Cell-Type Specific Protein Purification and Identification from Complex Tissues Using a Mutant Methionine tRNA Synthetase Mouse Line
Summary
This protocol describes how to perform cell-type-specific protein labeling with azidonorleucine (ANL) using a mouse line expressing a mutant L274G-Methionine tRNA synthetase (MetRS*) and the necessary steps for labeled cell-type-specific proteins isolation. We outline two possible ANL administration routes in live mice by (1) drinking water and (2) intraperitoneal injections.
Abstract
Understanding protein homeostasis in vivo is key to knowing how the cells work in both physiological and disease conditions. The present protocol describes in vivo labeling and subsequent purification of newly synthesized proteins using an engineered mouse line to direct protein labeling to specific cellular populations. It is an inducible line by Cre recombinase expression of L274G-Methionine tRNA synthetase (MetRS*), enabling azidonorleucine (ANL) incorporation to the proteins, which otherwise will not occur. Using the method described here, it is possible to purify cell-type-specific proteomes labeled in vivo and detect subtle changes in protein content due to sample complexity reduction.
Introduction
Aberrant protein homeostasis is caused by an imbalance in protein synthesis and degradation. Several diseases are related to alterations in protein homeostasis. The hallmark of some diseases is the presence of aggregates in different subcellular locations and brain areas. Protein homeostasis is not only important in disease but also plays a crucial role in normal organ and cellular function1. For example, protein synthesis is necessary for many forms of neuronal plasticity2,3, as determined by the use of chemical inhibitors that block protein synthesis4. However, it is neither clear in which cell-types the proteome is altered to support learning and memory, nor is it understood which specific proteins in each cell-type increase or decrease in their synthesis or degradation. Thus, a comprehensive study of protein homeostasis requires the capability to differentiate proteomes coming from specific cell-types. Indeed, the identification of cell-type-specific proteomes to study cellular processes occurring in a multicellular environment has been an important hurdle in proteomics. For this reason, we developed a technique using MetRS* expression combined with bio-orthogonal methods that has proven to be an effective way to identify and purify cell-type specific proteomes, filling this gap5,6,7.
The expression of a mutant MetRS* (MetRS L274G) allows for the loading of the non-canonical methionine analog ANL into the corresponding tRNA8,9 and its subsequent incorporation into proteins. When MetRS* expression is regulated by a cell-type-specific promoter, the non-canonical amino acid will be incorporated into the proteins in a cell-selective manner. Once ANL is incorporated in the proteins, it can be selectively functionalized by click-chemistry and subsequently either visualized by imaging (FUNCAT) or by Western Immunoblot (BONCAT). Alternatively, proteins can be selectively purified and identified by mass spectrometry (MS). Using this technology, we created a mouse line expressing the MetRS* protein under the control of the Cre recombinase. Considering the increasing number of available Cre-mouse lines, the MetRS* system can be used in any field to study any cell-type from any tissue for which there is an existing Cre-line. Protein labeling with ANL is possible in vitro or in vivo, and does not alter mouse behavior or protein integrity6. Labeling timespan can be adapted to the scientific question of each researcher, labeling newly synthesized proteins (shorter labeling times) or entire proteomes (longer labeling times). The use of this technique is limited by the number of cells of the type that the researcher is willing to study; hence protein isolation from cell-types with low numbers or low metabolic rates is not possible by this method. The goal of the presented method is to identify cell-type-specific proteins/proteomes labeled in vivo. In this protocol, we describe how to label cell-type-specific proteomes with ANL in live mice and purify the labeled proteins. After purification, proteins can be identified by routine mass spectrometry protocols5,10. The reduction of sample complexity achieved in this method by the selective purification of proteins from specific cellular populations allows the experimenter to detect subtle changes in proteomes, for example, in response to environmental changes. Purification of the labeled proteins can be achieved in ~10 days, not including the MS analysis or the labeling period. Here, we describe two methods for ANL administration to MetRS* expressing mice, namely (1) adding the amino acid in the drinking water, and (2) introducing ANL by intraperitoneal injections. Regardless of the method chosen for ANL administration, the isolation and purification steps are the same (from step 2 on).
Protocol
All experiments with animals were performed with permission from the local government offices in Germany (RP Darmstadt; protocols: V54-19c20/15-F122/14, V54-19c20/15-F126/1012) or Spain (Committee of Animal Experiments at the UCM and Environmental Counselling of the Comunidad de Madrid, protocol number: PROEX 005.0/21) and are compliant with the Max Planck Society rules and Spanish regulations and follow the EU guidelines for animal welfare.
1. In vivo metabolic labeling with ANL
- Drinking water:
- Dissolve ANL and maltose in the regular drinking water of the mice. The recommended maltose concentration is 0.7% (wt/vol). The maximum amount of ANL added to the drinking water is 1% (wt/vol). Once the mixture is ready, sterilize it by filtration and store it at 4 °C.
- Provide the mixture prepared in step 1.1 to the mice. Change bottles frequently (e.g., every 3 days) to avoid contaminations, and check them every day to look for possible contamination or clogging. Monitor the amount of drunk water daily by weighing it, to know mice's ANL intake.
NOTE: The maximum labeling period evaluated here is 3 weeks using an ANL concentration of 1% in the drinking water, which resulted in well-detectable labeling in the brain. Good labeling can also be achieved in 2 weeks. Neither shorter labeling times, nor longer time points, nor other ANL amounts have been studied by us using this administration method, which does not imply that the foregoing would not work. ANL incorporation rates can vary depending on the studied tissue or cell-type. The time of labeling can vary depending on the scientific question; for example, if proteomes were to be labeled, then labeling time should be calculated in function of the average half-lives of the proteins in the studied tissue. If the interest is only to identify newly synthesized proteins, the times can be shortened. The labeling periods and ANL dosages have to be empirically tested for each experimental condition and cell-type of interest.
- Intraperitoneal administration:
- Dissolve ANL in physiological NaCl solution to 400 mM, phosphate saline buffer (PBS), or water.
- Make sure that the osmolarity of the solution is within the physiological range. Here, 10 mL/kg body weight of ANL solution is administered to the mice.
NOTE: Good labeling in the excitatory neurons of the brain is successfully obtained by one daily intraperitoneal injection (IP) with 400 mM ANL for 1 week. Neither lower ANL concentrations nor other labeling periods by IP injections have been tested in this study, which does not imply they would not work. ANL is supplied as hydrochloride by some companies. In this case, the pH of the solutions must be adjusted to the adequate pH (depending on the administration route). ANL can also be synthesized following the method published by Link et al.11 with some modifications5. ANL labeling can be performed using other ANL concentrations, timespans, and administration routes. For tissues/cell-types with low metabolic rates, a low content methionine diet can be used, always starting 1 week before ANL administration. When 400 mM ANL is used, it can precipitate while stored at 4 °C; heating up to 37 °C brings ANL back into the solution. Let it reach room temperature before injection. In most world regions, ANL administration to mice is an animal experiment and needs to be approved by the pertinent authorities.
2. Tissue harvesting, lysis, and protein extraction
- Tissue dissection: Dissect the area of tissue containing the cell-type of interest and note the weight of the collected piece of tissue.
NOTE: Samples can be stored at -80 °C for several months (stop point). In the present protocol, mice cortex and hippocampus were dissected. - Tissue lysis: Homogenize the tissue at room temperature by adding a volume 12-15 times the wet weight of the tissue of the lysis buffer (PBS pH 7.4, 1% wt/vol SDS, 1% (vol/vol) Triton X-100, Benzonase (1:1000 vol/vol), a protease inhibitor (PI) (EDTA free 1:4000)). For example, for a 20 mg tissue piece, add 240-300 μL of lysis buffer. Triturate the tissue until it is homogenized.
NOTE: Samples can be stored at -80 °C for several months (stop point). For direct homogenization in 1.5 mL tubes, the use of a handheld, battery-operated homogenizer is recommended, especially when small pieces of tissue are processed. For bigger pieces, a Dounce homogenizer can be used. To every buffer where protease inhibitors (PI) are included, they should be added immediately before use and not earlier. - Protein denaturation: Heat the homogenate to 75 °C for 15 min and centrifuge at 17,000 x g for 15 min at 10 °C. Transfer the supernatant to a new tube.
- Protein content measurement: Measure the protein concentration of each sample, and adjust all the samples to be compared to the same protein concentration; adjust the concentration by adding lysis buffer. An optimal concentration range is between 2-4 μg/μL.
NOTE: Samples can be stored at -80 °C for several months (stop point). - Alkylation
- Dilute the homogenized samples by 2-3 times in PBS (pH 7.8) + PI (EDTA free 1:4000).
- Add iodoacetamide (IAA) to a final concentration of 20 mM (stock solution 500 mM).
- Leave the samples for 1-2 h at 20 °C in the dark. Do steps 2.5.2-2.5.3 twice.
NOTE: For some tissues, if the non-specific click in the negative control remains high, it might be necessary to perform a reduction step prior to alkylation12. Prepare the IAA stock freshly just before adding it to the samples. Samples can be stored at -80 °C for several months (stop point).
- Buffer exchange: Equilibrate buffer exchange columns using the click chemistry exchange buffer (PBS pH 7.8, 0.04% (wt/vol) SDS, 0.08% (vol/vol) Triton X-100 and PI (1:4000)). Follow the manufacturer's instructions. Exchange all alkylated samples.
NOTE: Eluted samples can be stored at -80 °C for several months (stop point). In this step, the IAA is eliminated, and the buffer is exchanged by the click chemistry buffer. Protein can be measured again after the buffer exchange step to make sure that the proteins were not lost during the buffer exchange process. Depending on the type of columns used for buffer exchange, protein can be massively lost. Use the recommended columns to avoid protein loss. For sample storage, the preparation of aliquots is recommended. - Click reaction to evaluate ANL incorporation in the experiment
- Take 40 μL of the eluted samples in step 2.6 and add PBS (pH 7.8) to a final volume of 120 µL.
- To set up the click chemistry reaction, add the following reagents in the given order and vortex for 20 s following each addition: 1.5 µL of triazole ligand (stock 40 mM), 1.5 µL of biotin alkyne (stock 5 mM) and 1 µL of Cu(I) bromide (stock 10 mg/mL, dissolved in DMSO by careful pipetting up and down, do not vortex). Add the reagents as fast as possible.
- Incubate the samples overnight in the dark at 4 °C with continuous rotation.
- Centrifuge the samples at 4 °C for 5 min at 17,000 x g. A slightly turquoise pellet will be visible. Transfer the supernatant to a new tube.
NOTE: Prepare Cu (I) bromide stock immediately before use to avoid copper oxidation. Keeping the ratio of volumes between the lysed sample and PBS constant between samples is essential to ensure the same final detergent concentration in each tube. To speed up the assembly of the click chemistry reaction, a table vortexer with racks for tubes is recommended. Store the clicked samples at -80 °C for several months, or at -20 °C for a couple of weeks until further processing (stop point).
- Western blot analysis to evaluate ANL incorporation in the experiment
- Run an SDS-PAGE with 20-40 μL of the supernatant from the click chemistry reaction (step 2.7). Use 12% acrylamide gels, run in 1% SDS, 250 mM Tris-HCl, 2 mM glycine. Run until the front is out of the gel.
- Transfer the proteins to a nitrocellulose or PVDF membrane13.
- Incubate the membrane overnight at 4 °C with GFP antibody (1:500, GFP protein is co-translated with MetRS*5), and biotin antibody (1:1000) for clicked alkyne detection, which is conjugated to biotin.
- Wash the primary antibody 2 times for 10 min and add the secondary antibody for 1.5 h.
- Wash the secondary antibody 2 times for 10 min and scan (if using fluorophore-conjugated antibodies) or expose to films (if using horseradish peroxidase-conjugated antibodies).
- Quantify the biotin signal using ImageJ or similar software, evaluate the general labeling of each sample of the experiment, and detect possible outliers. Evaluate the signal-to-noise ratio (ANL sample labeling to background from the negative controls) of the experiment and detect possible animals which failed to take up/incorporate ANL.
NOTE: For samples with high ANL incorporation, protein purification is theoretically possible using non-cleavable alkynes like the one used in this section for the evaluation of ANL incorporation. Using brain samples, the background obtained in the negative controls by MS after protein purification using non-cleavable alkynes is too high14. Therefore, only the easier-to-handle, non-cleavable alkynes were used for a first general sample and experiment evaluation. Regardless of the type of alkyne intended for protein purification, it is advisable to perform the steps for alkyne dosage optimization described in the following section (step 2.9).
- Click reaction for cleavable alkyne dosage optimization
- Prepare four tubes with 40 μL of one representative sample (obtained in step 2.6 and evaluated in step 2.8) and add PBS (pH 7.8) to a final volume of 120 μL.
- Set up the click chemistry reaction as explained in step 2.7 but this time, add four different amounts of the DST-alkyne. For said alkyne, testing 5 μM, 15 μM, 30 μM, and 60 μM is recommended.
- Repeat step 2.8 to decide which is the most suitable alkyne dosage for the specific experimental question under study, based on the highest labeling efficiency with the lowest background signal.
NOTE: Before subjecting the remaining samples to a click reaction, the optimal amount of any alkyne needs to be determined. There are several options of commercially available alkynes that can be used. They differ in the way of cleavage (e.g., light or reducing agents). Copper-free click chemistry using strain-promoted reagents (e.g., DBCO reagents) is also possible. Tiny changes in the amount of either alkynes or DBCO reagents often increase the signal in the negative control (background) significantly. Here, the protein purification using an alkyne with a disulfide bridge cleavable by reducing agents (Disulfide biotin alkyne or DST-alkyne15) is described. When using the DST-alkyne, for running the SDS-PAGE (step 8.1), avoid loading buffers with strong reducing agents such as DTT or β-Mercaptoethanol to prevent alkyne cleavage. In this study, heating at 72 °C, for 5 min, using 5 mM of N-ethylmaleimide (NEM) in the loading buffer does not affect DST-alkyne stability. Step 2.9.2 can be repeated, testing more fine-grained dosages in the range of the best one observed.
- Preparative click reaction for protein purification
- Click all the samples obtained in step 2.6 with the optimal alkyne dosage determined in step 2.9 and analyze a fraction by SDS-PAGE and Western blot (step 2.8).
NOTE: Make sure that the pipettes are properly calibrated to ensure sample upscaling accuracy. Clicked samples can be stored at -80 °C for several months (stop point).
- Click all the samples obtained in step 2.6 with the optimal alkyne dosage determined in step 2.9 and analyze a fraction by SDS-PAGE and Western blot (step 2.8).
3. Protein purification
- Buffer exchange
- Subject all clicked samples to a buffer exchange procedure as described in step 2.6, but use Neutravidin binding buffer as equilibration buffer (PBS pH 7.4, 0.15% (wt/vol) SDS, 1% (vol/vol) Triton X-100 and PI (1:2000)).
NOTE: Eluted samples can be stored at -80 °C for several months (stop point). In this step, the non-clicked free alkyne is eliminated, and the buffer is exchanged by the Neutravidin binding buffer.
- Subject all clicked samples to a buffer exchange procedure as described in step 2.6, but use Neutravidin binding buffer as equilibration buffer (PBS pH 7.4, 0.15% (wt/vol) SDS, 1% (vol/vol) Triton X-100 and PI (1:2000)).
- Binding to Neutravidin beads
- Wash the Neutravidin high-capacity beads with the Neutravidin binding buffer (see step 3.1); mix the beads with the buffer and centrifuge the mixture for 5 min at 3000 x g, and discard the supernatant. Repeat three times.
- Prepare a 1:1 slurry of Neutravidin beads by adding the same volume of dry beads and Neutravidin binding buffer.
- Set aside 20-40 μL of each sample as pre-purified lysate and store it at -20 °C.
- Measure protein concentration (see step 2.4), and mix 100 μg of protein with 4 μL of the bead slurry obtained in step 3.2.2.
- Incubate the mixture overnight at 4 °C with continuous rotation, enabling labeled proteins to bind to the beads.
NOTE: As for the click chemistry reaction, the basic affinity purification reaction described in step 3.2.4 can be upscaled. For example, for purification of proteins from the excitatory neurons of the hippocampus, 1 mg of protein lysate is mixed with 40 μL of Neutravidin beads (1:1 slurry). The amount of Neutravidin beads can be scaled up or down according to the amount of labeled proteins per mg of total protein, which will depend on the chosen cell-type and labeling period and needs to be determined empirically.
- Neutravidin beads washes and protein elution
- Collect the supernatants by centrifugation (see step 3.2.1). Set a 20-40 μl aliquot of each supernatant aside for later analysis and freeze the rest at -80 °C.
- Wash the beads with chilled Neutravidin washing buffer 1 (PBS pH 7.4, 0.2% (wt/vol) SDS, 1% (vol/vol) Triton X-100 and PI (1:2000)) by adding the buffer, sedimenting the beads, and discarding the supernatant. Repeat this step three times.
- Then, add the same buffer and incubate the beads for 10 min under continuous rotation at 4 °C before discarding the supernatant. Repeat this step three times.
- Wash the beads as explained in steps 3.3.2-3.3.3 but using Neutravidin washing buffer 2 (1x PBS, pH 7.4, and PI 1:2000).
- Wash the beads as explained the step 3.3.2-3.3.3 but using Neutravidin washing buffer 3 (50 mM ammonium bicarbonate and PI 1:2000).
- Elute the clicked proteins by incubating the beads for 30 min at 20 °C with a volume of Neutravidin elution buffer (5% (vol/vol) β-mercaptoethanol and 0.03% (wt/vol) SDS) corresponding to one volume of the dry beads used.
- Maintain the beads in suspension during elution by continuous agitation (1000 rpm) in a thermoblock shaker. Elute twice and combine both eluates.
NOTE: The centrifugation set up for separation of the solid fraction of the slurry (beads) and the aqueous phase (supernatant), as explained in step 3.2.1, applies to steps 3.3.1-3.3.7. The SDS amount can be increased if protein elution is not complete. However, with amounts above 0.08%-0.1% of SDS, proteins non-specifically bound to the beads start to be eluted. β-mercaptoethanol is volatile and toxic; the use of a hood for steps 3.3.6-3.3.7 is advisable. The samples collected in steps 3.2.3 and 3.3.1 should be run by SDS-PAGE and evaluated by Western blot (see step 2.8) for evaluating the efficiency of the affinity purification (step 3.3).
- Evaluation of eluted proteins
- Load 1/3 of the eluted samples to an SDS-PAGE (see step 2.8.1).
- Stain the gel to visualize proteins using a high sensitivity method, such as silver staining or a fluorescent method.
- If the samples have the expected quality, meaning that there is 3-4-fold more protein in the ANL labeled samples compared to the negative control, the eluted proteins can be identified by routine mass spectrometry methods like the one explained in reference5.
Representative Results
Following the described protocol (summarized in Figure 1), ANL was administered to mice either by daily intraperitoneal injections (400 mM ANL 10 mL/kg, Nex-Cre::MetRS*) for 7 days, or via drinking water (0.7% Maltose, 1% ANL, CamkII-Cre::MetRS*) for 21 days. After labeling, the corresponding brain areas were dissected, lysed, alkylated, and clicked. Click reactions were analyzed by SDS-PAGE and Western Immunoblot. Representative images of the experiments are shown in Figure 2 for ANL administration by IP injection and in Figure 3 for ANL administration via drinking water. Note that the aim of the figures is to show that both labeling protocols work, not to compare them. Comparison is not possible because different neuronal populations are labeled in the two experiments shown (excitatory neurons of the hippocampus and cortex, and the Purkinje neurons of the cerebellum).
A further experiment provides an example for the determination of the optimal DST-cleavable alkyne concentration (Figure 4). In this example, the fold change between labeled sample and control is highest at an alkyne concentration of 14 μM. This alkyne concentration is then applied to all samples. After verification of the labeling of each sample in the experiment with the chosen alkyne amount, all samples were subjected to the purification of ANL-labeled/biotin-clicked proteins by affinity binding using Neutravidin beads (Figure 5). In this step, proteins are bound to the beads, washed, and subsequently eluted by reducing the disulfide bridge present in the DST-alkyne. After this last step, the biotin and part of the alkyne remain bound to the beads. The ANL-containing proteins (bound to the rest of the alkyne) are recovered in the elution buffer. To evaluate the efficiency of the elution step, one-third of the eluate volume is loaded onto an SDS-PAGE gel and visualized using a sensitive total protein stain method. At least a 3-fold intensity difference of total protein stain between negative controls and ANL-labeled samples must be observed to achieve interpretable results with mass spectrometry. Completion of all steps described in this protocol will be followed by MS sample preparation, acquisition, and analysis5. Although there are no special requirements for MS (each lab may use its routine MS protocol5,10), it should be kept in mind that the amount of purified proteins will be generally low (in the order of nanograms). Figure 6 provides an example of MS results with a clear enrichment in the ANL-labeled sample as compared to the control (Figure 6A). This difference was already visible in the total protein stain shown in Figure 5. Besides changes in peptide intensity, there are also unique proteins found in both samples (Figure 6B).
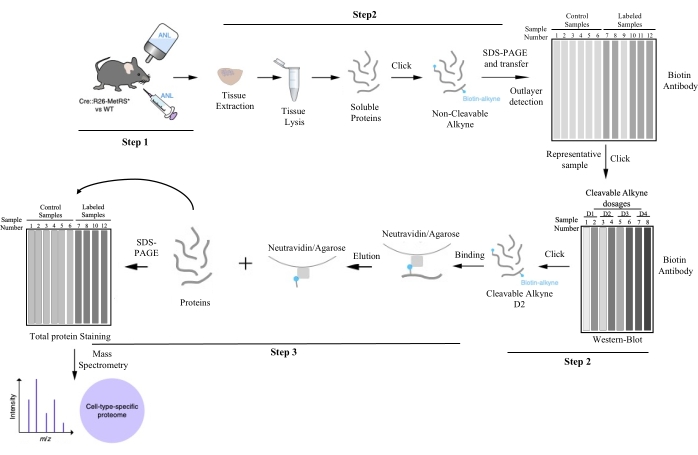
Figure 1: Work pipeline for cell-type-specific protein purification by BONCAT. Following protein labeling with ANL, the tissue of interest is dissected and lysed, tagged with biotin by click chemistry, and the amount of labeling for each sample is evaluated by BONCAT. Outliers (that failed to incorporate ANL) and representative samples can be differentiated in this step. One of the representative samples is clicked again to find the optimized cleavable alkyne dosage for subsequent protein purification. The alkyne dosage achieving the best signal-to-noise ratio is applied to every biological replicate. Cell-type-specific proteins are obtained by affinity purification. Purified samples are studied by mass spectrometry, and proteins are identified. This figure has been modified from Alvarez-Castelao et al. (2017)6. Please click here to view a larger version of this figure.
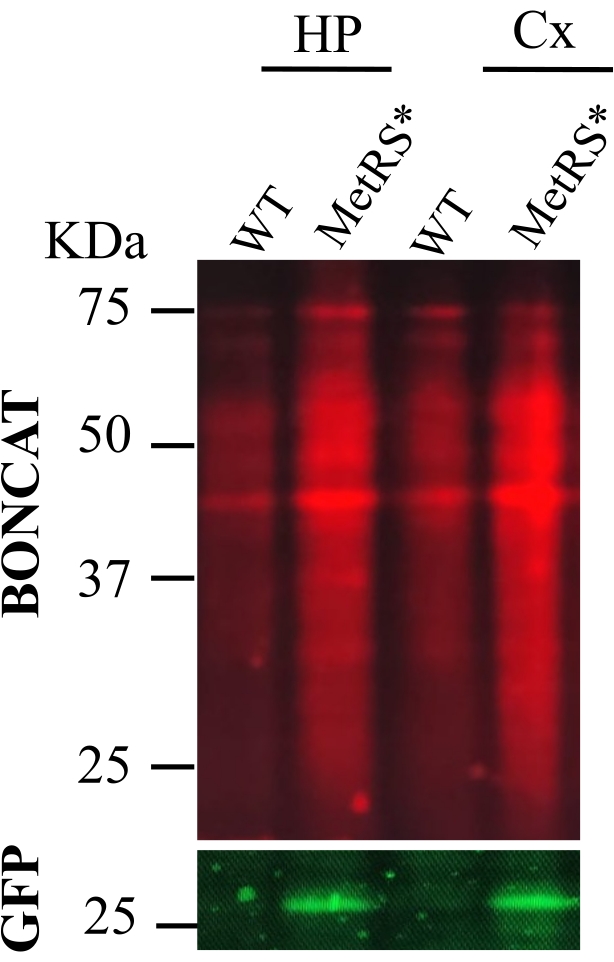
Figure 2: ANL administration by intraperitoneal injections (IP). BONCAT was performed to evaluate protein labeling in the hippocampus (HP) and cortex (CX) following metabolic labeling of proteins with ANL by daily IP injections for 7 days. Wild-type mice samples as negative control (wt) were clicked in parallel to the labeled samples (MetRS*). Labeled proteins are from the excitatory neurons using a Nex-Cre::MetRS* line16. Please click here to view a larger version of this figure.
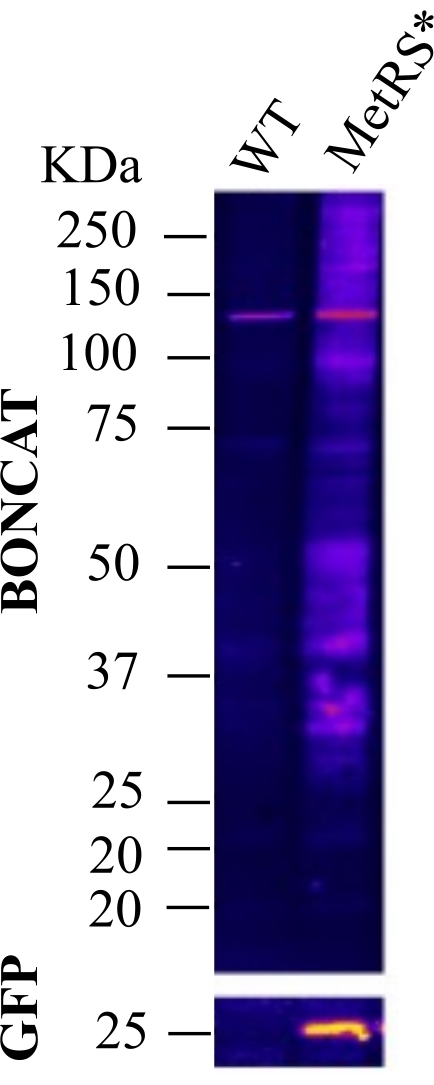
Figure 3: ANL administration by drinking water. BONCAT was performed to evaluate protein labeling in the Purkinje neurons of the cerebellum after administration of ANL to GAD-Cre::MetRS* mice via drinking water for 21 days. The figure shows a negative control (wt) and a labeled sample (MetRS*) lysed and clicked in parallel. This figure has been modified from Alvarez-Castelao et al. (2017)6. Please click here to view a larger version of this figure.
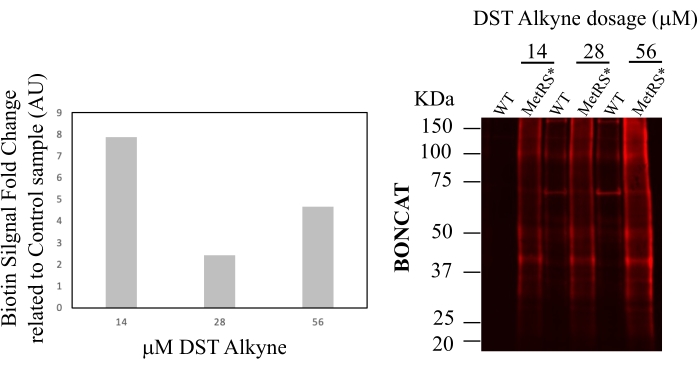
Figure 4: DST-alkyne dosage titration. One biological replicate per experiment is chosen as a representative sample to titrate the optimal alkyne concentration. Three concentrations (14, 28, and 56 μM) of the alkyne were tested here in the click reaction for BONCAT. The labeling ratio between the ANL-labelled sample (MetRS*) and the negative control (wt) determines the signal-to-noise ratio (shown in the graph). 14 μM is the best alkyne concentration obtained in this experiment. Cortex tissue from the ANL-labeled Nex::MetRS* mouse line was used for this experiment. Please click here to view a larger version of this figure.
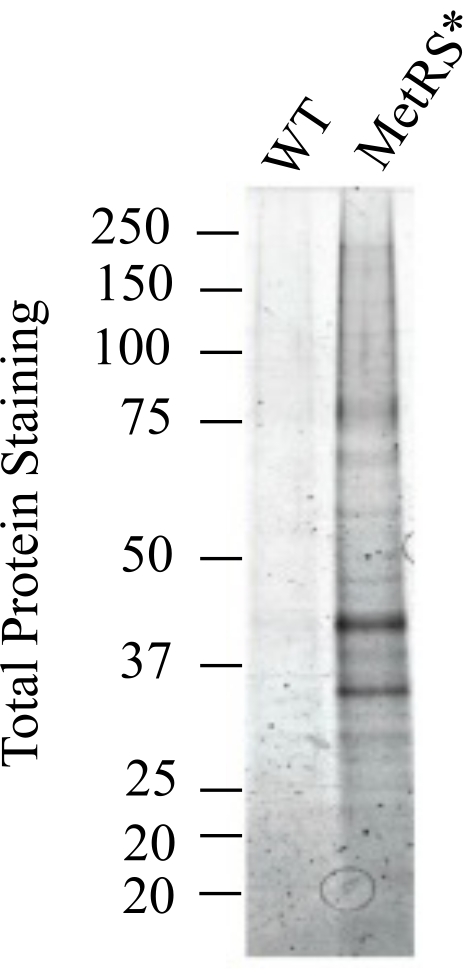
Figure 5: Purified proteins. Labeled proteins from Purkinje neurons were purified and stained using SYPRO Ruby. This figure has been modified from Alvarez-Castelao et al. (2017)6. Please click here to view a larger version of this figure.
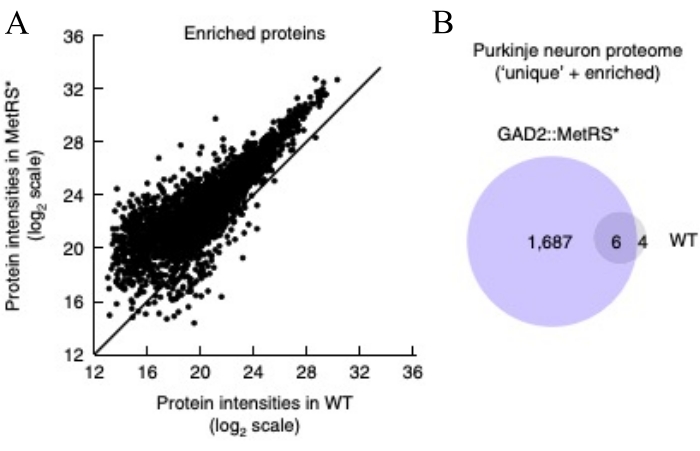
Figure 6: Protein identification and quantification. The Purkinje cell proteome was obtained by mass spectrometry using cerebellum from a GAD-Cre::MetRS* mouse line as starting material. (A) shows increased abundances (peptide intensity) of the identified proteins in ANL labeled samples compared to wild-type (wt) mice. (B) The Purkinje proteome was obtained by pooling proteins enriched in the ANL-labeled samples in A (defined by >3-fold intensities in MetRS* mice compared to wt), and unique proteins found in the MetRS*-expressing mice. This figure has been modified from Alvarez-Castelao et al. (2017)6. Please click here to view a larger version of this figure.
Discussion
The critical aspects of the protocol are; the inclusion of negative controls, having enough biological replicates, ANL administration route, amount, and duration, alkylation of the samples, alkyne concentration, and β-mercaptoethanol elimination when using DST-alkyne.
It is key to include negative control samples proceeding from ANL-labeled animals without Cre driver and therefore no MetRS* expression. These samples must be subjected to every step described in the protocol in parallel to the samples from ANL-labeled Cre-induced MetRS* mice. Non-clicked samples are not valid controls, as any results achieved using only this control may be due to non-specific click. We have not observed incorporation of ANL in cells not expressing the MetRS* gene, nor MetRS* expression in cells with no Cre; thus, wt animals labeled with ANL can be used as a negative control.
Given that the protocol hereby described consists of many steps where samples can be lost, it is recommended to calculate biological replicates bearing technical failure in mind. For brain samples, there is approximately 30% replicate loss.
We describe here two ANL administration routes and durations. This does not exclude that other administration routes (e.g., adding ANL to the food), work equally well. With respect to the administered ANL amount, the experiments shown here were performed using relatively high amounts of ANL. Labeling with lower amounts is also possible depending on the experimental set-up. The shortest time span of ANL labeling reported in this protocol is 1 week, and the longest 21 days. Shorter or longer labeling periods could be applied, but we have not determined it. Researchers should determine the ANL administration route, ANL dosage, and ANL labeling period adequate for the specific experimental question under study by considering the metabolic properties of the tissue, the cell-type of interest, and the experimental question under study.
Sample alkylation, also known as capping, is an important step to avoid background click17. When alkylation is properly done, the non-specific click as monitored by the control samples is reduced, and the difference between the negative control and ANL-labelled samples is larger. Elimination of free IAA after alkylation is also a key step. The presence of small amounts of IAA during the click reaction will limit its efficiency. Occasionally, the desalting step which also eliminates IAA (step 2.6), has to be repeated to ensure proper removal of IAA.
There are several biotin-alkynes and -DBCO reagents commercially available from a variety of companies. The main differences among them regard the polyethyleneglycol (PEG) linker chain length and the absence, presence, and type of cleavable groups. Regardless of the type used, excess amounts of alkyne lead to non-specific click to proteins bearing only natural amino acids. This can be easily prevented by precise and careful titration of the alkyne amount. As described, the best way to achieve this is using the actual lysed and alkylated samples to be used in the subsequent protein purification steps (step 2.6) and mass spectrometry analysis.
When DST-alkynes are used in the click reaction, β-mercaptoethanol reduces the disulfide bridge and dissociates the labeled proteins from the beads in the elution step (step 3.3). β-mercaptoethanol must be removed from the samples to allow the enzymatic digestion needed for MS. There are several ways to do this (e.g., lyophilization18, excision from a gel19, or cleaning using S-Trap columns20), and the choice should be made by the MS laboratory processing the samples.
Modifications and troubleshooting of the method should be directed to optimize the critical steps mentioned in the protocol. For instance, if there is too much variability, add more biological replicates; if the labeling is too low, increase ANL concentration, use longer labeling time spans, or test other ANL administration routes that could be more efficient for the studied tissue. For protein alkylation, a previous reducing step to the addition of IAA could be implemented.
The main limitation of the method is the purification of proteomes arising from small cell populations even if a small tissue area is dissected. Proteomes of more numerous cell populations that are, however, spread out over larger tissue areas are also difficult to access. Nevertheless, the in vivo labeling technique described here can still be applied to assess the referred cell-types by imaging (e.g., with FUNCAT or FUNCAT-PLA21).
This method is currently the only method available for in vivo study of cell-type-specific proteomes based on full-length proteins and for in situ visualization of cell-type-specific complete proteins. Other non-canonical amino acids such as azidohomoalanine (AHA) can also be used for in vivo protein labeling and purification of proteomes, but lack cell cell-type specificity22,23. Other approaches such as puromycin are suitable for providing a fixed picture of protein synthesis24,25. Nevertheless, using non-canonical amino acids for longer periods of labeling is possible, also reflecting protein degradation and showing a more accurate cellular proteome. BioID based methods are used to identify proteins in specific subcellular regions, regardless of their moment/place of synthesis26.
In the mouse line that we established (available from Jackson Lab, stock no. 028071), the mutant methionine tRNA synthetase (MetRS*) that allows for the incorporation of ANL into the cells is expressed in a Cre dependent manner. With this mouse line as a tool, it is possible to exclusively express the MetRS* in cell-types for which Cre-driver lines are available. This arrangement endows the method with considerable versatility, making it useful in almost every area of biomedical research.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
B.A-C is funded by the Spanish Ministry of Science and Innovation (Ramón y Cajal-RYC2018-024435-I), by the Autonomous Community of Madrid (Atracción de Talento-2019T1/BMD-14057), and MICINN (PID2020-113270RA-I00) grants. R. A-P is funded by Autonomous Community of Madrid (Atracción de Talento-2019T1/BMD-14057). E.M.S. is funded by the Max Planck Society, an Advanced Investigator award from the European Research Council (grant 743216), DFG CRC 1080: Molecular and Cellular Mechanisms of Neural Homeostasis, and DFG CRC 902: Molecular Principles of RNA-based Regulation. We thank D.C Dieterich and P. Landgraf for their technical advise and the synthesis of the DST-Alkyne. We thank E. Northrup, S. Zeissler, S. Gil Mast, and the animal facility of the MPI for Brain Research for their excellent support. We thank Sandra Goebbels for sharing the Nex-Cre mouse line. We thank Antonio G. Carroggio for his help with English editing. B.A-C. designed, conducted, and analyzed experiments. B.N-A, D.O.C, R.A-P, C. E., and S. t. D. conducted and analyzed experiments. B.A-C and E.M.S. designed experiments, and supervised the project, B.A-C wrote the paper. All authors edited the paper.
Materials
| 12% Acrylamide gels | GenScript | SurePAGE, Bis-Tris, 10 x 8, 12% | |
| β-Mercaptoethanol | Sigma | M6250 | Toxic; use a lab coat, gloves and a fume hood. |
| Ammonium bicarbonate | Sigma | 9830 | Toxic; use a lab coat, gloves and a fume hood |
| ANL | Synthesized as described previously for AHA (see references 5 and 11) | ||
| ANL-HCl | IrishBotech | HAA1625.0500 | |
| Benzonase | Sigma | E1014 | |
| Biotin alkyne | Thermo, | B10185 | |
| Chicken antibody anti-GFP | Aves | 1020 | |
| Complete EDTA-free protease inhibitor | Roche | 4693132001 | Toxic; use a lab coat and gloves. |
| Copper (I) bromide | Sigma | 254185 | 99.999% (wt/wt) |
| Disulfide tag (DST)-alkyne | Synthesized as reported in reference number 15, in which it is referred to as probe 20 | ||
| DMSO | Sigma | 276855 | |
| Filters | Merk | SCGP00525 | |
| Iodo acetamide (IAA) | Sigma | I1149 | |
| IR anti chicken 800 | LI-COR | IRDye 800CW | Donkey anti-Chicken Secondary Antibody |
| IR anti rabit 680 | LI-COR | IRDye 680RD | Goat anti-Rabbit IgG Secondary Antibody |
| Maltose | Sigma | M9171 | |
| Manual Mixer | BioSpec Products | 1083 | |
| NaCl | Sigma | S9888 | |
| N-ethylmaleimide | Sigma | 4259 | Toxic; use a lab coat, gloves and a fume hood. |
| NeutrAvidin beads | Pierce | 29200 | |
| Nitrocellulose membrane | Bio-rad | 1620112 | |
| PBS 1X | Thermo | J62036.K2 | |
| PBS 1X pH 7.8 | Preparation described in reference number 5 | ||
| PD SpinTrap G-25 columns | GE Healthcare | Buffer exchange | |
| Pierce BCA Protein Assay Kit | Thermo, | 23225 | Reagents in the Pierce BCA Protein Assay Kit are toxic to aquatic life. |
| Polyclonal rabbit anti-biotin antibody | Cell Signaling | 5597 | |
| PVDF membrane | Millipore | IPVH00010 | |
| SDS 10% | Sigma | 71736 | |
| SDS-PAGE Running buffer MOPS | GenScript | M00138 | |
| SYPRO Ruby stain | Sigma | S4942 | |
| Table automatic Vortexer | Eppendorf | Mixmate | |
| Triazole ligand | Sigma | 678937 | |
| Triton X-100 | Sigma | T9284 | |
| Water | Sigma | W4502 | Molecular biology grade |
Referenzen
- Alvarez-Castelao, B., Schuman, E. M. The regulation of synaptic protein turnover. Journal of Biological Chemistry. 290 (48), 28623-28630 (2015).
- Sutton, M. A., Schuman, E. M. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 127 (1), 49-58 (2006).
- Dorrbaum, A. R., Alvarez-Castelao, B., Nassim-Assir, B., Langer, J. D., Schuman, E. M. Proteome dynamics during homeostatic scaling in cultured neurons. Elife. 9, 52939 (2020).
- Flexner, J. B., Flexner, L. B., Stellar, E. Memory in mice as affected by intracerebral puromycin. Science. 141 (3575), 57-59 (1963).
- Alvarez-Castelao, B., Schanzenbacher, C. T., Langer, J. D., Schuman, E. M. Cell-type-specific metabolic labeling, detection and identification of nascent proteomes in vivo. Nature Protocols. 14 (2), 556-575 (2019).
- Alvarez-Castelao, B., et al. Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nature Biotechnology. 35 (12), 1196-1201 (2017).
- Ngo, J. T., et al. Cell-selective metabolic labeling of proteins. Nature Chemical Biology. 5 (10), 715-717 (2009).
- Mahdavi, A., et al. Engineered aminoacyl-tRNA synthetase for cell-selective analysis of mammalian protein synthesis. Journal of the American Chemical Society. 138 (13), 4278-4281 (2016).
- Yuet, K. P., et al. Cell-specific proteomic analysis in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 112 (9), 2705-2710 (2015).
- Patel, V. J., et al. A comparison of labeling and label-free mass spectrometry-based proteomics approaches. Journal of Proteome Research. 8 (7), 3752-3759 (2009).
- Link, A. J., Vink, M. K., Tirrell, D. A. Preparation of the functionalizable methionine surrogate azidohomoalanine via copper-catalyzed diazo transfer. Nature Protocols. 2 (8), 1879-1883 (2007).
- Landgraf, P., Antileo, E. R., Schuman, E. M., Dieterich, D. C. BONCAT: Metabolic labeling, click chemistry, and affinity purification of newly synthesized proteomes. Methods in Molecular Biology. 1266, 199-215 (2015).
- Kielkopf, C. L., Bauer, W., Urbatsch, I. L. Analysis of proteins by immunoblotting. Cold Spring Harbor Protocols. 2021 (12), (2021).
- Mellacheruvu, D., et al. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nature Methods. 10 (8), 730-736 (2013).
- Szychowski, J., et al. Cleavable biotin probes for labeling of biomolecules via azide-alkyne cycloaddition. Journal of the American Chemical Society. 132 (51), 18351-18360 (2010).
- Goebbels, S., et al. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 44 (12), 611-621 (2006).
- van Geel, R., Pruijn, G. J., van Delft, F. L., Boelens, W. C. Preventing thiol-yne addition improves the specificity of strain-promoted azide-alkyne cycloaddition. Bioconjugate Chemistry. 23 (3), 392-398 (2012).
- O’Fagain, C. Lyophilization of proteins. Methods in Molecular Biology. 244, 309-321 (2004).
- Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V., Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols. 1 (6), 2856-2860 (2006).
- Elinger, D., Gabashvili, A., Levin, Y. Suspension trapping (S-Trap) is compatible with typical protein extraction buffers and detergents for bottom-up proteomics. Journal of Proteome Research. 18 (3), 1441-1445 (2019).
- tom Dieck, S., et al. Direct visualization of newly synthesized target proteins in situ. Nature Methods. 12 (5), 411-414 (2015).
- McShane, E., et al. Kinetic analysis of protein stability reveals age-dependent degradation. Cell. 167 (3), 803-815 (2016).
- Calve, S., Witten, A. J., Ocken, A. R., Kinzer-Ursem, T. L. Incorporation of non-canonical amino acids into the developing murine proteome. Scientific Reports. 6, 32377 (2016).
- David, A., et al. Nuclear translation visualized by ribosome-bound nascent chain puromycylation. Journal of Cell Biology. 197 (1), 45-57 (2012).
- Schmidt, E. K., Clavarino, G., Ceppi, M., Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nature Methods. 6 (4), 275-277 (2009).
- Roux, K. J., Kim, D. I., Burke, B., May, D. G. BioID: A screen for protein-protein interactions. Current Protocols in Protein Science. 91, 11-15 (2018).

