 °C.
- Melt 1% Agarose and keep it in a water bath at 42 °C until use.
NOTE: The melted agarose can be kept in the water bath for a couple of hours. - Disinfect a GRIN lens (1 mm in diameter, 4.38 mm in length, Figure 2A) in 70% ethanol for 15 min, transfer it into a tube filled with saline to rinse it properly before implanting.
NOTE: GRIN lenses (see Table of Materials) are produced via silver and lithium-ion exchange in special glasses, rendering them non-toxic and neuron-friendly6,7. However, many commercially available GRIN lenses may leach toxic residuals, causing neurodegeneration, making them unsuitable for implantation in the live brain for long-term in vivo imaging studies. These GRIN lenses may require coating with biocompatible agents like parylene-C to prevent toxic side effects on neighboring neurons22.
- Weigh the mouse and anesthetize it by an intraperitoneal injection of Ketamine/Xylazine mixture (see Table of Materials) (Ketamine:100 mg/kg; Xylazine: 15 mg/kg).
NOTE: A mouse weighing 30 g requires 300 µL of Ketamine/Xylazine mixture (Ketamine 100 mg/mL and Xylazine 1.5 mg/mL) for the initial dose and 150 µL of Ketamine (10 mg/mL) for additional doses during surgery. To keep the mouse stay anesthetized during the entire surgical process, additional doses of Ketamine (50 mg/kg) need to be administered at least once per hour. The anesthesia stage of the mouse needs to be frequently monitored by assessing pedal reflexes. - Shave the hair off the surgical area with a shaver and clean the hair using a wet paper towel.
- Place the mouse in the stereotaxic stage and secure its position by tightening the nose clip and ear bars. Apply lubricating ophthalmic eye ointment on both eyes with a sterile cotton swab. Confirm that the mouse is fully anesthetized by assessing pedal reflexes.
- Disinfect the hairless area with 7.5% povidone-iodine solution and 70% ethanol three times each using sterile cotton swabs. Administer 2 mg/kg of Dexamethasone intramuscularly in the thigh to lower the risk of surgery-related swelling and inflammation.
- Inject 50 µL of 2% Lidocaine under the skin of the surgery area.
- Use a fine scissor to excise a 1.5 cm (height) x 1.0 cm (base) triangular skin area, from the anterior side between the eyes to the posterior side behind the lambda.
- Remove the periosteum tissue from the skull using fine forceps, micro-blade, and cotton swabs.
NOTE: The skull should be thoroughly cleaned and dried before pursuing the next step. - Apply cyanoacrylate (see Table of Materials) to the edges of the skin and attach the skin to the skull. Wait for 5 min until the cyanoacrylate dries.
- With the help of a 0.5 mm drill burr tip, align the bregma and lambda in the same horizontal plane by adjusting the height of the nose clip.
- Locate a dental drill burr (1.2 mm in diameter) to a position of A/P: 1.94 mm, M/L: 0.8 mm from the bregma. Drill through the skull. Remove the dura using a 30 G needle tip and clean all pieces of bone debris using 45° angled sharp forceps.
NOTE: This bone debris can block the subsequent aspiration step if not removed completely. - Attach a 27 G manually polished blunt-end needle (Figure 2B) to the needle holder coupled to a robotic arm tilted with an angle of 10° (Figure 2C). Connect the other end of the needle holder to the house vacuum system.
NOTE: The robotic arm (see Table of Materials) is developed by Dr. Lin's group at the NIDA/IRP and is controlled by a custom-developed, currently open-access software, AutoStereota (https://github.com/liang-bo/AutoStereota)23 - Locate the tip of the needle to just touching the bregma. Set the Z-coordinate of bregma to 0 by clicking on Bregma button on AutoStereota.
- Set Input X value to 0.8, Input Y value to 1.94, Input Z value to 1.0, then click on Find button to move the needle onto the top of the drilled hole on the skull.
- Adjust the needle's position to the center of the exposed brain tissue area via AutoStereota.
NOTE: To move the needle in lateral or medial directions, enter the Step value and click on Lateral or Medial buttons on AutoStereota. Similarly, to move the needle in anterior or posterior and dorsal or ventral directions, click on Rostral or Caudal and Dorsal or Ventral buttons, respectively. - Turn on the vacuum and start rinsing the exposed brain area with ACSF through a gravity-controlled tubing system (see Table of Materials) connected to a 30 G needle with a bent tip. ACSF gets continuously bubbled with a gas mixture of 95% O2 and 5% CO2 and filtered through a 0.2 µm filter.
NOTE: The ACSF flow rate is ~ 1.5 mL/min. The output pressure of the gas mixture is kept at ~3 psi. - Aspirate brain tissue layer-by-layer with the help of AutoStereota software (Figure 3).
NOTE: Aspiration of brain tissue is completed in 4 rounds so that a column pocket (1.8 mm in depth and 1 mm in diameter) is generated.- In "zStep" session, click and check the first and second rows. Set the values for the first row to 0.2 and 1. Set the values for the second row to 0.15 and 4 (Figure 3A).
- In "Mode" session, set Needle size 27 Gauge and 1.2, set Dims 0.9. All other values use default values (Figure 3A).
- Click sequentially on Set, Keep Zero and Start buttons to start aspiration.
NOTE: This is the 1st round of aspiration. Input values indicate that aspiration depth is 0.2 mm (from the Z-coordinate of 0) during the first step with 1 layer; during the second step, aspiration depth is 0.15 mm, continually repeated for 4 layers. The aspiration resolution is 1.2, and the diameter is 0.9 mm. During aspiration, the instant location of the needle tip can be monitored through the track graph panel. After completing this aspiration round, a column pocket with 0.8 mm in depth and 1 mm in diameter is generated. The needle tip will be back to the center with the Z-coordinate of 0. - In "zStep" session, click and check the first and second rows. Set the values for the first row to 1 and 1. Set the values for the second row to 0.15 and 4 (Figure 3B).
- In "Mode" session, keep all values the same as the previous round (Figure 3B).
- Click sequentially on Set, Keep Zero and Start buttons to start aspiration.
NOTE: This is the 2nd round of aspiration. Input values indicate that aspiration depth is 1 mm (from the Z-coordinate of 0) during the first step with 1 layer; during the second step, aspiration depth is 0.15 mm, continually repeated for 4 layers. After completing this aspiration round, the column pocket with 1.6 mm in depth and 1 mm in diameter is generated. - In "zStep" session, click and check the first row only. Set the values for the first row to 1.8 and 1 (Figure 3C).
- In "Mode" session, set Needle size 27 Gauge and 2.2. All other values remain the same as that of the previous round (Figure 3C).
- Click sequentially on Set, Keep Zero and Start buttons to start aspiration.
NOTE: This is the 3rd round of aspiration. Input values indicate aspiration depth is 1.8 mm (from the Z-coordinate of 0) with 1 layer. The aspiration resolution is 2.2. After completing this aspiration round, the column pocket with 1.8 mm in depth and 1 mm in diameter is generated. - In "zStep" session, click and check the first row only. Set the values for the first row to 1.6 and 1 (Figure 3D).
- In "Mode" session, set Needle size 27 Gauge and 2.2, set Dims 0.6 (Figure 3D).
- Click sequentially on Set, Keep Zero and Start buttons, to start aspiration.
NOTE: This is the 4th round of aspiration. The purpose of this step is to clean out blood accumulated in the pocket. Bleeding is common as small blood vessels get ruptured during the aspiration process. To thoroughly clean out blood, stop the irrigation of ACSF for 5 min and then turn on irrigation again. Repeat the 4th round of aspiration multiple times until the pocket is blood-free. This protocol can be used to target any other deep brain regions. For example, if the targeted brain area is the NAc, the final corresponding Z-coordinate of D/V should be 4.4 mm.
- Stop the vacuum and irrigation of ACSF. Bring the needle to +2 mm Z-coordinate and 0.5mm anterior to the center. Place the sterile 1mm GRIN lens into the pocket.
- Keep the tip of the needle in contact with the exposed GRIN lens to ensure the GRIN lens being settled at 10° angle. Gently press the upper surface of the GRIN lens with sterile soft tissue paper to ensure that the lower surface of the GRIN lens is in contact with the brain tissue.
- Apply the melted Agarose in the gap between the GRIN lens and brain tissue with the help of a spatula. After Agarose forms a gel, remove excess Agarose using a micro-blade.
- Clean the skull thoroughly with saline and cotton swabs. Allow the skull to dry before the subsequent dental cement (see Table of Materials) application.
- Take out the mixing well from -20 °C freezer. Mix the dental cement powder and catalyst liquid and apply a layer of self-curing adhesive resin cement on the skull; first, surround the GRIN lens and then cover the entire exposed skull.
- Wait for 5 min and allow it to harden completely. Remove the aspiration needle carefully.
- In a clean plastic well, mix dental cement powder, black charcoal with liquid, and apply a thin layer of the mixture on top of the 1st layer of dental cement. Wait for 5 min to let it harden.
- Protect the exposed GRIN lens by covering it with a customized cap made from a PCR tube (Figure 2D). Apply cyanoacrylate to attach the cap to the dental cement.
- Inject 1 mL of prewarmed saline subcutaneously into the mouse followed by 0.1 mg/kg of Buprenorphine. Place the mouse back in its home cage. Place the home cage in a 33 °C incubator and monitor the mouse until it is ambulatory. It usually takes 20-40 min for a mouse to wake up from anesthesia before it starts moving around.
- Administer nonsteroidal anti-inflammatory drugs and monitor the mouse for at least 3 postsurgical days. Let the mouse recover from the surgery for 30 days.
3. Affixing miniscope holder (base) to the mouse skull (Figure 1)
- Anesthetize the mouse in an induction chamber with 5% isoflurane and 1 L/min oxygen flow rate until its respiratory rate decreases to 1 breathe/s.
- Place the mouse in the stereotaxic stage and secure its position with ear bars and nose clips. Maintain a continuous flow of isoflurane (1.5%) and oxygen (0.5 L/min).
- Apply ophthalmic ointment on its eyes to keep them moist. Turn on the heating pad and maintain the temperature at 35 °C. Confirm that the mouse is fully anesthetized by assessing pedal reflexes.
- Remove the cap that covers the GRIN lens gently using pointed forceps. Drill off the dried cyanoacrylate residues from the dental cement completely using a microdrill (see Table of Materials).
- Cut the hair around the dental cement area with small scissors. Clean out the debris with the help of a compressed-air duster. Use an acetone dipped cotton swab to clean the upper surface of the GRIN lens.
- Prepare the miniscope with its holder (base).
- Place a #00-90 hex nut (see Table of Materials) into the slot present in the base and apply cyanoacrylate to secure it there (Figure 2E).
- Apply a polytetrafluoroethylene (PTFE) tape tightly around the thread of the miniscope and trim off the extra tape (Figure 2F).
- Fasten the miniscope to the base and use the locking screw to secure the miniscope to the base (Figure 2G).
- Connect the miniscope to its cable and turn on the custom-developed, currently open-access software, NeuView (see Table of Materials).
NOTE: NeuView software is custom-developed for miniscope in vivo calcium imaging from Dr. Lin's group at the NIDA/IRP7,12. It is open access (https://github.com/giovannibarbera/miniscope_v1.0). - In NeuView, click Hardware, check LED1, and click the Stream button to view the live images (Figure 4A). To stop live streaming, click on Stop button.
- Settle the miniscope into a custom-built miniscope holding arm (Figure 2H) whose XYZ position can be manipulated using motorized controllers (Figure 2I).
- Locate the miniscope just above the exposed GRIN lens and make it parallel to the surface of the lens. Slowly bring down the miniscope towards the GRIN lens and adjust its Z position until the best plane of focus is found.
NOTE: The best focal plane is determined by comparing several possible Z positions and selecting the focal plane with a clear visualization of most cell bodies. - Apply the first layer of dental cement around the miniscope base carefully without altering the position of the miniscope. After the cement is hardened, gently remove the holding arm such that the miniscope can stand on its own on the mouse head.
NOTE: The dental cement tends to shrink after hardening, and it will drag down the miniscope away from the original plane of focus. Typically the miniscope Z position is slightly lifted above the original focal plane before applying dental cement to compensate for the potential change. - Apply a second layer of dental cement around the base to fill all the gaps and ensure there is no LED light leakage from the gaps. Let the dental cement harden.
- Remove the mouse from the stereotaxic stage. Loosen the locking screw and detach the miniscope from the base. Put a 3D printed protective cap into the base to protect the exposed GRIN lens and tighten the locking screw in the base (Figure 2J).
- Place the mouse back in its home cage.
NOTE: It usually takes 10-15 min for a mouse to wake up from isoflurane anesthesia before it starts moving around.
4. Miniscope mounting and in vivo Ca2+ imaging (Figure 1)
- Mount the miniscope to its base
- Anesthetize the mouse briefly in the induction chamber (5% isoflurane and 1 L/min oxygen flow rate). Place the mouse on a clean bench surface.
- Loosen the locking screw with a small screwdriver, remove the protective cap and clean the surface of the GRIN lens with an acetone-soaked cotton swab.
- Wrap a PTFE tape tightly around the miniscope thread and fasten the miniscope to its base on the mouse head.
- Connect the miniscope to the cable (Figure 2K), turn on the NeuView Software.
- In NeuView, click Hardware, check LED1, and click the Stream button to view the live images (Figure 4A).
- Identify the best focal plane by adjusting the position of the miniscope relative to the base, either slightly tightening or loosening with the help of blunt forceps.
NOTE: The best focal plane is determined by comparing several possible locations and selecting the one with a clear visualization of most cell bodies. - Tighten the locking screw and place the mouse back in its home cage.
- In NeuView, adjust the LED light power to the optimal level. Click on Capture and then Trigger buttons to start recording and hit Stop after 150 frames.
NOTE: The lowest possible power determines the optimal level of the LED power for achieving a bright enough image. The purpose of recording a short video is to facilitate identifying the same focal plane in the future for repetitive imaging. - Disconnect the cable from the miniscope. Let the mouse recover for at least 30 min before starting the experiment.
NOTE: To protect the miniscope, typically, the water bottle and the wire food feeder are removed during this short period of time. If the mouse needs to stay in its home cage for longer, it is recommended to supply commercially available diet gel (see Table of Materials) in the home cage.
- Data acquisition for in vivo calcium imaging
NOTE: In vivo calcium Imaging can be simultaneously conducted along with any behavior tests that the researcher desires. For the demonstration purpose, the example here is in vivo Ca2+ imaging during an open field test. To accomplish this goal, two computers are required. One computer is equipped with commercial software (see Table of Materials) to control a camera to record mouse behavior automatically. The other computer is equipped with NeuView to control the miniscope and to record the Ca2+ images.- Turn on the behavior camera software (see Table of Materials) to view the mouse behavior arena through a Livestream function. Manually adjust the focus of the top camera (Figure 2L).
- Select Trigger/Strobe and check "Enable/disable trigger" (Figure 4B). Click Record button, use Durchsuchen to select the location where behavior recordings will be saved, select the desired image format.
NOTE: "Trigger Control" function is enabled to allow the behavior recording to be triggered by the NeuView software so that the behavior frames and the corresponding calcium imaging frames are temporally coupled together. The behavior recording can be saved in any desired format. Behavior recording is generally saved in JPEG format in the designated folder. - Bring the mouse close to the arena and connect the miniscope to the cable linked to the Data Acquisition System (Figure 2L) (see Table of Materials). The mouse is then placed in the center of the arena.
- With the Livestream function of NeuView software, adjust LED power to optimize the brightness of calcium image.
NOTE: For calcium imaging, the recording frame rate is 10 frames/s by default. - In NeuView, uncheck LED1, click on Capture and then Trigger buttons to start recording, and hit Stop after 100 frames.
NOTE: This is to record background images for about 100 frames with LED light off. - In the behavior camera software, click Start Recording (Figure 4C). In NeuView, check LED1, click on Capture, and then Trigger buttons to start recording. Hit Stop after 3000 frames.
NOTE: This is to record calcium imaging and behavior simultaneously. The open-field test is 15 min long, typically broken down into three recording sessions each of 5 min. This is to prevent the miniscope from overheating due to continuous use. - Save both the calcium imaging and behavior recordings in the designated folders. Repeat the recording for two more sessions while recording the background before each session and save all the recordings in the designated folder.
- Detach miniscope from its base.
- After the recording is completed, disconnect the cable from the miniscope.
- Anesthetize the mouse briefly in the induction chamber (5% isoflurane and 1L/min of oxygen flow rate). Place the mouse on a clean, warm surface.
- Unscrew the locking screw in the base and detach the miniscope from the base. Put back the protective cap over the base and tighten the locking screw. Put the mouse back in its home cage.
Representative Results
Figure 1 shows the schematic experimental procedure, including viral injection, GRIN lens implantation, affixation of the miniscope base to the mouse skull, and in vivo calcium imaging via a miniscope. The entire procedure takes ~2 months. Figure 2 shows the major components described in the protocol for miniscope in vivo calcium imaging. Figure 3 displays the interfaces of AutoStereota software during GRIN lens implantation. Figure 4 displays the interfaces of NeuView and behavior recording software during in vivo calcium imaging.
The outcome of in vivo calcium imaging is dependent on the success of both viral injection and GRIN lens implantation surgeries. Figure 5 shows a range of outcomes (i.e., unsuccessful, suboptimal, and good) from in vivo calcium imaging recordings. In unsuccessful cases, the calcium image could appear either dark or bright but usually reveals no or very few active neurons. We typically do not pursue in vivo calcium recording experiments if there are fewer than five active neurons. A good in vivo calcium imaging typically reveals several hundreds of active neurons. If a recording contains less than a hundred active neurons, we consider it a suboptimal recording.
In both suboptimal and good recordings in vivo calcium imaging experiments were pursued, and the subsequent data analysis was performed. Movie 1 shows a representative in vivo calcium imaging recording from the mouse mPFC. Behavior videos and calcium imaging data are usually processed separately. Mouse behavior videos can be manually scored. Calcium imaging files are processed using the CaImAn calcium image processing toolbox24. Figure 6 shows a representative cell map and several calcium traces from a good in vivo calcium imaging recording.
After completing in vivo calcium imaging, the final step is to confirm whether the viral injection and GRIN lens implantation has occurred in the desired brain region. For this purpose, the mouse was perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA). The mouse brain was harvested, postfixed in 4% PFA for 12 h, and stored in PBS at 4 °C. The mouse brain was then sectioned in 50 µm thick slices with a vibratome. The brain slices were stained with DAPI and observed under the microscope (not described in the protocol)12. Figure 7 is a mouse brain slice ~1.94 mm anterior to bregma from an experimental mouse, showing the track where the GRIN lens was implanted. The green fluorescence region beneath and around the GRIN lens track indicates the expression of GCaMP6f in the mPFC region.
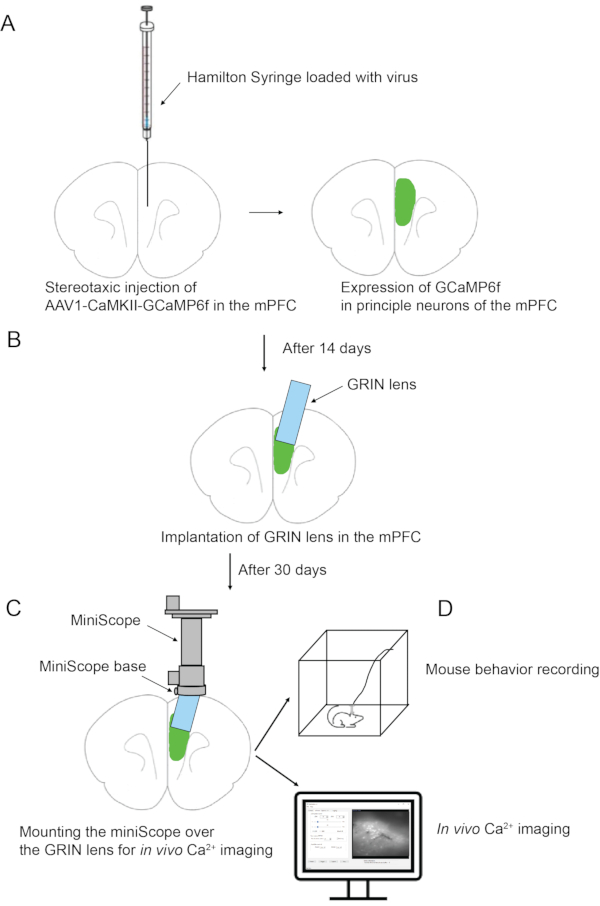
Figure 1: Schematic overview of the experimental procedure. (A) Stereotaxic injection of virus in the mPFC. (B) GRIN lens implantation in the mPFC. (C) Affixing miniscope base to the mouse skull. (D) Miniscope mounting and in vivo calcium imaging. Please click here to view a larger version of this figure.
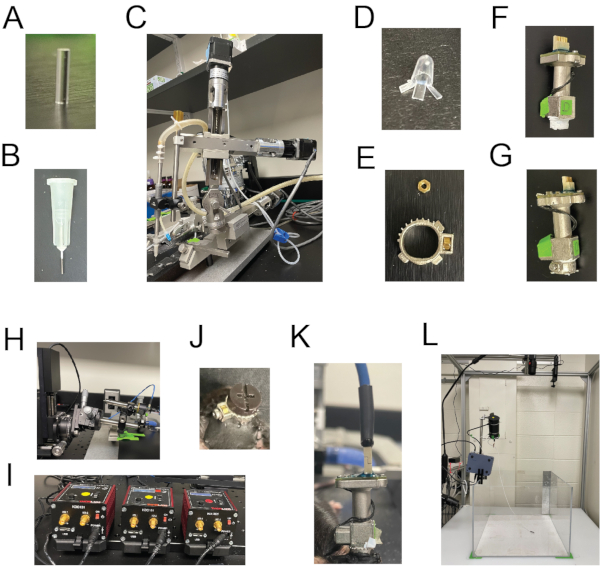
Figure 2: Main components needed for in vivo calcium imaging. (A) A GRIN lens with 1 mm diameter and 4.38 mm length. (B) A 27 G manually polished blunt-end needle used for brain tissue aspiration. (C) The robotic arm coupled to the needle holder. (D) A custom-made cap from a PCR tube to protect the exposed GRIN lens until the affixation of miniscope base on the mouse skull. (E) The miniscope holder (base) with a hex nut. (F) A miniscope whose thread part is wrapped with the PTFE tape. (G) A miniscope fastened to its base with a locking screw. (H) The miniscope holding arm. (I) A custom-built 3D motorized controller used to facilitate the movement of miniscope in XYZ positions. (J) A protective cap fastened onto the base to protect the exposed GRIN lens while the mouse is not performing in vivo calcium imaging. (K) The miniscope connected to the cable. (L) The Data Acquisition System for in vivo Ca2+ imaging. Please click here to view a larger version of this figure.
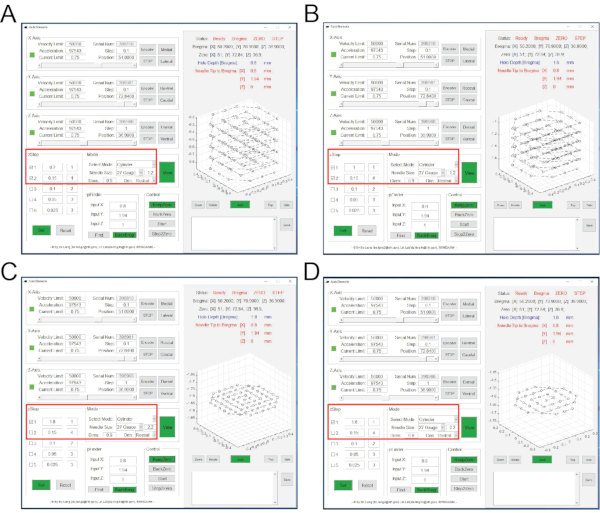
Figure 3: Interfaces of AutoStereota software during layer-by-layer brain tissue aspiration. (A) The interface corresponding to steps 2.17.1 to 2.17.3. (B) The interface corresponding to steps 2.17.4 to 2.17.6. (C) The interface corresponding to steps 2.17.7 to 2.17.9. (D) The interface corresponding to steps 2.17.10 to 2.17.12. Red boxes highlight the input values. Please click here to view a larger version of this figure.
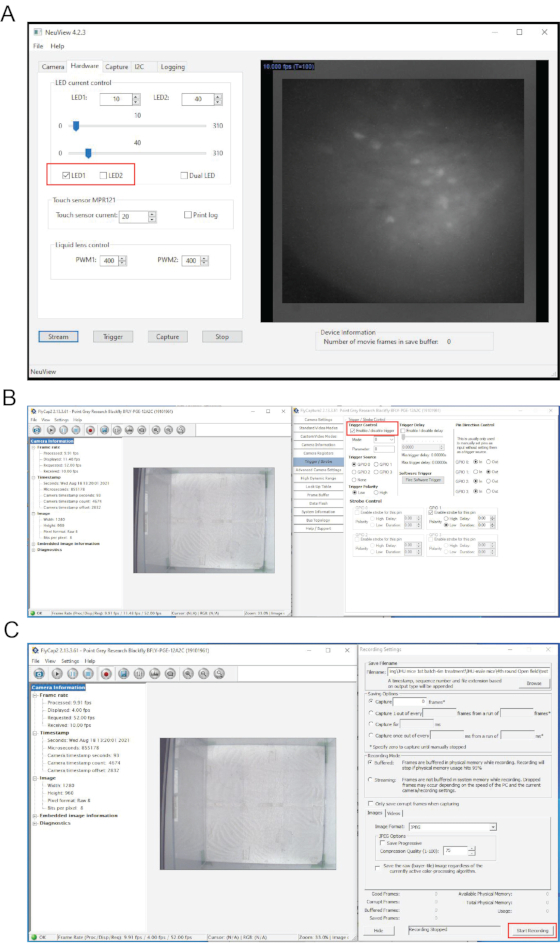
Figure 4: Interfaces of NeuView software and the behavior recording software during in vivo calcium imaging. (A) The interface of NeuView. (B,C) The interfaces of the behavior recording software. Red boxes highlight buttons that need to be clicked. Please click here to view a larger version of this figure.
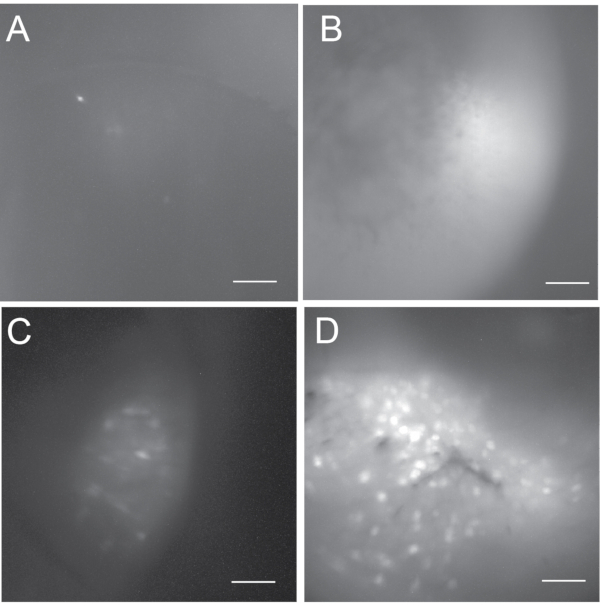
Figure 5: Maximum projection fluorescence cell maps to show the range of possible outcomes. (A,B) Unsuccessful in vivo calcium imaging that is not acceptable for subsequent data analysis. (A) is dark and contains less than 5 active neurons. (B) is bright but has no active neurons. (C) The cell map from a suboptimal in vivo calcium imaging that contains some active neurons. (D) The cell map from a good in vivo calcium imaging that includes several hundreds of active neurons. Scale bar: 100 μm. Please click here to view a larger version of this figure.
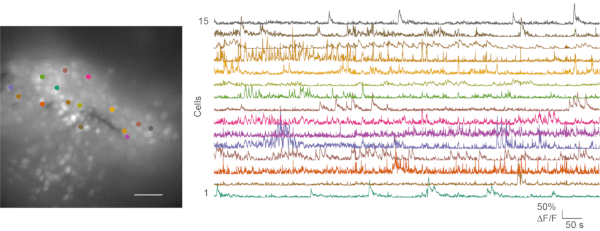
Figure 6: Representative cell map and calcium transients from a successful in vivo calcium imaging. The left panel is the maximum projection fluorescence cell map from an in vivo calcium imaging recording in the mPFC during an open field test. The recording lasts for 5 min. The right panel shows calcium transients from 15 regions of interest (color-matched). Scale bar: 100 μm. Please click here to view a larger version of this figure.
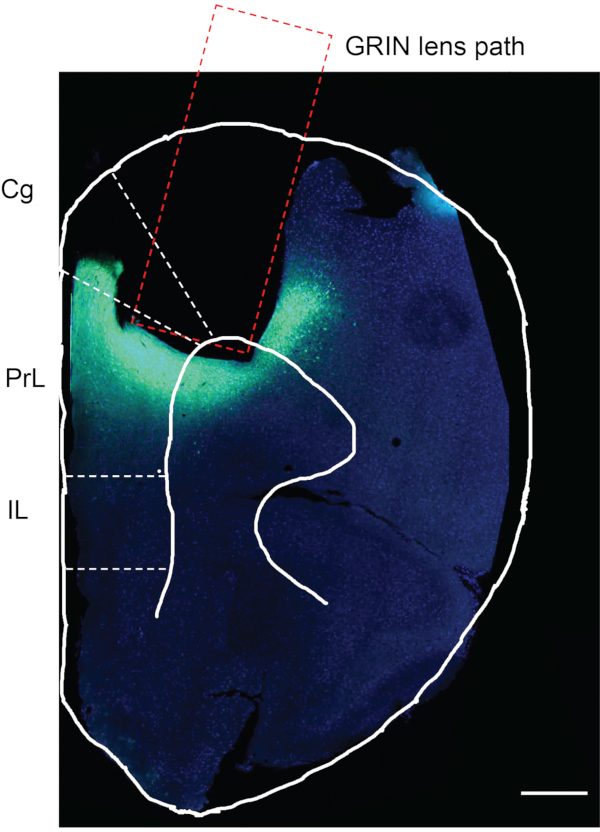
Figure 7: Postmortem assessment of an experimental mouse. The postmortem assessment for GCaMP6f expression and GRIN lens implantation in the mPFC of an experimental mouse. The rectangular area indicates the path for GRIN lens implantation. The green area under the GRIN lens implanted region confirms that GCaMP6f was expressed, and the GRIN lens was implanted precisely in the desired brain region. Cg, cingulate cortex; PrL, prelimbic cortex; IL, infralimbic cortex. Scale bar: 400 μm. Please click here to view a larger version of this figure.
Table 1: Comparisons of the custom-built miniscope system at the NIDA with other miniscope systems 7,8,9,10,11,13,25,26. Please click here to download this Table.
Movie 1: An in vivo calcium imaging recording from the mouse mPFC during an open field test. For demonstration purposes, this video shows only 1 min recording. The original recording frame rate is 10 frames/s. The video is 6 times faster than the original recording. Please click here to download this Movie.
Discussion
A central question in neuroscience is understanding how neural dynamics and circuits encode and store information, and how they are altered in brain diseases. Using a miniscope in vivo Ca2+ imaging system, individual neural activity from several hundreds of neurons within a local microcircuit can be simultaneously monitored from a freely behaving animal. Here, a detailed surgery protocol for viral injection and GRIN lens implantation is described to prepare rodents for a deep brain in vivo Ca2+ imaging via a custom-developed miniscope recording system. Table 1 shows the comparisons of our miniscope system with other commercially available and custom-built miniscope systems7,8,9,10,11,13,25,26. It is worth noting that GRIN lens implantation using the present surgical protocol is compatible with any commercial or custom-built single-photon and two-photon imaging systems for a deep brain in vivo calcium imaging.
From viral injection to data acquisition of miniscope in vivo calcium imaging, the entire experimental procedure takes at least 2 months to complete. It is a complicated and labor-intensive process. The ultimate success of the experiment depends on multiple factors, including proper choice of GECIs, injection of virus accurately in the targeted brain area, sufficient viral expression in the desired neural population, implantation of GRIN lens precisely in the desired location, adequate recovery from surgeries, as well as, whether severe inflammation occurs post-surgery, and whether animal's behavior is severely affected by surgeries, and so on.
Two critical steps include stereotaxic injection of virus and GRIN lens implantation. For the demonstration purpose, the stereotaxic microinjection was performed in the mouse mPFC, with adeno-associated virus (AAV1) encoding GCaMP6f under the control of CaMKII promoter that selectively labels pyramidal neurons in the mPFC. GCaMP6f was chosen as it is one of the fastest and most sensitive calcium indicators with a half-decay time of 71 ms15. In addition, AAV viral expression of GCaMP6f is long-lasting (i.e., several months), making it ideal for performing repetitive in vivo Ca2+ imaging over a long period for longitudinal studies in mouse models of neurodegenerative diseases27. The current surgery protocol can be adapted for targeting different cell populations in any other brain region. Various available viral tools allow selective labeling of specific neural populations in the desired brain region at the desired age. In addition, researchers can take advantage of the Cre-LoxP recombination system and various available transgenic mouse models to carry out genetic modifications and study the behavioral and neural circuitry outcome28,29.
One unique feature of the presented protocol is that the automated layer-by-layer brain tissue aspiration was performed before the GRIN lens (1 mm in diameter) implantation. This is achieved through a 27 G needle connected to a vacuum system, controlled by a custom-built robotic arm and software23. Based on our experience, this method generates a uniform surface for the GRIN lens to contact and causes less damage to the neighboring tissue than manual tissue aspiration23. For this reason, this procedure brings an obvious advantage for GRIN lenses with a relatively wider diameter (e.g., 1 mm). However, tissue aspiration may not be necessary for implanting a GRIN lens with a smaller diameter (0.5 mm or 0.25 mm). Instead, it can be directly planted along the leading track made with a 30 G needle21.
Besides the two critical steps discussed above, many other factors must be carefully considered for a successful operation. (1) All the instruments that contact the brain should be sterilized to prevent infection. (2) All surgery steps need to be performed to minimize damage to the brain to prevent further inflammation and excessive scar tissue formation. (3) The anesthesia doses given initially and maintained during the surgery, especially those administered intraperitoneally, need to be carefully considered. The anesthesia doses may be modified according to different mouse strains, as some may be more susceptible. (4) The condition of the mouse needs to be constantly monitored during surgery. Lastly, (5) the mice need to be regularly monitored post-surgery, as many complications may occur after the surgery.
Although a chunk of brain tissue is removed unilaterally during the GRIN lens implantation step, we did not observe any obvious behavior deficits7,12. The weight of the miniscope is around 2 grams and the cable is custom-designed to make it light and to ensure that the mouse can easily carry it. The miniscope and cable are only attached to the animal prior to in vivo imaging and detached after imaging. The entire imaging process usually takes no longer than 30 minutes. Therefore, these instrumentations do not prevent the mouse from freely behaving. The miniscope installation and deinstallation steps need a brief anesthesia (less than 2 minutes) with isoflurane for the purpose of animal restraining. We typically let the mouse recover from the brief exposure of isoflurane for 30 minutes before performing in vivo imaging. We have performed miniscope in vivo calcium imaging once per week for a few weeks without noticing any impact on mouse health and mouse social behavior12.
One major limitation of the current miniscope recording system is the need to connect the microscope to a cable for data acquisition. The presence of the cable sometimes restricts the mouse task performance and limits the recording of one animal at a time. Recently, a wireless miniscope has been developed25,26. This will broaden the task performance and allow simultaneous in vivo imaging from multiple animals in a group. Moreover, developing more sensitive GECIs with spectrally separable wavelengths combined with a dual-color miniscope will offer more exciting possibilities for neuroscience research.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work is supported by grants from the National Institute of Health (NIH) 5P20GM121310, R61NS115161, and UG3NS115608.
Materials
| 0.6mm and 1.2mm drill burrs | KF technology | 8000037800 | For craniotomy |
| 27-G and 30-G needle | BD PrecisionGlide Needle | REF 305109 and REF305106 | For both surgeries |
| 45 angled forceps | Fine Science tools | 11251-35 | For surgeries |
| 7.5% povidone-iodine solution (Betadine) | Purdue Products L.P. | NDC 67618-151-17 | Surface disinfectant |
| Acetone | Sigma-Aldrich | 179124-1L | GRIN lens cleaner |
| Agarose | Sigma-Aldrich | A9539-25G | For GRIN lens implantation |
| Antibiotic ointment | HeliDerm Technology | 81073087 | For virus injection |
| Anti-inflamatory drug (Ibuprofen) | Johnson & Johnson Consumer Inc | 30043308 | Acts as pain killer after surgeries |
| AutoStereota | NIDA/IRP | github.com/liang-bo/autostereota | For GRIN lens implantation |
| Behavior Recoding Software (Point Grey FlyCap2) | Point Grey | Point Grey Research Blackfly BFLY-PGE-12A2C | For recording behavior |
| Brass hex nut | McMASTER-CARR | 92736A112 | For GRIN lens implantation |
| Buprenorphine | Par Pharmaceuticals | NDC 4202317905 | For GRIN lens implantation |
| Calcium chloride | Sigma | 10043-52-4 | For preparing aCSF |
| Commutator | NIDA/IRP | Custom-designed | Component of image acquisition system |
| Compressed Oxygen and Caxbondioxide tank | Rocky Mountain Air Solutions | BI-OX-CD5C-K | For GRIN lens implantation |
| Compressed Oxygen tank | Rocky Mountain Air Solutions | OX-M-K | For virus injection |
| Cordless Microdrill | KF technology | 8000037800 | For craniotomy |
| Cyanoacrylate | Henkel Coorporation | # 1811182 | For GRIN lens implantation |
| Data acquisition controller | NIDA/IRP | Custom-designed | Component of image acquisition system |
| Data transmission cable | NIDA/IRP | Custom-designed | Component of image acquisition system |
| Dental cement set | C&B Metabond and Catalyst | A00253revA306 and A00168revB306 | For GRIN lens implantation |
| Dental cement set | Duralay | 2249D | For GRIN lens implantation |
| Dexamethasone | VETone | NDC 1398503702 | For GRIN lens implantation |
| Dextrose | Sigma | 50-99-7 | For preparing aCSF |
| Diet gel | Clear H20 | 72-06-5022 | Diet Supplement for mouse |
| GRIN lens | GRINTECH | NEM-100-25-10-860-S | For GRIN lens implantation |
| Heating Pad | Physitemp Instruments LLC. | #10023 | To keep the mouse body warm during surgeries |
| Isoflurane | VETone | V1 502017 | Anesthesia |
| Ketamine | VETone | V1 501072 | For GRIN lens implantation |
| Lidocaine | WEST-WARD | NDC 0143-9575-01 | Local anesthesia |
| Magnesium chloride hexahydrate | Sigma | 7791-18-6 | For preparing aCSF |
| Microliter syringe (Hamilton) | Hamilton | 7653-01 | For virus injection |
| MicroSyringe Pump Controller | World Precision Instrument | #178647 | For virus injection |
| Miniscope | NIDA/IRP | Custom-designed | For imaging |
| Miniscope base | Protolabs | Custom-designed | For mounting the base |
| Miniscope holding arm | NIDA/IRP | Custom-designed | For mounting the base |
| Miniscope protection cap | Protolabs | Custom-designed | For protecting the miniscope |
| Motorized controller | Thorlabs | KMTS50E | For mounting the base |
| NeuView | NIDA/IRP | https://github.com/giovannibarbera/miniscope_v1.0 | For in vivo imaging |
| Ophthalmic ointment | Puralube Vet Ointment | NDC 17033-211-38 | Ophthalmic |
| PCR tube | Thermo Scientific | AB-0622 | For GRIN lens implantation |
| Pinch Clamp | World Precision Instrument | 14040 | For clamping the tubing |
| Polytetrafluoroethylene (PTFE) tape | TegaSeal PTFE Tape | A-A-58092 | For fastening miniScope to the base |
| Potassium chloride | Sigma | 7447-40-7 | For preparing aCSF |
| Robotic arm | NIDA/IRP | Custom-designed | For GRIN lens implantation |
| Saline | Hospira | RL 7302 | For both surgeries |
| Set screw | DECORAH LLC. | 3BT-P9005-00-0025 | For screwing the brass hex nut in miniscope base |
| Silicone Rubber tubing, 0.062”ID, 1/8”OD | McMaster | 2124T3 | For irrigation of aCSF |
| Sodium bicarbonate | Sigma | 144-55-8 | For preparing aCSF |
| Sodium chloride | Sigma | 7647-14-5 | For preparing aCSF |
| Sodium phosphate monobasic | Sigma | 7558-80-7 | For preparing aCSF |
| Stereotaxic stage | KOPF | Model 962 Dual Ultra Precise Small Animal Stereotaxic | For both surgeries |
| Sterile cotton swab | Puritan | REF 806-WC | For both surgeries |
| Surgical tools | Fine Science tools | 11251-35 | For surgeries |
| Suture | Sofsilk | REF SS683 | For virus injection |
| Syringe filter (0.22 µm) | Millex | SLGVR33RS | For filtering aCSF during GRIN lens implantation |
| Viral suspension (AAV1-CamKII-GCamp6f) | Addgene | 100834-AAV1 | For virus injection |
| Titre: 2.8 X 10^13 GC/ml | |||
| Xylazine | VETone | V1 510650 | For GRIN lens implantation |
Referenzen
- Clapham, D. E. Calcium signaling. Cell. 131 (6), 1047-1058 (2007).
- Brini, M., Cali, T., Ottolini, D., Carafoli, E. Neuronal calcium signaling: function and dysfunction. Cellular and Molecular Life Sciences. 71 (15), 2787-2814 (2014).
- Chen, T. W., Li, N., Daie, K., Svoboda, K. A Map of Anticipatory Activity in Mouse Motor Cortex. Neuron. 94 (4), 866-879 (2017).
- Denk, W., Strickler, J. H., Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science. 248 (4951), 73-76 (1990).
- Komiyama, T., et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 464 (7292), 1182-1186 (2010).
- Peters, A. J., Lee, J., Hedrick, N. G., O’Neil, K., Komiyama, T. Reorganization of corticospinal output during motor learning. Nature Neuroscience. 20 (8), 1133-1141 (2017).
- Barbera, G., et al. Spatially compact neural clusters in the dorsal striatum encode locomotion relevant information. Neuron. 92 (1), 202-213 (2016).
- Cai, D. J., et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 534 (7605), 115-118 (2016).
- de Groot, A., et al. NINscope, a versatile miniscope for multi-region circuit investigations. Elife. 9, 49987 (2020).
- Ghosh, K. K., et al. Miniaturized integration of a fluorescence microscope. Nature Methods. 8 (10), 871-878 (2011).
- Jacob, A. D., et al. A compact head-mounted endoscope for in vivo calcium imaging in freely behaving mice. Current Protocols in Neuroscience. 84 (1), 51 (2018).
- Liang, B., et al. Distinct and Dynamic ON and OFF neural ensembles in the prefrontal cortex code social exploration. Neuron. 100 (3), 700-714 (2018).
- Liberti, W. A., et al. Unstable neurons underlie a stable learned behavior. Nature Neuroscience. 19 (12), 1665-1671 (2016).
- Stosiek, C., Garaschuk, O., Holthoff, K., Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proceedings of the National Academy of Sciences of the United States of America. 100 (12), 7319-7324 (2003).
- Chen, T. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Miyawaki, A., et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 388 (6645), 882-887 (1997).
- Bassett, J. J., Monteith, G. R. Genetically encoded calcium indicators as probes to assess the role of calcium channels in disease and for high-throughput drug discovery. Advances in Pharmacology. 79, 141-171 (2017).
- Lin, M. Z., Schnitzer, M. J. Genetically encoded indicators of neuronal activity. Nature Neuroscience. 19 (9), 1142-1153 (2016).
- Tian, L., Akerboom, J., Schreiter, E. R., Looger, L. L. Neural activity imaging with genetically encoded calcium indicators. Progress in Brain Research. 196, 79-94 (2012).
- Moore, D. T. Gradient-index optics: A review. Applied Optics. 19 (7), 1035-1038 (1980).
- Zhang, L., et al. Miniscope GRIN Lens System for Calcium Imaging of Neuronal Activity from Deep Brain Structures in Behaving Animals. Current Protocols in Neuroscience. 86 (1), 56 (2019).
- Yang, Y., et al. A two-step GRIN lens coating for in vivo brain imaging. Neuroscience Bulletin. 35 (3), 419-424 (2019).
- Liang, B., Zhang, L., Moffitt, C., Li, Y., Lin, D. T. An open-source automated surgical instrument for microendoscope implantation. Journal of Neuroscience Methods. 311, 83-88 (2019).
- Giovannucci, A., et al. CaImAn an open source tool for scalable calcium imaging data analysis. Elife. 8, 38173 (2019).
- Barbera, G., Liang, B., Zhang, L., Li, Y., Lin, D. T. A wireless miniScope for deep brain imaging in freely moving mice. Journal of Neuroscience Methods. 323, 56-60 (2019).
- Shuman, T., et al. Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nature Neuroscience. 23 (2), 229-238 (2020).
- Werner, C. T., Williams, C. J., Fermelia, M. R., Lin, D. T., Li, Y. Circuit mechanisms of neurodegenerative diseases: A new frontier with miniature fluorescence microscopy. Frontiers in Neuroscience. 13, 1174 (2019).
- Brault, V., Besson, V., Magnol, L., Duchon, A., Herault, Y. Cre/loxP-mediated chromosome engineering of the mouse genome. Handbook of Experimental Pharmacology. (178), 29-48 (2007).
- McLellan, M. A., Rosenthal, N. A., Pinto, A. R. Cre-loxP-mediated recombination: General principles and experimental considerations. Current Protocols in Mouse Biology. 7 (1), 1-12 (2017).

