Characterization of Blood Outgrowth Endothelial Cells (BOEC) from Porcine Peripheral Blood
Abstract
The endothelium is a dynamic integrated structure that plays an important role in many physiological functions such as angiogenesis, hemostasis, inflammation, and homeostasis. The endothelium also plays an important role in pathophysiologies such as atherosclerosis, hypertension, and diabetes. Endothelial cells form the inner lining of blood and lymphatic vessels and display heterogeneity in structure and function. Various groups have evaluated the functionality of endothelial cells derived from human peripheral blood with a focus on endothelial progenitor cells derived from hematopoietic stem cells or mature blood outgrowth endothelial cells (or endothelial colony-forming cells). These cells provide an autologous resource for therapeutics and disease modeling. Xenogeneic cells may provide an alternative source of therapeutics due to their availability and homogeneity achieved by using genetically similar animals raised in similar conditions. Hence, a robust protocol for the isolation and expansion of highly proliferative blood outgrowth endothelial cells from porcine peripheral blood has been presented. These cells can be used for numerous applications such as cardiovascular tissue engineering, cell therapy, disease modeling, drug screening, studying endothelial cell biology, and in vitro co-cultures to investigate inflammatory and coagulation responses in xenotransplantation.
Introduction
The endothelium is a highly complex, dynamic structure and a vital component of the vascular wall. It lines the inner surface of blood vessels to provide a physical interface between circulating blood and surrounding tissues. This heterogeneous structure is known to perform various functions such as angiogenesis, inflammation, vasoregulation, and hemostasis1,2,3,4. Human umbilical vein endothelial cells are a widely studied cell type to assess the functionality of endothelial cells. However, the patient-specific batch variability, inconsistent phenotype, and minimum splitting efficiency suggest a need to determine a cell source that could improve upon all of these features5.
Obtaining a homogenous population of primary endothelial cells can be technically challenging, and primary endothelial cells do not possess high proliferative capacity6. Hence, to study vascular regeneration and evaluate pathophysiological processes, various groups have tried to obtain and assess different types of endothelial cells derived from peripheral blood, e.g., endothelial progenitor cells (EPCs) or blood outgrowth endothelial cells (BOECs)6,7,8,9. The spindle-shaped early EPCs originate from hematopoietic stem cells (HSCs) and have limited growth potency and limited angiogenic ability to produce mature endothelial cells. Furthermore, they closely resemble inflammatory monocytes. In addition, their capacity to further differentiate into functional, proliferating, mature endothelial cells is still debatable6,7,9,10. The continuous culture of peripheral blood mononuclear cells (PBMCs) can give rise to a secondary population of cells known as late-outgrowth EPCs, BOECs, or endothelial colony-forming cells (ECFCs)6,7,9,10. Medina et al. in 2018, acknowledged the limitations of EPCs, the ambiguity of their nomenclature, along with a general lack of concordance with many distinct cell types continuously grouped under EPCs11. In contrast, BOECs have become recognized for their role in vascular repair, health and disease, and cell therapy. Further study and therapeutic use of these cells will rely on protocols to consistently derive these cell types from circulating progenitor cells.
Primary cells such as BOECs can be used as a surrogate to obtain highly proliferative mature endothelial cells6. BOECs are phenotypically distinct from early EPCs and exhibit typical endothelial features such as cobblestone morphology and expression of adherens junctions and caveolae12. Gene profiling by Hebbel et al.13,14,15 found that BOECs or ECFCs are the true endothelial cells as they promote microvascular and large vessel formation. Thus, BOECs can be used as a tool to evaluate pathophysiological processes and genetic variation16. They are also considered an excellent cell source for cell therapy for vascular regeneration17. Hence, a standardized protocol to consistently derive these highly proliferative cells is essential.
While BOECs provide a powerful tool for studying human pathophysiological and genetic variation, a more homogenous source of BOECs may provide more robust and reliable experimental and therapeutic outcomes. Superior homogeneity can be achieved by using xenogeneic cell sources derived from genetically similar animals raised in similar conditions18. While xenogeneic cell sources are prone to eliciting a host immune response, immunomodulation strategies are being developed with the goal of generating immunocompatible animals and animal products, including cells. Pigs, in particular, are an abundant source of peripheral blood and are commonly used to study medical devices and other therapies due to anatomical and physiological similarities to humans. Hence, this study refines the protocol for the isolation and expansion of highly proliferative BOECs from porcine peripheral blood.The protocol detailed below is a straightforward and reliable method to obtain a large number of BOECs from a relatively small volume of blood. The cultures can be expanded through several passages to generate millions of cells from a single blood sample.
Protocol
All animal studies were approved by the respective Institutional Animal Care and Use Committees (IACUC) at the Medical College of Wisconsin and Mayo Clinic.
NOTE: In this study, Yorkshire/Landrace/Duroc cross domestic pigs (Sus domesticus), male and female, 40-80 kg, 3-6 months old, were used.
1. Collection of porcine peripheral blood
- Prepare materials.
- Dilute heparin solution to 100 U/mL in sterile saline.
- Add 3-4 mL of heparin solution to each of two 50 mL conical tubes and two 60 mL syringes.
- Draw undiluted heparin solution (1,000 U/mL) into an extension tube.
- Connect a 19 G needle to one end of the heparin-filled extension tube and a heparin-containing 60 mL syringe to the other end.
- Anesthetize a pig according to Institutional policies
- Administer an IM injection of atropine (0.05 mg/kg), tiletamine/zolazepam (2-5 mg/kg), and xylazine (2 mg/kg) to induce anesthesia.
NOTE: adequate anesthesia is achieved once the animal is unconscious and the mandibular muscles are non-rigid. - Lay the animal in the supine position and place rolled-up towels on both sides to provide stability.
- Apply an ophthalmic ointment to the eyes to prevent dryness while under anesthesia.
- Provide thermal support during anesthesia including blankets, heating pads, and/or heated procedure table.
- Administer an IM injection of atropine (0.05 mg/kg), tiletamine/zolazepam (2-5 mg/kg), and xylazine (2 mg/kg) to induce anesthesia.
- Clean the femoral groin area with betadine scrub solution.
- Puncture the femoral vein or artery with the 19 G needle and slowly (~1-2 mL/s) draw 50 mL of blood into the syringe.
NOTE: Drawing too fast can damage the cells. - Leaving the needle in place, immediately kink the extension tube and disconnect the 60 mL syringe
- Slowly transfer the blood to a heparin-containing 50 mL conical tube.
- Tightly cap the 50 mL conical tube, gently invert a few times to mix, and place on ice.
- Connect the remaining heparin-containing 60 mL syringe to the extension tube, unkink the extension tube, and slowly draw an additional 50 mL of blood into the syringe.
- Immediately remove the needle from the artery or vein and slowly draw the remaining blood from the extension tube into the syringe.
- Disconnect the 60 mL syringe from the extension tube and slowly transfer the blood to the remaining heparin-containing 50 mL conical tube.
- Tightly cap the 50 mL conical tube, gently invert a few times to mix, and place on ice.
- Apply pressure hemostasis to the pig's femoral groin area.
- Recover the pig according to Institutional policies. Continuously monitor the animal until it regains sufficient consciousness to maintain sternal recumbency and return to the regular housing/kennel area.
2. Isolation of mononuclear cells
- Dilute the blood 1:1 with phosphate-buffered saline (PBS) in four 50 mL conical tubes.
NOTE: This work needs to be performed in a cell culture hood using an aseptic technique to avoid contaminating the cell culture. - Add 25 mL of room temperature ready-to-use density gradient solution to eight 50 mL conical tubes.
- Very slowly pipette 25 mL of blood/saline solution along the inside of each density gradient solution containing 50 mL conical tube by holding the tube at a sharp angle to the pipette tip.
NOTE: The blood solution should gently layer on top of the density gradient solution and maintain a well-defined interface. - Centrifuge the tubes for 30 min at 560 x g and room temperature (RT).
NOTE: For best results, disable the centrifuge brake if possible. - Use a thin pipette to carefully collect the buffy coat (cloudy layer of mononuclear cells above the density gradient solution and below the clear plasma) from each tube and distribute evenly into two new 50 mL conical tubes. Discard the density gradient solution containing tubes.
NOTE: Collect as much of the buffy coat as possible while collecting as little of the density gradient solution and plasma as possible.
3. Washing and plating of cells
- Coat a 6-well plate with fibronectin.
- Make a stock solution of human plasma fibronectin by diluting it to 1 mg/mL in sterile water. Store the aliquots at -20 °C.
- Make 3.6 mL of a working solution of fibronectin by diluting 600 µL of fibronectin stock solution in 3 mL of PBS.
- Transfer 600 µL of the fibronectin working solution into each well of a 6-well plate and gently rock until evenly coated.
- Wait 30 min for the fibronectin to coat the wells and gently aspirate the solution out of each well.
- While waiting for the fibronectin to coat, wash the mononuclear cells
- Top up the tubes with up to 45 mL of PBS
- Centrifuge for 5 min at 560 x g and 4 °C with low brake. Aspirate the supernatant from both tubes.
- Resuspend each cell pellet in 25 mL of PBS.
- Repeat to wash a second time.
- Resuspend each cell pellet in 5 mL of PBS and add 15 mL of 0.8% ammonium chloride solution to each tube.
NOTE: This step is to lyse the remaining red blood cells. - Incubate on ice for 10 min.
- Wash the cells as before by topping up tubes with PBS and centrifuge for 5 min at 560 x g and 4 °C with low brake. Aspirate the supernatant from both tubes.
- Resuspend each cell pellet in 25 mL of PBS and repeat to wash a second time.
- Resuspend each cell pellet in 6 mL of EGM-2 culture medium supplemented with 10% fetal bovine serum (FBS) and 1x antibiotic/antimycotic solution containing 10,000 U/mL penicillin, 10 mg/mL streptomycin and 25 µg/mL amphotericin.
NOTE: EGM-2 culture medium consists of EBM-2 culture medium supplemented with 200 µL of hydrocortisone, 2 mL of human fibroblast growth factor (hFGF-B), 500 µL of vascular endothelial growth factor (VEGF), 500 µL of recombinant analog of insulin-like growth factor (R3 IGF-1), 500 µL of ascorbic acid, 500 µL of human epidermal growth factor (hEGF), 500 µL of gentamicin/amphotericin, and 500 µL of heparin per 500 mL. - Gently transfer 2 mL of the cell suspension to each well of the fibronectin-coated 6-well plate and gently rock until evenly coated.
NOTE: The goal is to seed as many cells as possible to maximize the number of endothelial colonies that will form.
4. Cell culture
- Incubate the cells at 37 °C, 5% CO2, and 100% humidity.
- On the next day, gently add 1 mL of fresh culture medium to each well.
NOTE: it is important to be gentle not to disturb cells that are loosely adherent to the fibronectin coating. - On the next day, perform a half culture medium change by gently aspirating 1.5 mL of culture medium from each well and replacing it with 1.5 mL of fresh culture medium.
- On each of the next 5 days, gently perform a full culture medium change to each well (2 mL of fresh culture medium per well).
- After that, gently change the culture medium three times per week.
- Regularly visualize the cell cultures under low power (4x objective) light microscopy.
NOTE: Adherent blood outgrowth endothelial cell (BOEC) colonies will begin to appear in the wells 7-10 days after plating. Non-adherent cell types will be discarded with medium changes, and other adherent cell types will gradually dissipate as the BOEC colonies grow. - Passage the BOECs into a fibronectin-coated T-75 flask when ~70%-80% confluent and continue changing culture medium three times per week.
NOTE: Seeding density can be controlled by passaging colonies into a T25 flask instead. Subsequent passages do not require fibronectin-coating, and culture medium changes can be reduced to two times per week. - Cells from passages 1-3 may be used for experiments or transferred to a freezing medium and stored in liquid nitrogen for future use.
Representative Results
Morphology of the cultured cells was observed from the start of the culture until BOEC colonies were observed (Figure 1). A smaller population of adherent cells started to attach to the culture dishes and grow, while non-adherent cells were removed with culture medium changes (Figure 1B). Colonies first appeared on day 6 as a collection of endothelial-like cells proliferating radially outward from a central point (Figure 1D). As the culture progressed, cell colonies became more dense and displayed a cobblestone morphology similar to mature endothelial cells (Figure 1F). The typical endothelial cobblestone morphology provides an easy preliminary identification of BOECs using a light microscope.
Endothelial cell colonies are typically ready for passaging at 10-14 days of culture. At this time, the 6-well plate will typically yield ~1 million cells with percent viability of 93%-98%. During the first passage, endothelial cells will expand to yield ~6-10 million cells in a T75 flask.
To assess the morphogenesis of BOECs, the basement membrane matrix (e.g., Matrigel) was plated on 15 well angiogenesis plates at 10 µL/well and incubated at 37 °C for 30 min to allow polymerization. Passage 2 cells were seeded on basement membrane matrix-coated plates and imaged at 14 h and 24 h time points. A density of approximately 3,500 cells per well was able to form a network and tube-like structures. Tube formation was noticed within 14 h for serum free medium (EGM-2 with supplementals except for FBS) and within 24 h for complete growth medium (EGM-2 with supplements including FBS). Adding FBS may have diluted the endothelial cell-specific growth factors (e.g., VEGF) and introduced other signaling factors resulting in the delay of the tube formation process. 2D phase-contrast microscopy was used to image tube formation in serum-free medium (Figure 2A,B) and complete growth medium (Figure 2C,D). Cells in both media conditions underwent morphological differentiation and rapidly organized into extensive networks of capillary tube-like structures. These structures were composed of organized cellular cords resembling in vivo capillary networks. Furthermore, micrographs at 20x magnification illustrate the complex multicellular organization of endothelial cells and their morphological differentiation in detail. There were no morphological differences observed between the media conditions. The extensive network of capillary tube-like structures strongly suggests the differentiation of cultured cells into mature and functional endothelial cells.
BOECs were further characterized by the expression of mature endothelial cell marker CD31 or platelet endothelial cell adhesion molecule-1 (PECAM1) (Figure 3A,B). BOECs showed uniform expression of CD31. Furthermore, flow cytometry analysis confirmed the absence of EPCs as the cells stained negative for monocyte marker CD14 compared to the positive control group of PBMCs (Figure 3C,D).
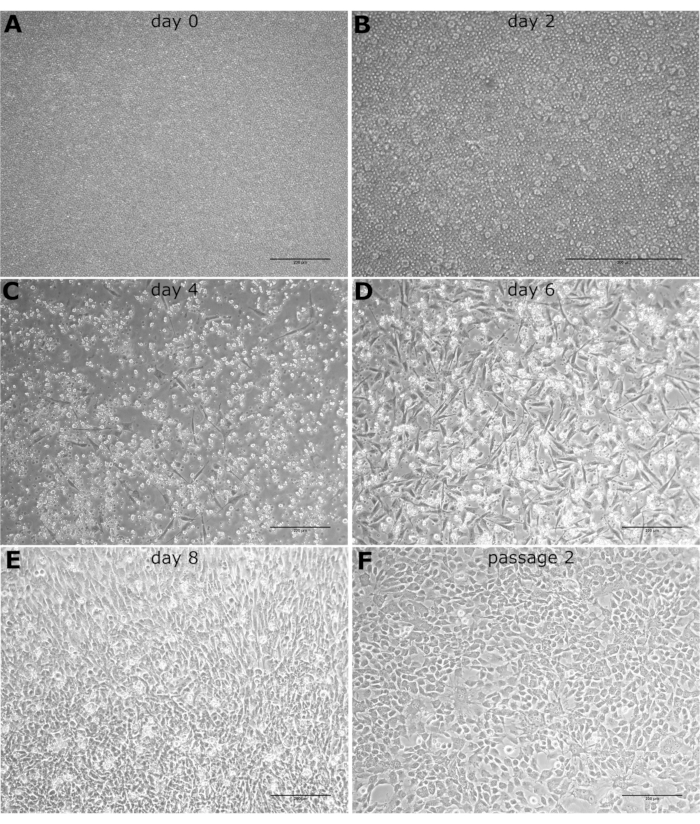
Figure 1: Phenotype light microscopy images. The light microscopy images of cultured BOECs observed at 10x magnification on (A) day 0, (B) day 2, (C) day 4, (D) day 6, (E) day 8, and (F) after the first passage. Scale bars: 200 µm. Please click here to view a larger version of this figure.
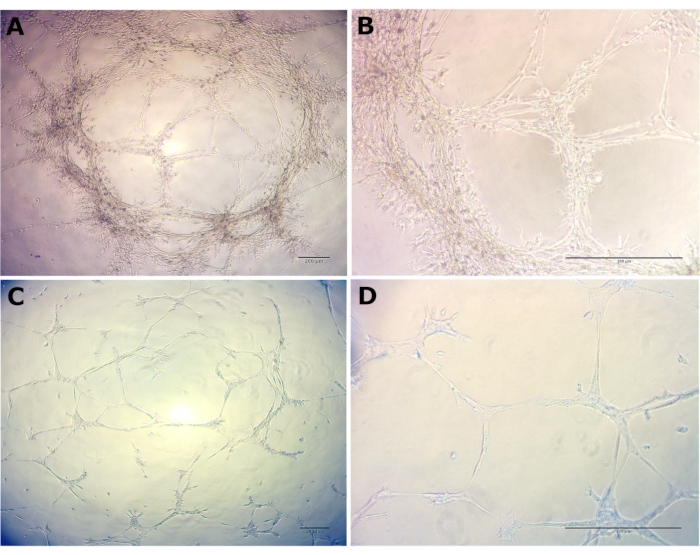
Figure 2: Morphological differentiation and organization of passage 2 BOECs. Morphological differentiation and organization of passage 2 BOECs into capillary tube-like structures was noticed in the basement membrane matrix within 14 h for serum-free medium and within 24 h for complete growth medium. (A) Serum-free medium at 4x, (B) Serum-free medium at 20x, (C) Complete growth medium at 4x, and (D) Complete growth medium at 20x. Scale bars: 200 µm. Please click here to view a larger version of this figure.
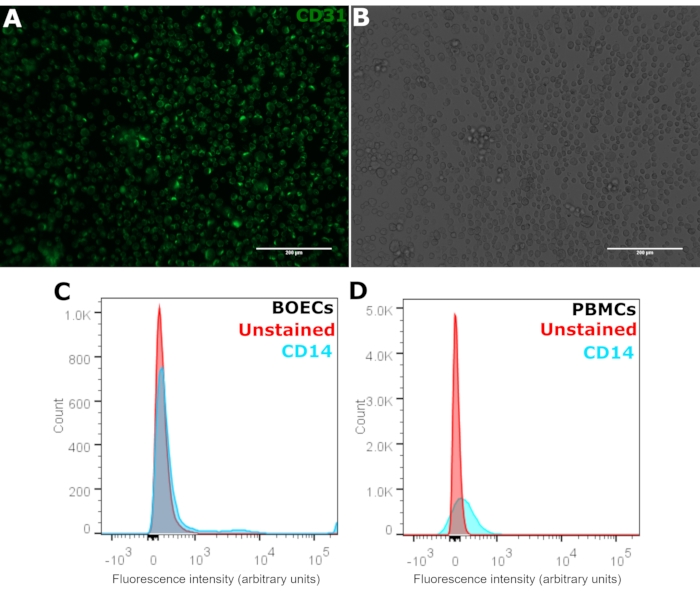
Figure 3: Characterization of BOECs with CD31 and CD14. (A) Passage 2-3 BOECs were stained for PECAM1 using anti-CD31-FITC antibody seen in green and (B) corresponding phase-contrast microscopy images. Cells were stained in suspension, and the suspension was imaged on a microscope slide. Scale bars: 200 µm. (C) Flow cytometry analysis of BOECs with CD14-AF700 antibody compared to (D) positive control peripheral blood mononuclear cells (PBMCs). Positive CD14 staining shown in blue and unstained cells shown in red. Please click here to view a larger version of this figure.
Discussion
BOECs are a powerful tool that may be used in various scientific and therapeutic approaches7,8,16. BOECs have been used to analyze EC gene expression to elucidate the key factors responsible for the development of vascular diseases and cancer5,19,20,21. BOECs have also been used in therapeutic applications such as vascular regeneration and gene delivery22,23,24. Genetically modified BOECs have been known for their antiangiogenic tumor therapeutic ability25,26. Further, the angiogenic potential of BOECs makes them excellent candidates for cellular therapies aiming at vascularization and neovascularization17,27. Even though several studies describe how to obtain BOECs from human peripheral blood6,7,8,9, the variability of human cell sources due to underlying pathophysiological and genetic variation suggests a critical need to obtain BOECs from healthy xenogenic sources9,13,18.
A detailed protocol to efficiently derive BOECs from porcine peripheral blood mononuclear cells has been presented. Critical steps include processing blood samples as soon as possible and efficient harvesting of buffy coat layers after density gradient centrifugation to maximize the number of viable PBMCs going into the culture. The initial plating density and timely passaging of cells are important considerations for obtaining consistent isolation of these cell types. A starting blood volume of 100 mL provides an appropriate number of PBMCs for a single six-well plate. It should be noted that the maximum volume of blood that can be safely collected from a pig will vary with animal body weight. A suggested safe one-time maximum blood volume collection for pigs is 8 mL per kg of body weight. In addition, timely passaging of initial colonies from passage 0 to passage 1 is also essential. The cells cease to proliferate if seeding density is very low or if the cells reach confluency. As the cells become confluent, they start transforming into an elongated mesenchymal morphology and phenotype. Seeding density can also be controlled by passaging colonies into a T25 flask rather than a T75 flask. Careful consideration is required not to lose the adherent cell types during initial media change as it is very imperative to capture as many adherent cells as possible while discarding the non-adherent EPCs. This protocol produces outgrowth cell colonies in approximately 1 week.
The protocol is highly reproducible and reliable. Expanding endothelial cell colonies are achieved in an estimated 90%-95% of cultures. Failure is typically due to lack of formation of endothelial cell colonies, meaning if at least one endothelial cell colony is formed, they will nearly always proceed to expand in culture. Sources of failure include random variability, excessive shearing of cells during blood collection, contaminating microbes in the culture, and high loss of mononuclear cells during the processing steps. After an unsuccessful culture, a second culture will typically be successful, indicating that variability between animals is not a major factor. The protocol is even successful when collecting blood immediately after euthanasia, indicating the washing steps efficiently reduce the euthanasia agents to innocuous levels.
Irrespective of the heterogeneity in the circulating blood cell population, BOECs that are very distinct from EPCs were obtained. BOECs are derived from a small subset of adherent PBMCs that lack CD14 expression. This was confirmed by the absence of CD14 staining (Figure 3C,D). The clearance of monocytic EPCs from BOEC cultures in early passages may be explained either by cell death or non-adherence. The cobblestone morphology of BOECs (Figure 1E,F) suggests a pronounced endothelial phenotype. In addition, the uniform expression of surface marker CD31 (Figure 3A) implies a homogenous population of mature endothelial cells. Further, the capillary morphogenesis of BOECs both in serum-free (Figure 2A,B) and complete growth media (Figure 2C,D) shows their inherent angiogenic capability6,8. Previous analysis of porcine BOECs generated using this protocol has shown these cells are additionally positive for von Willebrand factor expression and lectin binding28.
The abundance of PBMCs in circulating blood, from which BOECs emerge, supports the feasibility of a robust method to obtain a virtually limitless supply of therapeutic BOECs from animal sources. Once the technique is mastered, BOECs can be used for several applications, including vascular repair and regeneration, disease modeling, drug screening, and endothelial cell biology studies. Ongoing studies are needed to confirm the homogeneity of porcine BOECs and their compatibility as a therapeutic source in humans.
One limitation of this protocol is the risk of expanding endothelial cell colonies failing to generate. The failure rate is estimated to be 5%-10%, and in these instances, a second attempt is necessary. When assurance of success is important, twice the amount of blood can be collected, and the protocol can be followed in parallel to yield two independent cultures. Furthermore, our experience is limited to healthy Yorkshire/Landrace/Duroc cross domestic pigs (Sus domesticus), male and female, 40-80 kg, 3-6 months old. Success rates with other breeds, ages, and experimental conditions are unknown.
Another limitation of the protocol is that a definitive marker for BOEC phenotype does not exist. Here and in previous work28, the porcine BOECs generated from this protocol have been characterized for several BOEC markers, but the understanding of BOEC phenotype continues to evolve. BOECs are also characterized at passages 2-3 and phenotype at higher passages is unknown. An advantage of porcine BOECs compared to humans is the ready supply of pig blood, which allows for the generation of more frequent cultures and a robust supply of consistent, low passage cells.
One of the major limitations of using porcine cells in therapeutic approaches is the host immune response29. Several immunomodulation strategies are being investigated to mitigate the immunogenicity of xenogeneic cells for human transplantation, such as anti-inflammatory agents and CRISPR/Cas genetic manipulation30,31,32. More recent studies have proposed the expression of human complementary regulatory proteins (hCRPs)33 and the introduction of α1,3- galactosyltransferase deleted (GTKO)32,34 pigs. These advancements hold promise for the therapeutic application of xenogeneic cells, including porcine BOECs.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors wish to acknowledge funding from NIH/NHLBI R00 HL129068.
Materials
| 19 G needle | Covidien | 1188818112 | |
| 50 mL conical tubes | Corning | 352098 | |
| 6 well plate | BD Falcon | 353046 | |
| 60 mL syringes | Covidien | 8881560125 | |
| Ammonium chloride solution (0.8%) | Stemcell Technologies | 07850 | |
| Antibiotic/antimycotic solution (100x) | Gibco | 15240-062 | |
| Centrifuge | Thermo Scientific | 75-253-839 | |
| EGM-2 culture medium | Lonza Walkersville | CC-3162 | |
| Extension tube | Hanna Pharmaceutical Supply Co. | 03382C6227 | |
| Fetal bovine serum (FBS) | Atlas Biologicals | F-0500-A | |
| Ficoll-Paque 1077 | Cytiva | 17144003 | Density gradient solution |
| Heparin sodium injection (1,000 units/mL) | Pfizer | 00069-0058-01 | |
| Human plasma fibronectin | Gibco | 33016-015 | |
| Ice | N/A | N/A | |
| Phosphate-buffered saline (PBS) | Gibco | 10010-023 | |
| Pipette set | Eppendorf | 2231300004 | |
| Sterile water | Gibco | 15230-162 | |
| Thin pipette | Celltreat Scientific | 229280 |
Referenzen
- Aird, W. C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circulation Research. 100 (2), 174-190 (2007).
- Aird, W. C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation Research. 100 (2), 158-173 (2007).
- Pober, J. S., Tellides, G. Participation of blood vessel cells in human adaptive immune responses. Trends in Immunology. 33 (1), 49-57 (2012).
- Navarro, S., et al. The endothelial cell protein C receptor: its role in thrombosis. Thrombosis Research. 128 (5), 410-416 (2011).
- Hasstedt, S. J., et al. Cell adhesion molecule 1: a novel risk factor for venous thrombosis. Blood. 114 (14), 3084-3091 (2009).
- Ormiston, M. L., et al. Generation and culture of blood outgrowth endothelial cells from human peripheral blood. Journal of Visualized Experiments: JoVE. (106), e53384 (2015).
- Lin, Y., Weisdorf, D. J., Solovey, A., Hebbel, R. P. Origins of circulating endothelial cells and endothelial outgrowth from blood. Journal of Clinical Investigation. 105 (1), 71-77 (2000).
- Martin-Ramirez, J., Hofman, M., Biggelaar, M. V. D., Hebbel, R. P., Voorberg, J. Establishment of outgrowth endothelial cells from peripheral blood. Nature Protocols. 7 (9), 1709-1715 (2012).
- Gulati, R., et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circulation Research. 93 (11), 1023-1025 (2003).
- Hebbel, R. P. Blood endothelial cells: utility from ambiguity. The Journal of Clinical Investigation. 127 (5), 1613-1615 (2017).
- Medina, R. J., et al. Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Translational Medicine. 6 (5), 1316-1320 (2018).
- Medina, R. J., et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Medical Genomics. 3, 18 (2010).
- Jiang, A., Pan, W., Milbauer, L. C., Shyr, Y., Hebbel, R. P. A practical question based on cross-platform microarray data normalization: are BOEC more like large vessel or microvascular endothelial cells or neither of them. Journal of Bioinformatics and Computational Biology. 5 (4), 875-893 (2007).
- Pan, W., Shen, X., Jiang, A., Hebbel, R. P. Semi-supervised learning via penalized mixture model with application to microarray sample classification. Bioinformatics. 22 (19), 2388-2395 (2006).
- Hirschi, K. K., Ingram, D. A., Yoder, M. C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 28 (9), 1584-1595 (2008).
- Fernandez, L. A., et al. Blood outgrowth endothelial cells from hereditary haemorrhagic telangiectasia patients reveal abnormalities compatible with vascular lesions. Cardiovascular Research. 68 (2), 235-248 (2005).
- Critser, P. J., Yoder, M. C. Endothelial colony-forming cell role in neoangiogenesis and tissue repair. Current Opinion in Organ Transplantation. 15 (1), 68-72 (2010).
- Zhao, Y., et al. Isolation and culture of primary aortic endothelial cells from miniature pigs. Journal of Visualized Experiments: JoVE. (150), e59673 (2019).
- Chang Milbauer, L., et al. Genetic endothelial systems biology of sickle stroke risk. Blood. 111 (7), 3872-3879 (2008).
- Wei, P., et al. Differential endothelial cell gene expression by African Americans versusCaucasian Americans: a possible contribution to health disparity in vascular disease and cancer. BMC Medicine. 9 (1), 2 (2011).
- Hasstedt, S. J., et al. Cell adhesion molecule 1: a novel risk factor for venous thrombosis. Blood, The Journal of the American Society of Hematology. 114 (14), 3084-3091 (2009).
- Milbauer, L. C., et al. Blood outgrowth endothelial cell migration and trapping in vivo: a window into gene therapy. Translational Research. 153 (4), 179-189 (2009).
- Matsui, H., et al. Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo factor VIII expression from lentivirally engineered endothelial progenitors. Stem Cells. 25 (10), 2660-2669 (2007).
- De Meyer, S. F., et al. Phenotypic correction of von Willebrand disease type 3 blood-derived endothelial cells with lentiviral vectors expressing von Willebrand factor. Blood. 107 (12), 4728-4736 (2006).
- Bodempudi, V., et al. Blood outgrowth endothelial cell-based systemic delivery of antiangiogenic gene therapy for solid tumors. Cancer Gene Therapy. 17 (12), 855-863 (2010).
- Dudek, A. Z., et al. Systemic inhibition of tumour angiogenesis by endothelial cell-based gene therapy. British Journal of Cancer. 97 (4), 513-522 (2007).
- Moubarik, C., et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Reviews and Reports. 7 (1), 208-220 (2011).
- Pislaru Sorin, V., et al. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation. 114 (1), 314 (2006).
- Satyananda, V., et al. New concepts of immune modulation in xenotransplantation. Transplantation. 96 (11), 937-945 (2013).
- Klymiuk, N., Aigner, B., Brem, G., Wolf, E. Genetic modification of pigs as organ donors for xenotransplantation. Molecular Reproduction and Development. 77 (3), 209-221 (2010).
- Ryczek, N., Hryhorowicz, M., Zeyland, J., Lipiński, D., Słomski, R. CRISPR/Cas technology in pig-to-human xenotransplantation research. International Journal of Molecular Sciences. 22 (6), 3196 (2021).
- Cooper, D. K., Koren, E., Oriol, R. Genetically engineered pigs. Lancet. 342 (8872), 682-683 (1993).
- Cozzi, E., White, D. J. G. The generation of transgenic pigs as potential organ donors for humans. Nature Medicine. 1 (9), 964-966 (1995).
- Phelps, C. J., et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 299 (5605), 411-414 (2003).

