Fertility Sparing Procedure using Carbon Dioxide Fiber Laser Vaporization of Ovarian Endometrioma
Summary
In this protocol, CO2 fiber laser technique is demonstrated for the surgical treatment of ovarian endometriosis, which represents a viable alternative in terms of fertility preservation with the major advantage of not being dependent on the surgeon’s skills and personal experience.
Abstract
The surgical management of endometrioma is still a matter of debate. Cystectomy, which is recognized as the standard technique, seems to be associated with a potential reduction in the ovarian reserve due to the inadvertent removal and thermal damage of healthy ovarian tissue. New ablative techniques with reduced tissue penetration depth and less thermal spread to the surrounding parenchyma may represent a viable alternative to cystectomy. For these reasons, the aim of this manuscript is to demonstrate the ablation of the endometrioma capsule using a CO2 fiber laser technique and discuss the clinical outcomes. Once the cyst has been drained and washed, a biopsy is taken. After cyst eversion, vaporization of the inner surface of the cyst is performed using a CO2 fiber laser. The technique is simple and reproducible as even young surgeons without any surgical experience were more confident in performing laser CO2 vaporization instead of cystectomy. The positive effects of CO2 technology are reported in a randomized controlled trial, where the postoperative changes in the antral follicular count (AFC) and antimullerian hormone (AMH) levels were compared between patients who had their endometrioma excised (cystectomy) and those who had undergone endometrioma vaporization with CO2 laser. The patients treated with CO2 laser showed significantly increased AFC without a reduction in serum AMH levels as compared to the cystectomy group, in which both parameters were significantly reduced. The postoperative pregnancy rate was also assessed, and comparable pregnancy rates were found after both treatments. On the contrary, patients treated with the CO2 fiber laser technique had more favorable in-vitro fertilization (IVF) outcomes compared to cystectomy.
In conclusion, the CO2 fiber laser technique may represent a viable alternative to cystectomy in the surgical treatment of endometrioma in terms of ovarian preservation, pregnancy rates, and IVF outcomes. Moreover, it has the advantage of being independent of the surgeon’s skills and personal experience.
Introduction
The best surgical treatment for ovarian endometriosis, especially when fertility preservation is a priority for women with a desire for offspring, is still a matter of debate. Although cystectomy is still the recommended technique1, previous studies have raised some concerns about its possible detrimental effect on ovarian reserve and reproductive outcomes due to the inadvertent removal of healthy ovarian parenchyma2,3,4.
Indeed, unlike non-endometriotic cysts, endometrioma is a pseudocyst not surrounded by a real anatomic capsule5, in which inflammation caused by free iron and reactive oxygen species (ROS) plays a role in the substitution of the surrounding normal ovarian cortical tissue with fibrous tissue6. Thus, the absence of a clear cleavage plan may lead to an increased risk of removing healthy ovarian parenchyma, even when cystectomy is performed by experienced surgeons7,8.
Moreover, cystectomy-mediated injuries could lead to compromised vascularization due to the diffusion of thermal damage to the surrounding healthy ovarian parenchyma during coagulation, as shown by previous findings where adverse changes in the ovarian artery blood flow were reported after cystectomy9,10,11.
At our institution, concerns about ovarian damage after cystectomy led to the introduction of CO2 fiber laser technology since in 2015. This surgical procedure, which can deliver energy with a controlled tissue penetration depth and little thermal spread, was inspired by the work of Jacques Donnez more than 20 years ago12.
Although ablative techniques involving CO2 fiber laser technology do not represent a novelty in the surgical management of endometrioma, many surgeons may not feel confident with the procedure. Indeed, only a few studies have investigated the impact of this technique on ovarian reserve, pregnancy outcome, and the rate of recurrence of endometriosis. The aim of this protocol is to provide an overview of the promising results obtained using the CO2 fiber laser technology since its introduction in 2015 and to describe the simplicity and reproducibility of this technique.
Firstly, in order to assess the impact of CO2 fiber laser vaporization and cystectomy on ovarian reserve markers, a multicenter randomized trial was conducted between 2017 and 2018. A total of 60 patients were randomly assigned either to Group 1 (cystectomy: 30 patients) or Group 2 (CO2 laser vaporization: 30 patients) at a ratio of 1:1, by using a computer-generated randomization list that used the simple randomization method13. To investigate postoperative spontaneous conception, a prospective observational study was conducted between 2015 and 2019 on 142 women, comparing cystectomy and laser vaporization14. When pregnancy was not achieved after CO2 fiber laser vaporization, patients were referred to in-vitro fertilization (IVF) clinics and were then included (n = 26) in a prospective observational study to investigate ovarian responsiveness to controlled ovarian stimulation15. Following this, a retrospective analysis of a larger sample size study population (n = 125, women with or without offspring desire), who were treated between 2015 and 2018 and whose follow-up lasted at least 12 months, was performed to assess the recurrence rate of a cyst and/or pain symptoms after both the surgical techniques16.
Protocol
All the studies were conducted in compliance with the Declaration of Helsinki, as outlined in the International Conference on Harmonization Guidelines for Good Clinical Practice. Written informed consent for data collection and anonymous publication of disease-related information is routinely obtained at the institution during patient interviews preceding surgical treatment. Women participating in the randomized controlled study signed a specific informed consent form. The Institutional Review Board of the institution approved all the studies. A diagram of the studies' protocols is shown in Figure 1.
1. Patient selection
- Only include women with primary unilateral or bilateral endometriomas, with the largest cyst diameter between 3-8 cm, of reproductive age, and who have undergone surgery at San Raffaele Scientific Institute for pain or infertility.
- Additionally, exclude patients aged ≥ 40 years; with previous surgery of the ovaries, unilateral oophorectomy, salpingectomy, or hysterectomy; with endocrine diseases; undergoing hormonal treatment within 3 months of ovarian reserve assessment; or with suspicion of ovarian atypical endometriosis at the pre-operative ultrasound evaluation stage.
2. Patient characteristics
- Identify the basal patient characteristics as follows. At baseline (before surgery), perform a detailed medical interview and pelvic ultrasound scan. In particular, collect data regarding age, surgical indication, offspring desire, body mass index (BMI), volume (expressed in cm3) and mean diameter (expressed in cm) of unilateral or bilateral endometrioma, and the volume of each ovary.
- For ovarian reserve analysis, check the antral follicular count (AFC) and antimullerian hormone (AMH) levels. At baseline (during the first 2-3 days of the menstrual cycle), evaluate the AFC by pelvic ultrasound, and collect blood samples to determine AMH levels. Assess the AFC in both ovaries, before and after surgery, by counting the number of follicles with an average diameter of 2-10 mm.
3. Surgical technique
NOTE: A team of surgeons with extensive experience in the treatment of endometriosis is required.
- First, place the patient on the operating table with their legs placed into stirrups.
- After administering general anesthesia, place the patient in the lithotomic position, a variation of the supine position in which the legs are separated from the midline in a 30° to 45° abduction with the hips flexed until the thighs form an angle between 80° and 100°.
- Establish a sterile field by cleaning the following areas with a sponge soaked in antiseptic solution: the apex of the umbilicus, the abdomen, the perineum, and the top third of the thighs. Then, scrub with a gauze drenched in iodine solution the vulva and, when possible, the vaginal interior up to the cervix and discard it. Repeat this step 3x.
- With a new sponge drenched in iodine solution, swab the anus twice and discard it. Dry the prepared external areas with a sterile towel and place sterile drapes. Insert a urethral catheter for continuous bladder drainage.
- When possible, with the use of an anterior and posterior vaginal retractor, expose the cervix and insert the uterine manipulator into the cervix. Create a pneumoperitoneum by either a Verres needle inserted at an angle of 45° in non-obese patients to 90° in obese patients) or using the open technique (a small, 1 cm incision is made below the umbilicus on the midline). Keep the insufflation pressure between 12 mmHg and 14 mmHg.
- Insert a laparoscope and inspect the upper and lower abdomen. After positioning the patient in a slight Trendelenburg position, place the other laparoscopic access (usually two or three). Perform either CO2 fiber laser ablation or stripping technique as described below.
- One-step CO2 fiber laser vaporization
- Firstly, mobilize both adnexa to restore the normal anatomy of the pelvis. Then, by using an aspiration or irrigation device, drain the cyst content and irrigate and inspect its inner wall. Take a biopsy of the cyst wall using scissors and send it for routine histological examination to confirm the diagnosis of endometriosis.
- Select the basic operation mode and set the device to fiber laser mode with the continuous wave and constant timed-exposure mode at a power density of 13-15 W13.
- Evert the cyst with grasping forceps in order to expose the inner cystic wall and completely vaporize the inner wall with a CO2 fiber laser in a radial way, starting from the center to the periphery, keeping the tip of the fiber at a distance of at least 1 cm from the cystic surface (see Table of Materials).
- Do not suture the ovary after vaporization. Carefully control any source of bleeding at the end of the procedure using the water test (i.e., washing the bleeding sites to visualize and achieve hemostasis selectively) or by slightly reducing the pneumoperitoneum.
- Cystectomy
- Start with adhesiolysis in order to free the ovaries from the surrounding structures. Make a sharp cortical incision on the thinnest part of the cyst, just enough to identify the correct cleavage plane. Avoid making the incision close to the fallopian tube or fimbriae.
- Take the edges of the incision with two grasping forceps and strip out the cyst from the healthy ovarian parenchyma by delicate traction and counter traction maneuvers.
- After removal of the cyst, perform selective hemostasis with bipolar coagulation using the water test, mainly on the edges of the ovary, to reduce the risk of ovarian damage13.
- At the end of the surgery, carefully remove the uterine manipulator if positioned. Suture the fascia with a medium absorption rate braided suture size 0 and the skin with a quick absorption rate suture size 3-0. Place patches on all the incisions and remove the urethral catheter the day after the surgical procedure.
4. Post-operative follow-up and testing
- Identify the endometriosis stage. Perform endometriosis staging according to the revised American Society for Reproductive Medicine (r-ASRM) classification17. Calculate the lesion score and total score at the end of surgery based on the surgery report, according to the standards of the r-ASRM classification, and report these as the r-ASRM score.
- Follow-up 30 days after the surgical procedure. After surgery, refer the patient to the endometriosis outpatient clinic for follow-up. In the case of patients with no immediate pregnancy intention, prescribe hormonal therapy (progestins or estroprogestins).
- Assess recurrence rate.
NOTE: To assess the recurrence rate, the minimum follow-up duration is 12 months.- Perform periodic gynecological examinations at intervals ranging from 3-12 months, according to patients' offspring desire or onset of symptoms (i.e., dysmenorrhea, chronic pelvic pain, dyspareunia).
- At every follow-up, perform a gynecological examination as well as a transvaginal ultrasound to check for recurrence of the endometriotic cyst. In case of the identification of a cyst with a typical sonographic aspect and a diameter of more than 10 mm arising on the operated ovary, confirmed by transvaginal ultrasound, consider it as cyst recurrence. Assess the symptoms of cyst recurrence by interviewing the patient.
- Assess pregnancy outcomes. Allow patients wishing to conceive to attempt spontaneous conception for a period of 6-9 months in the case of CO2 fiber laser vaporization and for a period of 12 months after cystectomy. If spontaneous conception fails, refer the patients immediately for assisted reproductive techniques (ART).
NOTE: Pregnancy is defined as the evidence of a vital embryo in utero by transvaginal ultrasound at 6 weeks of pregnancy. As suggested by usual clinical practice, in case of the absence of fetal cardiac activity at 6 weeks of pregnancy, wait for at least 7-10 days to diagnose a pregnancy loss. The time taken for spontaneous conception is defined as the interval between surgery and spontaneous conception (or the first IVF when natural conception fails). Do not include patients who become pregnant following oocyte donor-IVF.
Representative Results
The details of the outcomes of the included studies are shown in Table 1.
Ovarian reserve after one-step laser vaporization versus cystectomy in the treatment of ovarian endometrioma13
The aim of this randomized controlled study was to compare the two surgical procedures for endometrioma treatment (cystectomy versus CO2 laser vaporization) in terms of their impact on ovarian reserve markers (AFC and serum AMH concentrations) and ovarian volume before and 3 months after treatment. The results of the study are summarized in Table 1. In the case of unilateral endometrioma, the change in AFC (ΔAFC) of the operated ovary was found to be significantly higher after one-step CO2 fiber laser vaporization compared with cystectomy. Conversely, serum AMH levels were significantly reduced at 3 months in the cystectomy group, compared with no reduction in the CO2 fiber laser group. The increase in AFC following CO2 fiber laser vaporization at 3-month echographic evaluation may be due to the effect of the laser stimulating the ovarian microenvironment, thus allowing for neovascularization. Higher AFC 3 months after CO2 ablation compared with baseline could be because of the removal of the mechanical distortion of the cyst on the surrounding healthy ovarian parenchyma. Moreover, it is known that serum AMH levels represent a predictive value for ovarian response to hormonal stimulation in ART rather than a unique marker of ovarian reserve.
Ovarian volume was similar in both the operated ovary and the contralateral non-operated ovary after CO2 fiber laser vaporization, while after cystectomy it was reduced compared to the contralateral non-operated ovary.
Fertility outcome after CO2 laser vaporization versus cystectomy in women with ovarian endometrioma14
This retrospective study aimed to investigate the spontaneous pregnancy rate in women undergoing surgery (cystectomy versus one-step CO2 fiber laser vaporization) for symptomatic endometriomas. The results of the study are shown in Table 1. No differences were found in terms of spontaneous pregnancy rates between the two groups. Two factors namely, age at the time of surgery and duration of infertility, were identified as the only independent pregnancy indicators. The other factors such as, size of endometrioma size at the time of surgery, unilateral versus bilateral involvement, the r-ASRM score, concomitant deep endometriosis, type of surgery performed (cystectomy versus ablation with CO2 fiber laser), and recurrence of disease did not have any significant predictive value for pregnancy.
Recurrence rate of ovarian endometriosis after one-step CO2 fiber laser vaporization versus cystectomy16
This retrospective study investigated postoperative recurrence rates in patients with endometriomas managed by either one-step CO2 fiber laser vaporization or cystectomy. In particular, the recurrence rate in terms of recurrence of the cyst on the operated ovary or recurrence of pain symptoms was assessed. The results are summarized in Table 1.
Recurrence of ovarian endometriosis was recorded in 6.3% of patients that were treated with cystectomy and in 4.9% of patients treated with CO2 fiber laser16. All recurrent patients in the CO2 fiber laser vaporization group were found to be not receiving any medical therapy because of pregnancy intention, whereas two out of the four recurrent patients (50%) in the cystectomy group were under hormonal treatment. The only independent poor prognostic indicator for cyst recurrence was mean endometrioma diameter (>5 cm) at the time of surgery.
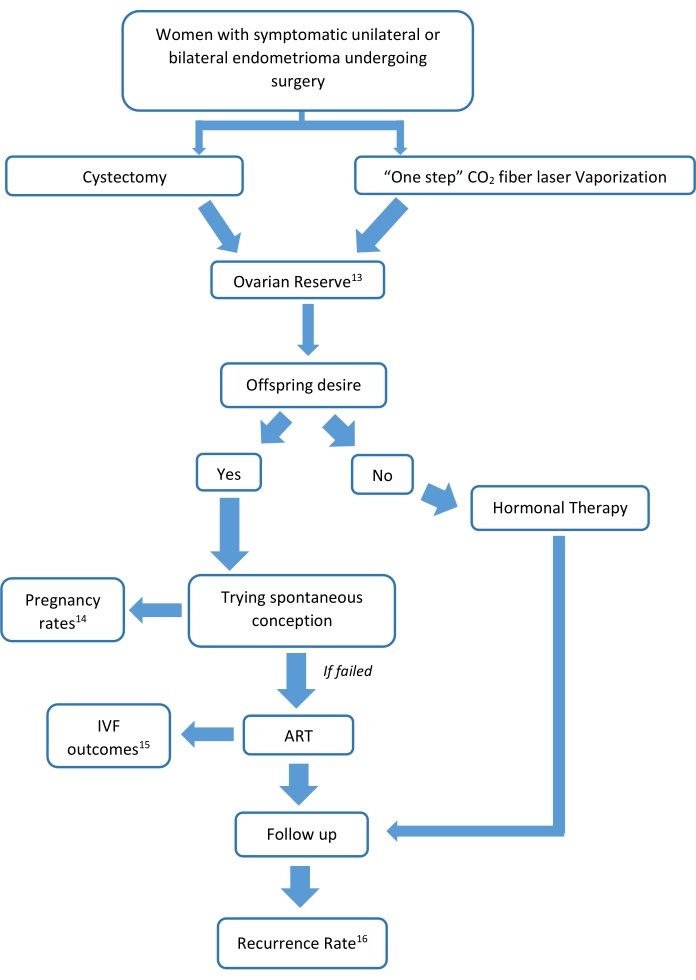
Figure 1: Diagram of the studies' protocols. The figure represents a study flow-chart of how the analysis proceeded, along with all the aspects of the CO2 fiber laser technique. All of the studies have their own study population, whose characteristics are represented in more detail in the original publications13,14,15,16. Please click here to view a larger version of this figure.
Table 1: Details of the outcomes of the included studies conducted from 2015 to 2020 with CO2 fiber laser vaporization. Data was taken from our previous studies and analyzed, and a summary of the previous studies' results is shown13,14,16. Please click here to download this Table.
Discussion
The aim of this method is to provide a comprehensive overview of our experience with CO2 fiber laser technology at San Raffaele Scientific Institute since 2015, when the use of this technique first started for the surgical management of endometriomas. Since endometriosis is a chronic benign gynecological condition affecting women of reproductive age with potential offspring desire, surgical techniques need to be as fertility-sparing as possible.
A Cochrane review18 comparing ablative and stripping techniques reported better outcomes in terms of spontaneous pregnancy and recurrence rate in the cystectomy group. However, these results have been questioned since the ablation group consisted only of bipolar energy, which is known to be burdened by a deeper thermal effect compared to other types of energy, such as CO2 fiber laser and plasma energy12,19,20,21,22,23. Moreover, many concerns have been raised about the possible detrimental effect of cystectomy on ovarian reserve. Increasing evidence suggests that the risk of inadvertent removal of healthy ovarian parenchyma exists and that this risk is inversely correlated with surgical expertise and cyst size8,24,25. Conversely, CO2 fiber laser technology has been shown to be safe and effective according to Donnez et al.26. Moreover, the tissue penetration during the laser ablation technique cannot go deeper than 1.0-1.5 mm, allowing only the destruction of the filmy superficial internal lining of the cyst and protecting the deeper healthy ovarian parenchyma.
Based on this evidence, we adopted the CO2 fiber laser in 2015 due to promising results in terms of ovarian reserve, reproductive outcomes, and recurrence rate. This technique has turned out to be simple and easily reproducible since residents with no surgical experience performed better with a flexible CO2 fiber laser delivery system compared with the standard in-line-of-sight CO2 laser system after a 2-month training period with a gynecological laparoscopic box. These findings demonstrate that CO2 fiber laser is more technically accessible to all surgeons compared to the stripping technique27.
The first multicenter randomized study13 aimed to assess the impact of cystectomy and CO2 laser vaporization on ovarian reserve. In particular, a significant improvement was observed in the AFC of the operated ovary after CO2 laser vaporization compared to cystectomy. Similar results were obtained by Pados et al.28, where an increase in the AFC was found in the treated ovary after 6 months of the three-stage procedure. Donnez also reported similar AFC results between the operated ovary and the contralateral after using a combination of excisional and ablative techniques26. Anti-mullerian hormone levels were found to be significantly decreased in the cystectomy group as compared to CO2 laser vaporization. These findings are in-line with those reported by Tsolakidis et al.29. Moreover, no differences were found in ovarian volume before and after surgery when using the CO2 fiber laser, suggesting that this technology could be able to better preserve the normal ovarian volume.
Starting from these findings, we aimed to assess the pregnancy rate in those wishing to conceive14. Although positive results were observed for ovarian reserve markers, we did not find differences between cystectomy and CO2 laser in terms of postoperative spontaneous pregnancy rate. However, due to concerns about the recurrence rate in this group, women treated with ablation were given less time to conceive spontaneously and were then referred to assisted reproductive technology. These different strategies could represent a limitation of the study since the different time restrictions could account for the lower spontaneous pregnancy rates in the CO2 laser treatment group. Moreover, CO2 fiber laser vaporization was introduced later in the clinic, hence some patients who underwent cystectomy and were treated before the introduction of the ablative technique had a long time to conceive spontaneously. All the women who did not conceive spontaneously were referred to IVF therapy, with good outcomes in terms of the number of retrieved oocytes, number of embryos, and cumulative pregnancy rates. Indeed, in a recent pilot study assessing the ovarian responsiveness to controlled ovarian stimulation in patients who underwent CO2 fiber laser vaporization, it was demonstrated that CO2 fiber laser ablation is associated with favorable ART outcomes and does not impair the number of recruited follicles in the operated ovary compared to the contralateral healthy one15. Alongside the small sample size, there is a lack of evidence in the current literature regarding this issue. Although not conclusive, these results are reassuring with regard to ovarian reserve, but it is necessary to design a more accurate case-control study with a larger sample size and an adequate control group.
To answer the question about the recurrence rate, we retrospectively reviewed the data generated between 2015 and 201816 with the aim of assessing the effectiveness of CO2 laser compared to the stripping technique in terms of recurrence of endometrioma and pain symptoms. The results suggested, for the first time, that ablation with one-step CO2 fiber laser technology is associated with recurrence rates similar to those observed after cystectomy in the surgical treatment of ovarian endometrioma. The real incidence of endometrioma recurrence is uncertain, and it is estimated to occur between 6% and 32% of cases12,20,30,31. This heterogeneity is attributable to the different definitions of recurrence and variability in the follow-up duration of different studies. After undergoing ablative techniques using CO2 laser in-line-of-sight or plasma energy, recurrence rates ranging from 8% through 30% have been reported12,20,30,31. The recurrence rate reported here is lower compared to earlier reported studies, owing to the surgeon's experience and to the technology itself. Indeed, its simplicity, the long arm of the flexible fiber that allows for accessibility to areas and anatomic spaces that are difficult to reach, and its reproducibility make the CO2 fiber laser a viable alternative to traditional cystectomy for gynecologists approaching endometrioma without specific skills in the field of reproductive and endometriosis surgery27. However, there are some critical steps during this procedure to be taken care of: during the eversion of the cyst ensure not to leave any area of the capsule unexposed and untreated; pay attention to the laser spot inside the abdominal cavity, avoiding the spread of the laser to the surrounding structures; and consider other surgical strategies in case of very large endometriomas (i.e., diameters >8 cm) because of increased risk of leaving untreated areas and the longer operation time.
Concerns have been raised about the costs of this procedure for the health care system. Undoubtedly, CO2 laser vaporization requires specific facilities (see Table of Materials) when compared to the cystectomy technique, though it should be noted that it can also be used for other treatments such as cosmetic surgery and otolaryngological and urogynecological dysfunctions. Except for the single, multicenter randomized controlled study reported in13, the external validity of these findings is limited due to the small sample size and the nature of the studies. However, in light of the results obtained, we believe that the CO2 fiber laser constitutes the next-best alternative to the standard technique in terms of ovarian preservation, pregnancy rates, and IVF outcomes. Multicenter randomized trials are needed in order to standardize endometrioma-related infertility treatment.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
No external funding was either sought or obtained for this study.
Materials
| CO2 fiber laser | UltraPulse Duo system, Lumenis Ltd | AC-1059590 | |
| Insufflation Needle | Covidien | 10065003 | |
| Laparoscopic Forceps | Erbe Elektromedizin GmbH | 20195-133 | |
| Manipulator | Lumenis Ltd | ||
| UltraPulse Duo | Lumenis Ltd | GA-2000000 | CO2 laser system |
| VIO 3 | Erbe Elektromedizin GmbH | 10160-000 | electrosurgical unit |
| Voluson S8 | GE Healthcare | 186958SU5 | ultrasound scan voluson system 8 |
Referenzen
- Bafort, C., Beebeejaun, Y., Tomassetti, C., Bosteels, J., Duffy, J. M. Laparoscopic surgery for endometriosis. The Cochrane Database of Systematic Reviews. 10 (10), (2020).
- Somigliana, E., et al. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertility and sterility. 98 (6), 1531-1538 (2012).
- Uncu, G., et al. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Human Reproduction. 28 (8), 2140-2145 (2013).
- Alborzi, S., Keramati, P., Younesi, M., Samsami, A., Dadras, N. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometriomas. Fertility and Sterility. 101 (2), 427-434 (2014).
- Muzii, L., Bianchi, A., Croce, C., Manci, N., Panici, P. B. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue-sparing procedure. Fertility and Sterility. 77 (3), 609-614 (2002).
- Sanchez, A. M., et al. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Human Reproduction Update. 20 (2), 217-230 (2014).
- Benaglia, L., et al. Rate of severe ovarian damage following surgery for endometriomas. Human Reproduction. 25 (3), 678-682 (2010).
- Muzii, L., et al. Histologic analysis of specimens from laparoscopic endometrioma excision performed by different surgeons: does the surgeon matter. Fertility and Sterility. 95 (6), 2116-2119 (2011).
- La Torre, R., et al. Ovarian blood flow before and after conservative laparoscopic treatment for endometrioma. Clinical and Experimental Obstetrics & Gynecology. 25 (1-2), 12-14 (1998).
- Loh, F. H., Tan, A. T., Kumar, J., Ng, S. C. Ovarian response after laparoscopic ovarian cystectomy for endometriotic cysts in 132 monitored cycles. Fertility and Sterility. 72 (2), 316-321 (1999).
- Candiani, M., et al. Ovarian recovery after laparoscopic enucleation of ovarian cysts: insights from echographic short-term postsurgical follow-up. Journal of Minimally Invasive Gynecology. 12 (5), 409-414 (2005).
- Donnez, J., et al. Large ovarian endometriomas. Human Reproduction. 11 (3), 641-646 (1996).
- Candiani, M., et al. Assessment of ovarian reserve after cystectomy versus ‘one-step’ laser vaporization in the treatment of ovarian endometrioma: a small randomized clinical trial. Human Reproduction. 33 (12), 2205-2211 (2018).
- Candiani, M., et al. Fertility outcome after CO2 laser vaporization versus cystectomy in women with ovarian endometrioma: a comparative study. Journal of Minimally Invasive Gynecology. 28 (1), 34-41 (2021).
- Ottolina, J., et al. Ovarian responsiveness in assisted reproductive technology after CO2 fiber laser vaporization for endometrioma treatment: preliminary data. Minerva Endocrinologica. 45 (4), 288-294 (2020).
- Candiani, M., et al. Recurrence rate after "one-step" CO2 fiber laser vaporization versus cystectomy for ovarian endometrioma: a 3-year follow-up study. Journal of Minimally Invasive Gynecology. 27 (4), 901-908 (2020).
- American Society for Reproductive Medicine. Revised American Fertility Society classification of endometriosis: 1996. Fertility and Sterility. 67 (5), 817-821 (1997).
- Hart, R. J., Hickey, M., Maouris, P., Buckett, W. Excisional surgery versus ablative surgery for ovarian endometriomata. The Cochrane Database of Systematic Reviews. (2), (2008).
- Daniell, J. F., Kurtz, B. R., Gurley, L. D. Laser laparoscopicmanagement of large endometriomas. Fertility and Sterility. 55 (4), 692-695 (1991).
- Sutton, C. J., Ewen, S. P., Jacobs, S. A., Whitelaw, N. L. Laser laparoscopic surgery in the treatment of ovarian endometriomas. The Journal of the American Association of Gynecologic Laparoscopists. 4 (3), 319-323 (1997).
- Donnez, J., Pirard, C., Smets, M., Jadoul, P., Squifflet, J. Surgical management of endometriosis. Best practice & research. Clinical Obstetrics & Gynaecology. 18 (2), 329-348 (2004).
- Sutton, C. J., Jones, K. D. Laser laparoscopy for endometriosis and endometriotic cysts. Surgical Endoscopy. 16 (11), 1513-1517 (2002).
- Roman, H., et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertility and Sterility. 96 (6), 1396-1400 (2011).
- Muzii, L., et al. Histologic analysis of endometriomas: what the surgeon needs to know. Fertility and Sterility. 87 (2), 362-366 (2007).
- Roman, H., et al. Vaporization of ovarian endometrioma using plasma energy: histologic findings of a pilot study. Fertility and Sterility. 95 (5), 1853 (2011).
- Donnez, J., Wyns, C., Nisolle, M. Does ovarian surgery for endometriomas impair the ovarian response to gonadotropin. Fertility and Sterility. 76 (4), 662-665 (2001).
- Vanni, V. S., et al. Flexible CO2 laser fiber: first look at the learning curve required in gynecological laparoscopy training. Minerva Ginecologica. 70 (1), 53-57 (2018).
- Pados, G., Tsolakidis, D., Assimakopoulos, E., Athanatos, D., Tarlatzis, B. Sonographic changes after laparoscopic cystectomy compared with three-stage management in patients with ovarian endometriomas: a prospective randomized study. Human Reproduction. 25 (3), 672-677 (2010).
- Tsolakidis, D., et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertility and Sterility. 94 (1), 71-77 (2010).
- Roman, H., et al. Postoperative recurrence and fertility after endometrioma ablation using plasma energy: retrospective assessment of a 3-year experience. Journal of Minimally Invasive Gynecology. 20 (5), 573-582 (2013).
- Carmona, F., Martínez-Zamora, M. A., Rabanal, A., Martínez-Román, S., Balasch, J. Ovarian cystectomy versus laser vaporization in the treatment of ovarian endometriomas: a randomized clinical trial with a five-year follow-up. Fertility and Sterility. 96 (1), 251-254 (2011).

