An Optimized Single-Molecule Pull-Down Assay for Quantification of Protein Phosphorylation
Summary
The present protocol describes sample preparation and data analysis to quantify protein phosphorylation using an improved single-molecule pull-down (SiMPull) assay.
Abstract
Phosphorylation is a necessary posttranslational modification that regulates protein function and directs cell signaling outcomes. Current methods to measure protein phosphorylation cannot preserve the heterogeneity in phosphorylation across individual proteins. The single-molecule pull-down (SiMPull) assay was developed to investigate the composition of macromolecular complexes via immunoprecipitation of proteins on a glass coverslip followed by single-molecule imaging. The current technique is an adaptation of SiMPull that provides robust quantification of the phosphorylation state of full-length membrane receptors at the single-molecule level. Imaging thousands of individual receptors in this way allows for quantifying protein phosphorylation patterns. The present protocol details the optimized SiMPull procedure, from sample preparation to imaging. Optimization of glass preparation and antibody fixation protocols further enhances data quality. The current protocol provides code for the single-molecule data analysis that calculates the fraction of receptors phosphorylated within a sample. While this work focuses on phosphorylation of the epidermal growth factor receptor (EGFR), the protocol can be generalized to other membrane receptors and cytosolic signaling molecules.
Introduction
Membrane-associated signaling is tuned by a combination of ligand-induced membrane receptor activation and recruitment of downstream accessory proteins that propagate the signal. Phosphorylation of key tyrosines in receptor cytoplasmic tails is critical to initiating the formation of signaling complexes, or signalosomes1,2. Therefore, an important question in biology is how phosphorylation patterns are created and maintained to recruit signaling partners and dictate cellular outcomes. This includes understanding the heterogeneity of receptor phosphorylation, both in abundance and in the specific phosphotyrosine patterns that can provide a means of manipulating signaling outputs by dictating the composition of the signalosome3,4,5,6,7. However, there are limitations in current methods to interrogate protein phosphorylation. Western blot analysis is excellent for describing trends of protein phosphorylation but is semi-quantitative8 and does not provide information on the heterogeneity of the system because thousands to millions of receptors are averaged together. While western blots allow probing a sample using phospho-specific antibodies to specific tyrosines, they cannot provide information on multisite phosphorylation patterns within the same protein. Quantitative phosphoproteomics report on phosphotyrosine abundance, but there are limitations to detecting multisite phosphorylation, as the residues of interest need to be located within the same peptide (typically 7-35 amino acids) that is generated by enzymatic digestion9,10,11.
To overcome the limitations mentioned above, the single-molecule pull-down (SiMPull) assay has been adapted to quantify the phosphorylation states of intact receptors at the single-molecule level. SiMPull was first demonstrated as a powerful tool for interrogating macromolecular complexes by Jain et al.12,13. In SiMPull, macromolecular complexes were immunoprecipitated (IP) on antibody-functionalized glass coverslips and then analyzed through single-molecule microscopy for protein subunit number and co-IP with complex components12. A modification by Kim et al.14, termed SiMBlot, was the first to use a variation of SiMPull to analyze phosphorylation of denatured proteins. The SiMBlot protocol relies on capturing biotinylated cell surface proteins using NeutrAvidin-coated coverslips, which are then probed for phosphorylation with phospho-specific antibody labeling14. Despite these advances, improvements were needed to make the quantification of posttranslational modification more robust and applicable to a broader range of proteins.
The present protocol describes an optimized SiMPull approach that was used to quantify phosphorylation patterns of intact epidermal growth factor receptor (EGFR) in response to a range of ligand conditions and oncogenic mutations15. While this work focuses on EGFR, this approach can be applied to any membrane receptor and cytosolic proteins of interest (POI), for which quality antibodies are available. The protocol includes steps to reduce sample autofluorescence, a sample array design that requires minimal sample volume with simultaneous preparation of up to 20 samples, and optimization of antibody labeling and fixation conditions. Data analysis algorithms have been developed for single-molecule detection and quantification of phosphorylated proteins.
Protocol
1. Coverslip preparation
NOTE: For this step, one needs to wear personal protective equipment (PPE), which includes a double layer of nitrile gloves, safety glasses or face shield, and a lab coat.
- Perform piranha etching to remove organic debris from the glass.
CAUTION: Piranha solution is a strong oxidizing agent that is corrosive and highly reactive when in contact with organic materials. Reaction with organic debris is exothermic and potentially explosive. Thus, the procedure must be performed in a chemical fume hood with the sash lowered. Pyrex glassware is required to handle the piranha solution.- Prepare the workspace inside a chemical fume hood. Arrange coverslips without overlap in the bottom of a 4 L glass beaker, the "reaction" beaker, and place the reaction beaker on a hotplate with gentle heat. Allow the glassware to warm for 10 min. Place a "waste" 1 L glass beaker with 500 mL of ddH2O proximal to the reaction beaker.
- Add 49 mL of 12 N sulfuric acid (H2SO4) slowly to the reaction beaker with a glass serological pipette. Rinse the pipette in the waste beaker before disposal.
- Add 21 mL of 30% hydrogen peroxide (H2O2) dropwise with a glass serological pipette to the reaction beaker. Slowly distribute the H2O2 droplets evenly across the bottom of the reaction flask to prevent localized quenching of the piranha etching reaction. Rinse the pipette in the waste beaker before disposal.
CAUTION: Always add H2O2 into the H2SO4, and never vice-versa. - Piranha etch the coverslips for 30 min. Gently agitate the contents of the reaction beaker every 5 min.
- Quench the piranha solution by pouring the contents of the waste beaker into the reaction beaker. Transfer the liquid slowly down the wall of the reaction beaker to minimize splashing. Remove the reaction beaker from the hotplate.
- When the reaction is quenched and cooled, pour the piranha solution back into the waste beaker for neutralization without removing the etched coverslips from the reaction beaker.
- Neutralize the piranha solution with the gradual addition of a weak base. For example, use an excessive mass 20 g of sodium bicarbonate (NaHCO3)/100 mL piranha solution.
CAUTION: Do not store piranha solutions in sealed waste containers. The solution must always be neutralized before disposal. The neutralization reaction produces vigorous bubbles and may be explosive if not controlled by the gradual addition of the weak base. - Stir the neutralized solution with a glass stir rod and let it react for 2 h. Raise the pH to >4 and dispose of the solution.
- Between the additions of weak base to the piranha solution, transfer the etched coverslips from the reaction beaker into a Buchner funnel with a glass stir rod and rinse for 5 min in running ddH2O.
NOTE: Proceed immediately to the next step or store the piranha etched coverslips for up to 2 weeks in ddH2O in a sealed glass jar or Petri dish (wrap with sealing film, see Table of Materials).
- Bath-sonicate the coverslips in organic solvents following the steps below.
- Place the coverslips in a glass Coplin jar (see Table of Materials) and cover with methanol (CH3OH). Seal the lid to the jar with sealing film and bath-sonicate for 10 min. Carefully pour the methanol out of the Coplin jar into a glass storage bottle.
- Fill the Coplin jar with acetone (C3H6O), seal the lid, and bath-sonicate for 10 min. Carefully pour the acetone out of the Coplin jar into a glass storage bottle.
CAUTION: Methanol is flammable and acutely toxic. Use in a chemical fume hood. Acetone is flammable and an irritant. Therefore, handle with and store it in glass, and use it in a chemical fume hood. Dispose of as hazardous waste according to local regulations and guidelines.
NOTE: Methanol and acetone can be reused up to five times each.
- Activate the coverslip surface for silane functionalization.
- Bath-sonicate with 1 M potassium hydroxide (KOH) for 20 min. Carefully pour the KOH out of the Coplin Jar into a 50 mL conical tube for reuse.
CAUTION: KOH is corrosive and an irritant. Use in a chemical fume hood and do not store in glass. Store it in polypropylene tubes. Dispose of as hazardous waste according to local regulations and guidelines.
NOTE: KOH can be reused up to five times. - Rinse twice with ddH2O. Drain ddH2O from the coverslips, and then heat each coverslip by waving through the flame of a Bunsen burner to drive off all surface moisture. Place the coverslips in a dry Coplin jar.
- Bath-sonicate with 1 M potassium hydroxide (KOH) for 20 min. Carefully pour the KOH out of the Coplin Jar into a 50 mL conical tube for reuse.
- Perform coverslip aminosilanization.
- Prepare the aminosilane solution by mixing 69.4 mL of methanol with 3.6 mL of acetic acid (CH3COOH) in a conical flask. Add 720 µL of N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (aminosilane) and mix well (see Table of Materials).
CAUTION: Acetic acid is flammable and corrosive. Handle with glass pipettes and store in glass. Work with acetic acid in a chemical fume hood. Aminosilane is an acutely toxic inhalation hazard, a sensitizer, and an irritant. It is harmful to aquatic life. Use in a chemical fume hood. Dispose of the chemicals as hazardous waste according to local regulations and guidelines.
NOTE: Aminosilane is photosensitive and hydrolyses rapidly in water. All steps with this reagent need to be performed under minimal light conditions to retain activity. Purge the bottle with nitrogen gas and apply sealing film before storage in a dark desiccator. Replace every 6-9 months. - Immediately add the aminosilane solution to the Coplin jar. Cover and apply the sealing film, continuing to protect from light.
- Incubate the coverslips in the aminosilane solution for 10 min in the dark at room temperature (RT). Bath-sonicate for 1 min and then incubate for another 10 min. Carefully pour the aminosilane solution into a waste container designated for CH3OH with trace aminosilane and CH3COOH.
- Rinse the coverslips with methanol and pour the solution into a waste container designated for methanol.
- Rinse the coverslips three times for 2 min each with ddH2O. Drain the coverslips, dab away excess moisture, and air dry completely for 10 min.
- Prepare the aminosilane solution by mixing 69.4 mL of methanol with 3.6 mL of acetic acid (CH3COOH) in a conical flask. Add 720 µL of N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (aminosilane) and mix well (see Table of Materials).
- Perform the array preparation/biotin-PEG functionalization of the coverslips.
- Prepare 1 M NaHCO3 (pH 8.5) working stock by dissolving 84.5 mg of NaHCO3 into 1 mL of ddH2O. For final concentration of 10 mM NaHCO3, dilute 1 M NaHCO3 into ddH2O (1:100).
- Draw a grid array on the dry aminosilanized coverslips with a hydrophobic barrier pen (see Table of Materials) and wait for the ink to dry. Write an identifier on the coverslip to mark the proper orientation. Place the coverslips in a humidified chamber.
NOTE: The array should consist of 16-20 squares, approximately 4 mm x 4 mm in size. - To make the biotin-PEG succinimidyl valerate (biotin-PEG)/mPEG succinimidyl valerate (mPEG) solution, first, remove mPEG and biotin-PEG (see Table of Materials) from the freezer and equilibrate to RT. Add 153 mg of mPEG and 3.9 mg of biotin-PEG (~1:39 biotin-PEG:mPEG) to a 1.5 mL microcentrifuge tube, and resuspend in 609 µL of 10 mM NaHCO3 by gentle pipetting. Centrifuge at 10,000 x g for 1 min at RT to remove bubbles.
NOTE: The hydrolysis half-life of succinimidyl valerate in pH 8.5 buffer is ~30 min. After adding the buffer to mPEG, proceed with the following steps as rapidly as possible. This step is critical. - Apply the biotin-PEG/mPEG solution to completely cover each square in the coverslip arrays, typically 10-15 µL per square. Do not allow liquid to overflow the defined space. Store the coverslips in a humidity chamber in the dark for 3-4 h at RT.
- Wash the coverslips with copious amounts of water by sequentially dipping them into 3x 250 mL glass beakers filled with ddH2O for 10 s each.
- Drive off all moisture from the coverslips with nitrogen gas. Store the coverslips back-to-back in a nitrogen-filled 50 mL conical tube wrapped with sealing film at -20 °C.
NOTE: Proceed immediately to step 2 or store coverslips at -20 °C for up to 1 week before use.
2. Preparation of SiMPull lysate
CAUTION: The required PPE for the remaining steps of the protocol are nitrile gloves, safety glasses, and lab coats.
NOTE: The lysates were prepared from the adherent CHO cells expressing EGFR-GFP. The cells were plated in a 60 mm tissue culture (TC60) dish overnight12,13. CHO cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1% L-glutamine, 1% Penicillin-Streptomycin, and 500 ng/mL of geneticin (see Table of Materials). Other adherent cell lines or suspension cells can also be used.
- Plate the cells following the steps below.
- Wash the culture dish (containing the cells) with 1 mL of 1x PBS. Add 1 mL of 1x Trypsin and incubate for 5 min at 37 °C to detach the cells. Using a pipette, transfer the detached cells from the dish to a 1.5 mL centrifuge tube.
- Take 10 µL of trypan blue and mix with 10 µL of cell suspension in a separate centrifuge tube. Count cells using 10 µL of the cell mixture in an automatic cell counter, according to the manufacturer's instructions (see Table of Materials).
- Plate 8 x 105 cells overnight in a TC60 petri dish. Plate one dish per condition.
NOTE: For the present study, the cells were either untreated or treated with Pervanadate and EGF as described in step 2.4.1.
- Prepare the following solutions for the cell lysate preparation.
- Prepare ice-cold 1x PBS (pH 7.4).
- Prepare lysis buffer, a solution of 1% non-ionic, non-degenerating detergent (see Table of Materials) in 50 mM Tris-HCl (pH 7.2) and 150 mM NaCl supplemented with Protease/Phosphatase Inhibitor (PPI) (1:100 from stock). Place tube on a nutator and allow buffer to mix for 15 min. Keep the prepared solution on ice.
- Prepare the Tyrode's buffer for a final concentration of 135 mM NaCl, 10 mM KCl, 0.4 mM MgCl2, 1 mM CaCl2, 10 mM HEPES (pH 7.2), 20 mM glucose, and 0.1% Bovine Serum Albumin (BSA) (see Table of Materials). Warm the solution to 37 °C.
- Prepare the positive control for phosphorylation – 1 mM pervanadate treatment.
NOTE: This step is optional. Pervandate is the peroxidized form of vanadate-an inhibitor of protein tyrosine phosphatases16. Preventing protein dephosphorylation by inhibiting phosphatase activity results in a highly phosphorylated sample.- Prepare a 200 mM stock of activated sodium orthovanadate (Na3VO4).
- To prepare 100 mL solution, add 3.89 g of Na3VO4 (see Table of Materials) to 90 mL of ddH2O and dissolve while stirring. Adjust the pH to 10 by adding HCl or NaOH dropwise. Adding HCl will turn the solution yellow.
- Bring volume to 100 mL with ddH2O. Boil the solution by heating it in the microwave. After boiling, the solution will be colorless.
- Cool the solution to RT and readjust pH to 10. Repeat the boiling, cooling, and pH adjustment two to four more times, until the pH stabilizes at 10. Aliquot and store at -20 °C.
- Prepare a stock of 30 mM pervanadate (PV) by mixing 20.4 µL of 3% H2O2 with 100 µL of 200 mM Na3VO4 and 546.8 µL of ddH2O (equimolar concentrations of H2O2 and activated Na3VO4). Incubate in the dark at RT for 15 min.
- Prepare 1 mM PV in Tyrode's buffer. For 10 mL solution, add 0.33 mL of 30 mM PV stock to 9.67 mL of 37 °C Tyrode's buffer. Treat cells immediately.
- Wash cells once with 3 mL of Tyrode's buffer. Add 3 mL of 1 mM PV in the Tyrode's buffer to the cells and incubate for 15 min at 37 °C.
- Prepare a 200 mM stock of activated sodium orthovanadate (Na3VO4).
- Perform the ligand stimulation.
- Stimulate cells with the ligand of interest using appropriate concentration, time, and temperature. For maximal EGFR stimulation, incubate with 1 mL of 50 nM Epidermal Growth Factor (EGF, see Table of Materials) + 1 mM of PV in Tyrode's buffer for 5 min at 37 °C.
- Perform cell lysis.
- After desired cell treatment, place the dish on ice and wash with ice-cold 1x PBS. Completely remove the full volume of PBS using a pipette.
- Add 180 µL of lysis buffer (step 2.2.2) to the plate. Use a cell scraper to pull buffer around the plate to cover the cells fully. Apply firm, consistent pressure with the cell scraper across the entire cultured surface to lyse the cells fully.
NOTE: The volume of lysis buffer needs to be kept at a minimum to ensure a high protein concentration. - Pipette the lysed cells and transfer them to a 1.5 mL tube. Keep the tube on ice for 30 min. Vortex the lysates every 5 min.
NOTE: If the POI consists of multiple subunits or is sensitive to dissociation, do not vortex the lysates. - Centrifuge the lysed cells at 16,000 x g for 20 min at 4 °C. Transfer the supernatant to a new 1.5 mL tube using a pipette. This contains the total protein lysate.
- Reserve 10 µL of the lysate and dilute it into 90 µL of the lysis buffer for bicinchoninic colorimetric assay (BCA) analysis17. Store the remaining total protein lysate at -80 °C.
- Determine lysate total protein concentration using BCA analysis (see Table of Materials).
NOTE: Total protein lysates can be prepared on the experiment day and used fresh or stored as single-use aliquots at -80 °C for up to 12 weeks. Do not freeze/thaw.
3. Functionalization of the array with the biotinylated antibody
- Prepare the following solutions.
- T50 Buffer, a solution of 10 mM Tris-HCl (pH 8.0) and 50 mM NaCl. The solution is stable for 1 month at RT.
- T50-BSA by supplementing T50 buffer with 0.1 mg/mL of BSA. Keep the prepared solution on ice.
- 10 mg/mL of sodium borohydride (NaBH4) in 1x PBS. Prepare this immediately before use.
- 0.2 mg/mL of NeutrAvidin (see Table of Materials) in T50 buffer.
CAUTION: NaBH4 is a reducing agent and is flammable. Always purge the container with nitrogen gas after use and store it in a desiccator.
- Functionalize the array with the biotinylated antibody.
- Remove the PEG-biotin functionalized arrays from the freezer and equilibrate the conical tube to RT before opening. Place the coverslip with the array oriented up onto a sealing film lined 100 mm tissue culture (TC100) dish.
NOTE: Minimize overhead lighting. All solutions should "bead up" on the squares defined by the hydrophobic array. Add an appropriate volume of solution to completely cover each square (typically 10-15 µL) and do not allow liquid to overflow the defined space. To rapidly remove liquids, use an in-house vacuum line attached to a vacuum flask to capture waste. Allow NaBH4 to degas for 1 h before disposal by leaving the tube open in the chemical fume hood. NaBH4 treatment is necessary to reduce background autofluorescence, thereby reducing false-positive single molecule detections. - Treat each square of the array with 10 mg/mL of NaBH4 in 1x PBS for 4 min at RT. Wash three times with PBS.
- Incubate each square for 5 min with 0.2 mg/mL of NeutrAvidin in T50. Wash three times with T50-BSA.
NOTE: NeutrAvidin binds to PEG-biotin and provides a binding site for biotinylated antibodies12,13,15. - Incubate each square for 10 min with 2 µg/mL of biotinylated POI-specific antibody in T50-BSA; wash three times with T50-BSA.
NOTE: The present protocol uses biotinylated anti-EGFR IgG (see Table of Materials) to capture EGFR-GFP.
- Remove the PEG-biotin functionalized arrays from the freezer and equilibrate the conical tube to RT before opening. Place the coverslip with the array oriented up onto a sealing film lined 100 mm tissue culture (TC100) dish.
4. SiMPull of POI from whole-cell lysates
NOTE: Place the TC100 dish of functionalized SiMPull arrays on ice for the remainder of the SiMPull preparation. This step is the pull-down of a POI from total protein lysate. The lysate must not be reused after thawing.
- Prepare the following solutions.
- Prepare 4% paraformaldehyde (PFA)/0.1% glutaraldehyde (GA) in 1x PBS.
CAUTION: PFA and GA are toxic chemical fixatives and potential carcinogens. Wear PPE. Dispose of the chemicals as hazardous waste according to local regulations and guidelines. - Prepare 10 mM Tris-HCl, pH 7.4.
- Prepare 4% paraformaldehyde (PFA)/0.1% glutaraldehyde (GA) in 1x PBS.
- Thaw and mix the lysate by gently pipetting up and down. Keep on ice.
- Dilute 1 µL of the lysate into 100 µL ice-cold T50-BSA/PPI.
NOTE: If needed, determine the appropriate dilution factor of the total protein lysate by applying a range of dilutions to the array. The optimal density of SiMPull receptors per array area is 0.04-0.08/µm2. Lysate dilutions can be assessed in step 6 (Data analysis). - Incubate the lysate on the array for 10 min; then wash four times with ice-cold T50-BSA/PPI.
- Dilute AF647-conjugated anti-phosphotyrosine antibody (see Table of Materials) in ice-cold T50-BSA/PPI and incubate on the array for 1 h.
NOTE: In the present protocol, a pan anti-pTyr (PY99)-AF647 IgG is used to identify the phosphorylated population of EGFR-GFP. The use of directly labeled antibodies removes the need for secondary antibodies, increasing the labeling options and improving the consistency of the results. Fluorescently-labeled antibodies can be obtained from commercial sources. If not commercially available, antibodies can be custom labeled using standard bioconjugation techniques, and commercial bioconjugation kits are available. Each batch of fluorescently labeled antibodies needs to be tested for optimal labeling conditions by performing SiMPull to measure a dose curve and find the saturation point. - Wash six times with ice-cold T50-BSA for a total of 6-8 min.
- Wash twice with ice-cold 1x PBS.
- Incubate the array with 4% PFA/0.1% GA solution for 10 min to prevent antibody dissociation.
- Wash twice for 5 min each with 10 mM Tris-HCl, pH 7.4/PBS to inactivate the fixatives.
NOTE: For experiments using more than one antibody, (e.g., detecting multiple phosphotyrosine sites), repeat steps 4.5-4.9. See step 6.2.9 for information on determining steric hindrance between two antibodies.
5. Image acquisition
NOTE: Single-molecule image acquisition is performed using a 150x TIRF objective and an image splitter that captures each spectral channel in a specific quadrant of the emCCD camera (see Table of Materials). Calibration images are first acquired to allow for channel registration and camera gain calibration with a nanopatterned channel alignment grid (nanogrid) that contains 20 x 20 arrays of 200 ± 50 nm holes at an intrahole distance of 3 ± 1 µm (total size ~60 µm × 60 µm).
- Perform channel registration following the steps below.
NOTE: Accurate channel registration is needed to properly calculate the colocalization of emitters. This step is critical.- Clean the oil objective and deposit a drop of oil on the objective. Place the nanogrid on the stage for imaging. Using transmitted white light, focus on the grid pattern.
NOTE: Images with the nanogrid are acquired using transmitted light, which passes through the nanogrid and is detected in all spectral channels. Alternatively, one can use multifluorescent beads that emit fluorescence detected in each channel. Image acquisition will need to be optimized according to each microscope setup. - Acquire a series of 20 images of the grid. Ensure that pixels are not saturated. Save the image series as "Fiducial."
- Defocus the nanogrid to create an Airy pattern18. Acquire a series of 20 images for gain calibrations. Save the image as "Gain".
- Acquire a series of 20 images for camera offset by blocking all light from going to the camera. Save the image as "Background".
- Clean the oil objective and deposit a drop of oil on the objective. Place the nanogrid on the stage for imaging. Using transmitted white light, focus on the grid pattern.
- Acquire SiMPull images.
NOTE: Before imaging the coverslip array, exchange the Tris solution for T50-BSA and equilibrate the array to RT.- Clean the oil objective and deposit additional oil on the objective. Secure the coverslip array on the microscope stage.
- Optimize each fluorophore's excitation power, TIRF angle, and camera integration time. The goal is to achieve the highest signal-to-noise while minimizing the photobleaching of the sample. Record the laser power for consistency in future measurements.
NOTE: The present study used 300 ms exposure time for the far-red channel and 1 s for the green channel. The 642 nm laser was used at approximately 500 µW laser power, while the 488 nm laser was used at 860 µW, measured before the tube lens. - Acquire images for each sample. Image the far-red channel first, followed by each lower wavelength fluorophore to reduce photobleaching. Due to the low volume used for each sample, check the buffer level every 30-45 min and replenish as needed.
6. Data analysis
- Download the demo codes.
NOTE: The provided demo code and example data sets demonstrate the full data analysis workflow (Supplementary Coding Files 1-4). The system requirements listed in SiMPullMain.m are found in Supplementary Coding File 1.- Unzip and Save into a personal Documents/MATLAB (MacOS/Linux) or DocumentsMATLAB (Windows) directory.
NOTE: This generates four new folders: SiMPull_class, smite, Sample Data, Sample Analysis Outputs. - Open the "ReadMe_Setup.txt" file found in the SiMPull_class folder.
- Install MATLAB and MATLAB Toolboxes: Curve Fitting Toolbox, Parallel Computing Toolbox, and Statistics and Machine Learning toolbox.
- Install DipImage19 according to the download instructions.
- Install "smite" single-molecule analysis package as described in ReadMe_setup.txt.
NOTE: "smite" is available on the GitHub repository (see Table of Materials). - Open SiMPullMain.m, found in SiMPull_class folder, by dragging the file into the MATLAB window.
- Change directory to …MATLABSample Data by clicking on the Browse for folder icon and selecting the folder.
- Unzip and Save into a personal Documents/MATLAB (MacOS/Linux) or DocumentsMATLAB (Windows) directory.
- Overview of data processing steps
- Run SiMPullMain.m – following instructions for each section. Execute each section individually by placing the cursor in that section and clicking the Run Section icon.
NOTE: The general steps for data analysis are described in this section. Detailed instructions are found in the accompanying SiMPullMain.m code. - Run the "Initialization" section to set the path to define spectral channels and image size.
- Run the "Find Camera Gain and Offset" section to convert camera gain to photons using the Gain and Background datasets.
- Run the "Channel Registration" section to calculate the local weighted mean transform used for image registration.
- Format and curate the data. Run the "Join Sequential Channels into a Quad Image" Section. Run "Remove Bad Frames".
- Run the "Fit Single Molecules and Find Overlapping Molecules" Section.
NOTE: This section executes multiple functions to localize single molecules in each channel and determine colocalization events between spectral channels. - To determine the minimum photon count per true GFP fit, run the "Optimize Minimum Photon Threshold". This is an iterative process.
- First, set smf.Thresholding_MinPhotons = [0, 0, 0] and run the section. Select "Blank Data" files when prompted. Repeat with the "CHO-EGFR-GFP" files.
- Select an appropriate minimum threshold value by comparing the two histograms. Set smf.Thresholding_MinPhotons = [475, 0, 0] and run the section again.
- Run the "Calculate Percentage of GFP fits Positive for FR Signal" section to correct background localizations and calculate final values.
- Perform Option 1 (step 6.2.10) or Option 2 (step 6.2.11) as per experimental need.
- Option 1: Correct for the number of receptors available at the plasma membrane for ligand binding, as described in Reference15.
- First, label surface receptors with saturating levels of fluorescent NHS Ester dye (NHS-AF647, see Table of Materials). Then perform a SiMPull experiment to determine the percentage of GFP localization that colocalizes with AF647.
NOTE: This provides an estimate of the fraction of receptors available for NHS labeling and the ratio of receptors on the surface (SR). - Apply the SR correction in the final calculation: NGFP = (NLOC – NBG)*SR, where NGFP is the corrected number of GFP localizations, NBG is the background localization number, and NLOC is total localizations.
NOTE: In the present example, this correction is not applied because Pervanadate is membrane permeable16 and, therefore, the action of phosphatase inhibition is not limited to surface receptors.
- First, label surface receptors with saturating levels of fluorescent NHS Ester dye (NHS-AF647, see Table of Materials). Then perform a SiMPull experiment to determine the percentage of GFP localization that colocalizes with AF647.
- Option 2: For multisite phosphorylation measurements, consider the potential for steric hindrance when two antibodies are used.
NOTE: Steric hindrance may cause a reduction in the observed percentage of phosphorylated receptors for Antibody 1 when in the presence of Antibody 2 (P12), compared to Antibody 1 alone (P1).- Use SiMPull to determine P1 and P12 and calculate the correction factor for steric hindrance, following previously published Reference15.
- Run SiMPullMain.m – following instructions for each section. Execute each section individually by placing the cursor in that section and clicking the Run Section icon.
Representative Results
A cartoon depicting the SiMPull process is shown in Figure 1A. Coverslips are functionalized using NeutrAvidin as an anchor for biotinylated anti-EGFR antibodies to capture EGFR-GFP from total protein lysates. After washing away unbound protein, the phosphorylated receptors are labeled with an anti-phosphotyrosine (anti-PY) antibody15. Figure 1B shows an image of the hydrophobic array, where multiple samples can be prepared and imaged on the same coverslip. One advantage of this sample holder is that minimal sample volumes of ~10 µL are required. The coverslip can be imaged by placing it directly on the microscope stage. However, it is helpful to stabilize the coverslip by using a coverslip holder. The coverslip holder shown in Figure 1B was created using a 3D printer, and the blueprint is provided in Supplementary Coding File 5. The autofluorescence of the hydrophobic ink is a useful guide to finding the focal plane of the sample (Figure 1C). An example of a multichannel raw image is shown in Figure 1D. An overlay of the raw green and far-red channels is shown in Figure 1E.
Figure 2 outlines the analysis workflow and provides representative data. Data acquisition first starts with acquiring fiducials for channel registration, which is used to overlay the individual spectral channels data (Figure 2A). Bright-field images are taken using a nanogrid pattern that passes white light and is detected in each spectral channel of the image splitter (not shown). The green channel acts as the reference channel, and the far-red channel is the shifted channel. The local weighted mean transform is calculated using the fitgeotrans20 function in MATLAB and is used to shift far-red coordinates into the coordinate frame of the green channel. This transform uses a second-order polynomial model at every control point. Multichannel data of the SiMPull array is then acquired. This workflow consisted of a semi-automated acquisition, where a starting ROI was selected for the specific sample square, and three regions around this area were imaged, such that each dataset contains the full quad-view image from three independent ROIs (Figure 1D). In each spectral channel, the emitter candidate locations are found by applying a difference of Gaussian filter to images and identifying local maxima. Subregions (boxes, Figure 2B) are drawn around local maxima, and emitter photon counts are estimated by assuming each subregion contains only one emitter. Subregions holding emitter candidates with photon counts above a minimum value are retained for fitting. A Gaussian point spread function (PSF) fits each emitter candidate within small subregions roughly centered around each emitter. The resulting localizations are thresholded based on their photon count, background, Cramér-Rao lower bound of the fit coordinates, PSF variance (i.e., PSF width), and a p-value describing the goodness of fit of the PSF model. A Gaussian image is created for each spectral channel, with uniform intensity Gaussian blobs placed at the coordinates for each good fit (Figure 2C). Colocalization is visualized by overlaying the Gaussian images from each spectral channel using the transform calculated from the fiducial sample (Figure 2D). It is important to fluorescently label the receptor for identification since there is still non-specific binding of the anti-phosphotyrosine antibodies to the surface when cell lysate is present. The EGFR-GFP (green channel) is used to generate a mask of the receptor locations, and only the AF647-anti-PY signal (far-red channel) within that mask is counted (Figure 2D). Pairs within 1 pixel (106.7 nm pixel size) are considered to be colocalized and saved to a list containing the reference channel coordinates. The percentage of AF647 colocalized with GFP is calculated to determine the fraction of phosphorylated receptors (Figure 2E).
There are several critical steps to ensure good data quality. One such effort is to incubate the coverslip array with NaBH4 as described in the protocol to quench autofluorescence in the green channel. This autofluorescence refers to the non-specific signal due to possible impurities on the glass, containing single or conjugated π bonds21. Such impurities are potentially from the aminosilane and PEG reagents used in the functionalization process or dust from the air, and tend to fluoresce in the green spectral channel. Despite efforts to keep glass stored under nitrogen, these molecules may also be generated through oxidation that occurs in storage. NaBH4 has also been used to reduce fluorescence from impurities on slides and microarrays, including those with silane coating16. Figure 3A shows the reduction in the number of background detections that occur when the piranha etched glass is treated with NaBH4. While NaBH4 reduces background fluorescence dramatically, some emitters are still detected in the green channel. One can correct this by acquiring background images from lysate-free samples (Figure 3D) and subtracting the average number of background localizations from the GFP-containing samples. Fluorescence from impurities was not detected in the far-red channel. If the receptor density is too high, multiple GFP emitters can be found within a single diffraction-limited spot (data not shown). Using step-photobleaching to identify the number of GFPs per spot, we found that a receptor density between 0.04-0.08 proteins/µm2 provided sufficient spacing between single emitters to remove the potential of finding multiple emitters per spot12. The receptor density can be optimized by varying the amount of IP antibody bound to the glass surface or the amount of lysate added. It is critical to ensure that the antibody targeting the POI is used at saturating levels. It is recommended to acquire an antibody concentration curve on phosphorylated samples to determine the appropriate labeling conditions (Figure 3B). In addition, the phospho-specificity of an antibody needs to be validated with resting samples and/or treatment with protein-specific kinase inhibitors (Figure 3B). Antibodies will dissociate from the receptor during the imaging time window. Treating the sample with a combination of PFA and GA prevented signal loss (Figure 3C).
Finally, it is important to optimize the single-molecule fitting parameters. The first "box finding" step that identifies potential emitter candidates (Figure 2B) needs to be generous to allow many candidates to undergo the Gaussian Fitting. Thus, the minimum photon threshold for box finding can be relatively low to capture all real emitters and some background spots. It is also important to not set the box size and overlap allowance too small. Keeping the box size within 5-7 pixels and allowing two-pixel overlap is ideal for emitters at the recommended density. After box finding, the minimum photons threshold in the fitting step needs to be optimized. The minimum photons parameter contributes to determining which Gaussian fitted emitters pass as a true fit. To determine the proper minimum photon threshold for true GFP fits, the code includes a histogram plotting function to examine the photons/localization in both background (no cell lysate) and GFP-containing (plus cell lysate) samples (Figure 3D). This step is important because, while NaBH4 reduces the amount of fluorescence from impurities, it does not remove all background localizations. Figure 3D demonstrates the need to set a minimum photon threshold to reduce the number of detections from impurities. To determine this threshold, a histogram of background emitter intensities is calculated from imaging a sample that is not exposed to cell lysate (Figure 3D, top left). The majority of the background emitters were found to have values less than 475 photons. In comparison, the sample containing true GFP emitters showed a significant fraction of the distribution above 475 (Figure 3D, top right). The threshold is chosen by inspection to remove as many background counts as possible while minimizing the amount of signal loss from the lysate sample (Figure 3D, bottom row). The remaining background count density at this threshold is accounted for in the quantitative analysis.
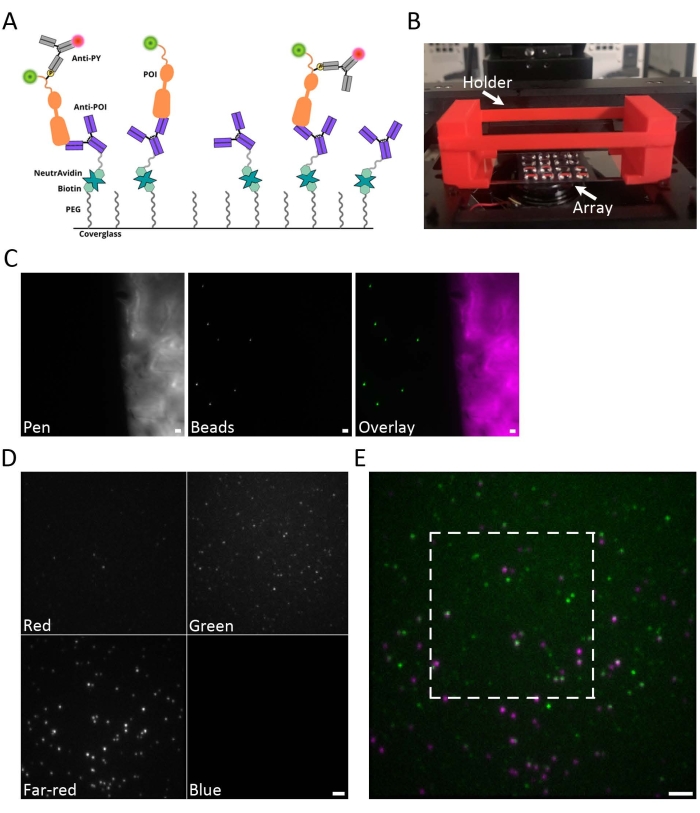
Figure 1: Overview of sample preparation. (A) Cartoon depicting the SiMPull approach. Coverslips are functionalized with an antibody that recognizes the POI to capture that POI from whole cell lysates. The glass is first coated with PEG and biotin-PEG. NeutrAvidin is then bound to the biotin-PEG and acts as an anchor for the biotinylated anti-POI antibody. Phosphorylated proteins are then detected with a fluorescently labeled anti-PY antibody. (B) Photograph of the coverslip holder (red) with coverslip array in place and mounted on the microscope stage. The multisample arrays are generated using hydrophobic ink to create up to 20 individual sample squares on a single glass coverslip. The coverslip is 60 mm x 24 mm. (C) Example images of the hydrophobic ink autofluorescence (magenta) and fluorescent beads (green). The autofluorescence of the hydrophobic ink is a useful guide to find the focal plane at the coverslip surface. (D) Example of a raw data image with spectral channels separated on the camera chip by the Quad-view image splitter. The Quad-view filter set includes the following emission filters: blue (445/45 nm), green (525/45 nm), red (600/37 nm), far-red (685/40 nm). (E) Raw overlay of green and far-red channels. The white box indicates the region further examined in Figure 2B–D. Scale bar (C-E) = 2 µm. Please click here to view a larger version of this figure.
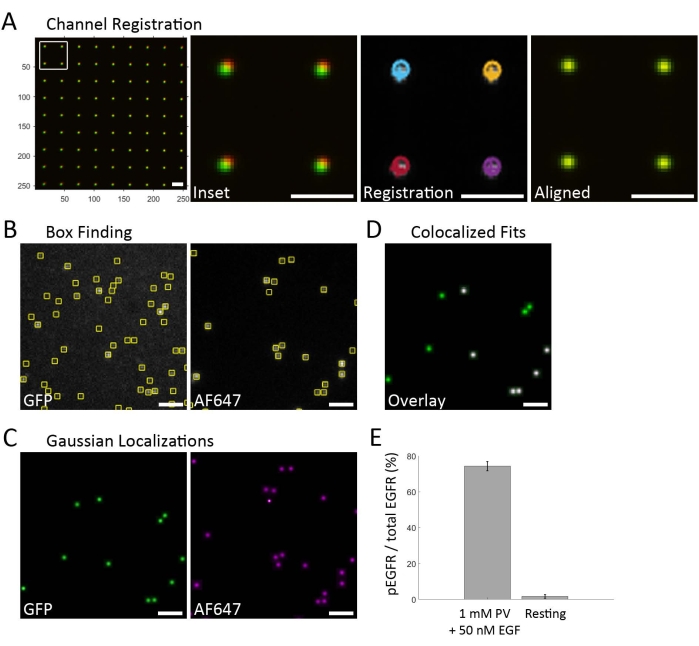
Figure 2: Data analysis workflow. (A) Channel registration is first performed on images acquired from the nanogrid. After cropping the two spectral channels of interest (here, green and far-red), the fiducial images for each channel are overlaid (left). Enlargement of the box in the left image (Inset) shows that the images are not yet truly registered. The emitters in each channel fit a Gaussian model and are localized (Registration). Localization of emitters is shown as circles for the far-red channel and crosses for the green channel. The final step is to apply a local weighted mean transform to shift the far-red channel localization coordinates into the green channel reference frame (Aligned). The calculated local weighted mean transform is then used to register the subsequent SiMPull data. (B) Representative images of the green/EGFR-GFP channel and the far-red/AF647-anti-PY channel. Single emitters above the background photon count are identified and marked with boxes. (C) The emission profile within each selected box is fit to a Gaussian model, and the emitters that fit the model of a single fluorophore PSF are kept. (D) A mask is created from the GFP emitters to identify the location of EGFR-GFP (green). Colocalization of EGFR-GFP and AF647-anti-PY identifies phosphorylated receptors (white). (E) The fraction of phosphorylated receptors is calculated from the colocalized EGFR-GFP and AF647-anti-PY fits. The bar graph compares PV + EGF treatment to resting cells, averaged for multiple measurements. Error bars represent standard error calculated assuming a binomial distribution. Scale bar = 2 µm. Please click here to view a larger version of this figure.
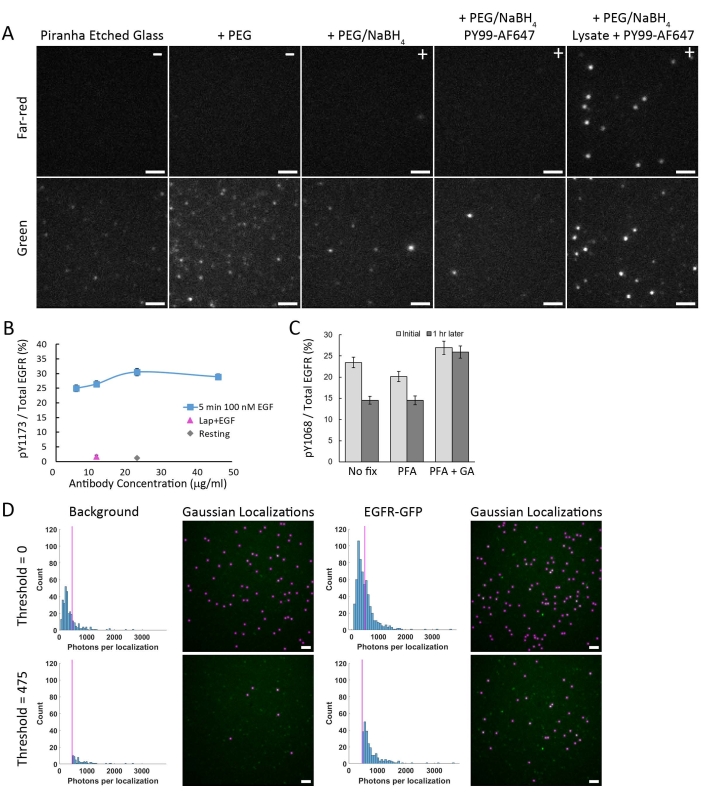
Figure 3: Critical steps to ensure data quality. (A) From left to right, the first three panels are representative images of the autofluorescence on glass under the respective conditions: after piranha etching, with PEG, and PEG plus NaBH4 treatment (indicated with +). Additionally, surface functionalization is retained after NaBH4 treatment as demonstrated by minimal non-specific PY99-AF647 binding while retaining robust binding of EGFR-GFP from the lysate. (B) A saturation curve must be acquired for each batch of antibodies used to ensure optimal antibody labeling. This figure shows the concentration curve for labeling EGFR with the site-specific phosphotyrosine antibody, anti-EGFR-pY1173. Minimal phosphorylation is detected in untreated cells (Resting, gray diamond). As a control for non-specific binding, cells were also treated with the EGFR kinase inhibitor, Lapatinib, before adding 100 nM of EGF (magenta triangle), which shows the expected prevention of EGFR phosphorylation. Error bars represent standard error assuming a binomial distribution. (C) Fixation of the sample with a combination of PFA and GA prevents antibody dissociation over time. Error bars represent standard error assuming a binomial distribution. (D) False positives are excluded by selecting the appropriate threshold for Gaussian fitting. Comparing the histogram of fit intensities at a low threshold (Threshold = 0; top) between background (no lysate) and real data (plus cell lysate) allows for selection of appropriate value (Threshold = 475; bottom) to remove fits from autofluorescent spots in the green channel. The vertical magenta line indicates a 475 photon threshold. Histograms are calculated from the same number of ROIs for each sample type (n = 3). Scale bar = 2 µm. Please click here to view a larger version of this figure.
Supplementary Coding File 1: zip file containing scripts and utilities for running SiMPull analysis. Please click here to download this File.
Supplementary Coding File 2: zip file containing the smite single-molecule analysis package. Please click here to download this File.
Supplementary Coding File 3: zip file containing the sample data. Please click here to download this File.
Supplementary Coding File 4: zip file containing representative sample data analysis outputs. Please click here to download this File.
Supplementary Coding File 5: Coverslip holder blueprint for 3-D printing. Please click here to download this File.
Discussion
The protocol described here was optimized to enable quantitative measurements of receptor phosphorylation at the single protein level. Several straightforward but important modifications to the SiMPull protocol were developed that improved the reliability of the measurement for phospho-tyrosine detection, including reduction of autofluorescence with NaBH4 treatment and postfixing of the sample to prevent antibody dissociation. Using the green channel mask to identify receptor locations for calculation of colocalization with the anti-PY antibody also improves the measurement accuracy by removing potential artifacts from the non-specific binding of the antibody to the cell lysate. The two-color imaging was utilized to detect the fraction of receptors phosphorylated. In this scenario, the receptor was genetically tagged with GFP, and the antibody was directly labeled with a far-red dye. The SiMPull approach applies to other protein targets for which specific antibodies are available, including intracellular proteins. In addition, because denaturing conditions are not required, multisubunit receptors/complexes can also be captured. However, denaturation may be incorporated if the PTMs of interest are located in structured regions of the protein14. Ultimately, SiMPull can be readily expanded to include simultaneous labeling of distinct phospho-tyrosines on individual receptors to quantify multisite phosphorylation patterns15. The interrogation of full-length, intact receptors in such a way cannot be achieved by other standard methods, including western blotting and phospho-mass spectrometry.
Along with the advantages of SiMPull, some limitations need to be considered. As with any antibody-based technique, the affinity and specificity of antibodies used are critical to the success of the measurement. Therefore, it is important to optimize antibody labeling conditions and ideally avoid secondary antibodies by using directly-labeled primary antibodies. Furthermore, the surface-bound antibodies will precipitate proteins localized to the plasma membrane and within cytosolic compartments. This can underestimate phosphorylation since cytosol-localized proteins are not accessible to the exogenously added ligand. Extra steps must be taken to correct the receptor surface levels (step 6.2.10). The anti-phosphotyrosine antibodies exhibited some non-specific binding once lysate was present. To avoid this artifact, the EGFR was genetically-tagged with GFP to identify the location of receptors, which allowed us to exclude the anti-PY signal off the receptor. If endogenous proteins are to be interrogated, then counterstaining with a total protein antibody can provide the mask image, with appropriate correction for any non-specific binding. Finally, while SiMPull provides information on heterogeneity at the protein level, the lysate generated in this protocol is from thousands of cells, and cell-to-cell variability is lost. However, advances towards single-cell SiMPull have been made using a flow chamber consisting of a coverslip and a microscope side with a 10 µm gap; bacteria were sparsely plated on the coverslip while the slide was functionalized with antibody to capture the desired proteins. Upon lysis of the bacteria, the proteins from each cell were captured in a confined area on the antibody-coated slide22. Similar single-cell SiMPull analysis of mammalian cells and protein phosphorylation may be possible in the future.
The SiMPull protocol contains several critical steps required to ensure high-quality data. For example, the protocol includes an elaborate preparation of the coverslip glass. Piranha etching coverslips thoroughly cleans the glass and increases hydroxyl groups and hydrophilicity, which are needed to optimize the coverslip surface. Following several washes with organic solvents, KOH treatment provides additional hydroxyl groups for aminosilanization13,23, which coats the glass with amine groups for PEG and biotin-PEG binding. Improper cleaning or functionalization at any of these steps will interfere with protein pull-down. Control of the molar ratio of PEG:biotin-PEG, along with lysate concentration, are key factors in obtaining appropriate protein IP density on the SiMPull substrate. As with any biological assay, there is variability between cell lysate preparations, and small differences between phosphorylation percentages may be seen between sample replicates. Therefore, it is important to measure the phosphorylation levels of different tyrosine sites within the same sample. The sample chamber described in this protocol provides a system to collect many data points in one imaging session and allows for averaging over multiple SiMPull experiments.
On the image acquisition side, it is important to obtain the fiducial sample to ensure an accurate channel overlay; otherwise, colocalization will not be accurate. It is also important to optimize laser power and camera settings to maximize signal-to-noise while at the same time minimizing photobleaching. Lastly, while the sample array requires a small amount of sample and reagents, the low volumes are susceptible to evaporation during the imaging session. It is important to periodically check the sample array (~30-45 min) and add buffer as needed to prevent samples from drying.
The present protocol demonstrated the use of SiMPull to quantify membrane receptor phosphorylation states. While focused on EGFR, the approach can be applied to other cell surface receptors and intracellular proteins and protein complexes, as long as appropriate antibodies are available. Another potential use for SiMPull is to interrogate the contents and phosphorylation status of phase-separated condensates. In addition, SiMPull can be used to measure other PTMs, such as ubiquitination. Therefore, SiMPull provides a unique tool for cell biologists to interrogate PTMs on intact proteins and correlate PTM patterns with cellular outcome.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health R35GM126934, R01AI153617, and R01CA248166 to DSL. EMB was supported through the ASERT-IRACDA program (NIH K12GM088021) and JAR by the UNM MARC program (NIH 2T34GM008751-20). We gratefully acknowledge using the University of New Mexico Comprehensive Cancer Center fluorescence microscopy shared resource, supported by NIH P30CA118100. We want to acknowledge Drs. Ankur Jain and Taekijip Ha, whose original development of SiMPull inspired this work.
ES-C present address: Immunodynamics Group, Laboratory of Integrative Cancer Immunology, Center for Cancer Research, National Cancer Institute, Bethesda
Materials
| 1.5 mL microcentrifuge tubes | MTC Bio | C2000 | |
| 10 mM Tris-HCl pH 7.4 | |||
| 10 mM Tris-HCl pH 8.0/ 50 mM NaCl | T50 Buffer | ||
| 100 mm Tissue Culture dish | CELLTREAT | 229620 | Storage of piranha etched glass/arrays |
| 15 mL conical tube | |||
| 16% Paraformaldehyde Aqueous Solution | Electron Microscopy Sciences | 15710 | Hazardous |
| 50 mL conical tube | Functionalized Glass storage/ KOH reuse | ||
| 50 mM Tris-HCl pH 7.2/150 mM NaCl | Lysis Buffer Component | ||
| 60 mm Tissue Culture dish | Corning | 430166 | |
| 8% Glutaraldehyde Aqueous Solution | Electron Microscopy Sciences | 16020 | Hazardous |
| Acetone (C3H6O) | Millipore Sigma | 270725 | Hazardous |
| Alexa Fluor 647 NHS Ester | Thermo Fisher Scientific | A-20006 | |
| Animal-Free Recombinant Human EGF | Peprotech | AF-100-15 | |
| Anti-Human EGFR (External Domain) – Biotin | Leinco Technologies, Inc | E101 | |
| Anti-p-Tyr Antibody (PY99) Alexa Fluor 647 | Santa Cruz Biotechnology | sc-7020 AF647 | |
| Bath-sonicator | Branson | 1200 | |
| BCA Protein Assay Kit | Pierce | 23227 | |
| Biotin-PEG | Laysan Bio | Biotin-PEG-SVA, MW 5,000 | |
| Bovine serum albumin | Gold Biotechnology | A-420-1 | Tyrode's Buffer Component |
| Buchner funnel | |||
| Bunsen burner | |||
| Calcium Chloride (CaCl2) | Millipore Sigma | C4901 | Tyrode's Buffer Component |
| Cell Scraper | Bioworld | 30900017-1 | |
| Conical Filtering Flask | Fisher Scientific | S15464 | |
| Coplin Jar | WHEATON | 900470 | |
| Countess II Automated Cell Counter | Thermo Fisher Scientific | AMQAX1000 | |
| Coverslips 24 x 60 #1.5 | Electron Microscopy Sciences | 63793 | |
| DipImage | https://diplib.org/ | ||
| DMEM | Caisson Labs | DML19-500 | |
| emCCD camera | Andor iXon | ||
| Fetal Bovine Serum, Optima | Bio-Techne | S12450H | Heat Inactivated |
| Fusion 360 software | Autodesk | ||
| Geneticin G418 Disulfate | Caisson Labs | G030-5GM | |
| Glacial Acetic Acid (CH3COOH) | JT Baker | JTB-9526-01 | Hazardous |
| Glass serological pipettes | |||
| Glass Stir Rod | |||
| Glucose (D-(+)-Glucose) | Millipore Sigma | D9434 | Tyrode's Buffer Component |
| Halt Phosphotase and Protease Inhibitor Cocktail (100X) | Thermo Fisher Scientific | 78446 | Lysis Buffer Component |
| HEPES | Millipore Sigma | H3375 | Tyrode's Buffer Component |
| Hydrochloric Acid (HCl) | VWR | BDH7204-1 | Hazardous |
| Hydrogen Peroxide (H2O2) (3%) | HX0645 | ||
| Hydrogen Peroxide (H2O2) (30%) | EMD Millipore | HX0635-2 | |
| Ice | |||
| IGEPAL CA-630 (NP-40) | Sigma Aldrich | I8896 | Lysis Buffer Component |
| ImmEdge Hydrophobic Barrier Pen | Vector Laboratories | H-4000 | |
| Immersol 518F immersion oil | Zeiss | 444960-0000-000 | |
| in-house vacuum line | |||
| L-glutamine | Thermo Fisher Scientific | 25030-164 | |
| Magnessium Chloride Hexahydrate (MgCl2-6H2O) | MPBio | 2191421 | Tyrode's Buffer Component |
| Matlab | Mathworks | Curve Fitting Toolbox, Parallel Computing Toolbox, and Statistics and Machine Learning toolbox | |
| Methanol (CH3OH) | IBIS Scientific | MX0486-1 | Hazardous |
| Milli-Q water | |||
| Mix-n-Stain CF Dye Antibody Labeling Kits | Biotium | 92245 | Suggested conjugation kit |
| mPEG | Laysan Bio | mPEG-succinimidyl valerate, MW 5,000 | |
| N-(2-aminoethyl)-3-aminopropyltrimethoxysilane | UCT United Chemical | A0700 | Hazardous |
| Nanogrid | Miraloma Tech | ||
| NeutrAvidin Biotin Binding Protein | Thermo Fisher Scientific | 31000 | |
| Nitrogen (compressed gas) | |||
| NVIDIA GPU with CUDA | Look for compatibility at https://www.mathworks.com/help/parallel-computing/gpu-support-by-release.html | ||
| Olympus iX71 Microscope | Olympus | ||
| Parafilm M Sealing Film | The Lab Depot | HS234526C | |
| PBS pH 7.4 | Caisson Labs | PBL06 | |
| PC-200 Analog Hot Plate | Corning | 6795-200 | |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | 15140-163 | |
| Phospho-EGF Receptor (Tyr1068) (1H12) Mouse mAb | Cell Signaling Technology | 2236BF | |
| Potassium Chloride (KCl) | Millipore Sigma | 529552 | Tyrode's Buffer Component |
| Potassium Hydroxide (KOH) | Millipore Sigma | 1050330500 | Hazardous |
| Premium PLA Filament, 1.75 mm diameter | Raise 3D | PMS:2035U/RAL:3028 | Printing temperature range: 205-235 °C |
| Pro2 3D printer | Raise 3D | ||
| Pyrex 1 L beaker | |||
| PYREX 100 mL storage bottles | Corning | 1395-100 | CH3OH/C3H6O reuse |
| Pyrex 250 mL beakers | |||
| Pyrex 4 L beaker | |||
| Quad-view Image Splitter | Photometrics | Model QV2 | |
| Refrigerated centrifuge | Eppendorf | EP-5415R | |
| RevCount Cell Counters, EVE Cell Counting Slides | VWR | 10027-446 | |
| Semrock emission filters: blue (445/45 nm), green (525/45 nm), red (600/37 nm), far-red (685/40 nm) | Semrock | LF405/488/561/635-4X4M-B-000 | |
| Serological pipette controller | |||
| Serological Pipettes | |||
| smite single molecule analysis package | https://github.com/LidkeLab/smite.git | ||
| Sodium Bicarbonate (NaHCO3) | Sigma Aldrich | S6014 | Hazardous |
| Sodium Borohydride (NaBH4) | Millipore Sigma | 452874 | Tyrode's Buffer Component |
| Sodium Chloride (NaCl) | Millipore Sigma | S9625 | Activate by successive heat and pH cycling |
| Sodium Hydroxide | VWR | BDH3247-1 | |
| Sodium Orthovanadate (Na3VO4) | Millipore Sigma | S6508 | Hazardous |
| Sulfuric Acid (H2SO4) | Millipore Sigma | 258105 | Hazardous |
| TetraSpeck Microspheres | Thermo Fisher Scientific | T7279 | multi-fluorescent beads |
| Tris (Trizma) base | Millipore Sigma | T1503 | |
| Trypan blue stain, 0.4% | Thermo Fisher Scientific | 15250061 | |
| Trypsin-EDTA 0.05% | Thermo Fisher Scientific | 25300120 |
Referenzen
- Lemmon, M. A., Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell. 141 (7), 1117-1134 (2010).
- Seet, B. T., Dikic, I., Zhou, M. M., Pawson, T. Reading protein modifications with interaction domains. Nature Reviews Molecular Cell Biology. 7 (7), 473-483 (2006).
- Coba, M. P., et al. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Science Signaling. 2 (68), (2009).
- Gibson, S. K., Parkes, J. H., Liebman, P. A. Phosphorylation modulates the affinity of light-activated rhodopsin for g protein and arrestin. Biochemie. 39 (19), 5738-5749 (2000).
- Stites, E. C., et al. Use of mechanistic models to integrate and analyze multiple proteomic datasets. Biophysical Journal. 108 (7), 1819-1829 (2015).
- Hause, R. J., et al. Comprehensive binary interaction mapping of SH2 domains via fluorescence polarization reveals novel functional diversification of ErbB receptors. PLOS ONE. 7 (9), 44471 (2012).
- Lau, E. K., et al. Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Science Signaling. 4 (185), (2011).
- Mishra, M., Tiwari, S., Gomes, A. V. Protein purification and analysis: next generation Western blotting techniques. Expert Review of Proteomics. 14 (11), 1037-1053 (2017).
- Brunner, A. M., et al. Benchmarking multiple fragmentation methods on an orbitrap fusion for top-down phospho-proteoform characterization. Analytical Chemistry. 87 (8), 4152-4158 (2015).
- Swaney, D. L., Wenger, C. D., Coon, J. J. Value of using multiple proteases for large-scale mass spectrometry-based proteomics. Journal of Proteome Research. 9 (3), 1323-1329 (2010).
- Curran, T. G., Zhang, Y., Ma, D. J., Sarkaria, J. N., White, F. M. MARQUIS: A multiplex method for absolute quantification of peptides and posttranslational modifications. Nature Communications. 6 (1), 1-11 (2015).
- Jain, A., et al. Probing cellular protein complexes using single-molecule pull-down. Nature. 473 (7348), 484-488 (2011).
- Jain, A., Liu, R., Xiang, Y. K., Ha, T. Single-molecule pull-down for studying protein interactions. Nature Protocols. 7 (3), 445-452 (2012).
- Kim, K. L., et al. Pairwise detection of site-specific receptor phosphorylations using single-molecule blotting. Nature Communications. 7 (1), 1-10 (2016).
- Salazar-Cavazos, E., et al. Multisite EGFR phosphorylation is regulated by adaptor protein abundances and dimer lifetimes. Molecular Biology of the Cell. 31 (7), 695 (2020).
- Huyer, G., et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. Journal of Biological Chemistry. 272 (2), 843-851 (1997).
- Smith, P. K., et al. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 150 (1), 76-85 (1985).
- Keller, H. E. Objective lenses for confocal microscopy. Handbook of Biological Confocal Microscopy. , 145-161 (2006).
- Hendriks, C. L. L., van Vliet, L. J., Rieger, B., van Kempen, G. M. P., van Ginkel, M. . Dipimage: a scientific image processing toolbox for MATLAB. , (1999).
- fitgeotrans: Fit geometric transformation to control point pairs. The MathWorks Inc Available from: https://www.mathworks.com/images/ref/fitgeotrans.html (2013)
- Raghavachari, N., Bao, Y. P., Li, G., Xie, X., Müller, U. R. Reduction of autofluorescence on DNA microarrays and slide surfaces by treatment with sodium borohydride. Analytical Biochemistry. 312 (2), 101-105 (2003).
- Wang, X., Park, S., Zeng, L., Jain, A., Ha, T. Toward single-cell single-molecule pull-down. Biophysical Journal. 115 (2), 283-288 (2018).
- Chandradoss, S. D., et al. Surface passivation for single-molecule protein studies. Journal of Visualized Experiments. (86), e50549 (2014).

